Abstract
Post-ischemic neurodegeneration may be accelerated by a cytokine-receptor mediated apoptotic pathway, as shown in a transgenic rat overexpressing tumor necrosis factor-alpha (TNFα) in brain. To further investigate the mechanism of ischemic cellular injury in this animal, we tested the hypothesis that increased synthesis of TNFα augments neuronal death by promoting mitochondrial dysfunction, calcium dysregulation, and oxidative stress. Adult male TNFα-transgenic (TNFα-Tg) and non-transgenic (non-Tg) littermates underwent reversible middle cerebral artery occlusion (MCAO) for 1 hour followed by 1 hour of reperfusion. Cortical mitochondria were isolated from injured (ipsilateral) and uninjured (contralateral) hemispheres of ischemic rats or from pooled hemispheres of control animals. ATP synthesis was attenuated in non-ischemic TNFα-Tg rats, demonstrated by reduction of State III and respiratory control ratio, increased production of reactive oxygen species, and earlier formation of the calcium-induced membrane permeability transition pore. After MCAO, mitochondrial dysfunction was augmented more significantly in ischemic TNFα-Tg brain mitochondria than in non-Tg rats. These results show that mitochondrial dysfunction may be caused by increased brain levels of TNFα without physiological stress, but will be exacerbated after MCAO. We conclude that ischemic stress and synthesis of inflammatory cytokines synergistically augment mitochondrial dysfunction to promote neuronal death.
Keywords: calcium homeostasis, brain ischemia, transgenic rats, tumor necrosis factor alpha, mitochondrial bioenergetics, mitochondrial permeability transition pore, reactive oxygen species
1. Introduction
Tumor necrosis factor-alpha (TNFα) is a pro-inflammatory cytokine that is synthesized at high levels in ischemic brain (Vila et al., 2000; Zaremba et al., 2001). After stroke, the level of TNFα has been shown to rise sequentially within the infarct core, the ischemic penumbra, and normally-perfused cerebral tissue to remain elevated in spinal fluid, astroglia, and macrophages for up to 40 days (Tomimoto et al., 1996). Using a rat model of middle cerebral artery occlusion (MCAO), Liu and co-workers (Liu et al., 1994) reported that TNFα mRNA was increased in cortex after three hours of focal ischemia, followed by elevated levels of TNFα protein observed at 6 hours. Intracerebroventricular administration of exogenous TNFα significantly expands infarct volume (Barone et al., 1997).
Although TNFα is well recognized as a pro-apoptotic mediator of cell death, recent evidence suggests that it may have an alternate, beneficial role in augmenting neural recovery. This multi-faceted capacity of the cytokine appears to be driven by complex interactions between TNFα in its active, soluble form, its less active precursor, and its principal receptors on mammalian cells, p55/tumor necrosis factor-receptor 1 (TNF-R1) and p75/TNF-R2 (Marchetti et al., 2004; Wajant et al., 2003). Iosif and colleagues (Iosif et al., 2006) demonstrated that progenitor cell proliferation is enhanced within the subventricular zone (SVZ) in the brain of TNF-R1-/- mice subjected to MCAO. This finding suggests that TNF-R1 is a negative regulator of SVZ progenitor growth and indicates that a selective TNF-R1 antagonist, if administered effectively in vivo, may augment neural cell replacement after ischemia.
In ischemic brain injury, excitotoxic neurotransmission increases Ca2+ conductance through ion pumps distributed in the neuronal plasma membrane (Dugan et al., 1995; Dykens, 1994; Starkov and Fiskum, 2003; Sullivan et al., 2005). The rising intracellular content of Ca2+ exerts numerous secondary effects on mitochondria, the intracellular organelle that acts as the energy power house for the neuron (Nicholls and Budd, 2000). The salient element of this sub-cellular injury cascade is destabilization of the mitochondrial membrane potential (Δψm) that contributes to the synthesis of ATP (Mitchell, 1961), promotes sequestration of calcium (Ca2+) from the cytosol into the mitochondrial matrix (Gunter et al., 2004), and modulates generation of reactive oxygen species (ROS) to temper oxidative stress (Kroemer and Reed, 2000; Starkov and Fiskum, 2003). The membrane permeability transition (mPT) is driven by the formation of a proteinaceous pore between the inner and outer mitochondrial membranes and is dependent on Δψm and calcium homeostasis. Opening of the mPT pore results from excess Ca2+ uptake and will increase ROS production, inhibit ATP synthesis, and facilitate release of pro-apoptotic cytochrome c (Sullivan et al., 2005). Once released through perforations in the outer mitochondrial membrane, cytochrome c and the Smac/DIABLO protein complex will initiate caspase 8- and caspase 3-dependent cell death (Srinivasula et al., 2000). Thus, mitochondrial failure and its derivative cell signaling cascades lead to integrated necrotic and apoptotic neuronal death (Dugan et al., 1995; Kroemer and Reed, 2000).
Current evidence suggests that neuronal mitochondria impaired by ischemic stress contribute synergistically to TNFα signaling-induced cell death pathways (Shakibaei et al., 2005). In this paper, we examined the effect of enhanced TNFα synthesis on mitochondrial function in normally perfused and ischemic brain, as represented in the TNFα-transgenic (TNFα-Tg) rat. Within the brain of this animal, TNFα protein is overexpressed at 5-fold higher levels than in non-Tg or wild type littermates (Pettigrew et al., 2008). Previously, we showed that TNFα-Tg rats had increased caspase 3 activity within the infarct core and ischemic penumbra, and were more susceptible to apoptotic cellular death after 24 hours of reperfusion (Pettigrew et al., 2008). In the present work, we tested the hypothesis that elevated TNFα protein in ischemic brain will augment mitochondrial dysfunction, intracellular calcium dysregulation, and oxidative stress, thereby contributing to neural cell death.
2. Results
Whole blood glucose and hematocrit were assayed before ischemia in all animals subjected to MCAO. Arterial blood gases were sampled before ischemia, at the conclusion of 1 hour of MCAO, and after 15 minutes of post-ischemic reperfusion. Mean arterial blood pressure was recorded continuously during MCAO and for 1 hour afterward until animals were euthanatized. In comparing TNFα-Tg and non-Tg rats, there were no significant differences in pre-ischemic whole blood glucose and hematocrit or in serially-sampled MABP and arterial blood pH, PaO2, and PaCO2 (see Table 1). The only significant, between-group difference was in mean body weight that was slightly lower in TNFα-Tg rats than in non-Tg littermates (298.4 ± 28.7 v. 317.5 ± 28.7; p ≤ 0.05, unpaired t-test). This observation implied that transgenic animals were subject to mild growth delay, when compared to non-Tg littermates. We noted that transgenic rats have lower weight gain per week, over an 8-week time course between birth and surgical preparation for MCAO. We did not observe loss of weight from a pre-ischemic peak level in TNFα-Tg rats, which would indicate the presence of a wasting syndrome.
Table 1.
Physiological Data.
| Weight (g) | Glucose (mmol/L) | Hematocrit (%) | Heart Rate (BPM) | Mean Arterial Blood Pressure (mmHg) | Arterial Blood pH (Units) | Arterial Carbon Dioxide Pressure (mmHg) | Arterial Oxygen Pressure (mmHg) | |
|---|---|---|---|---|---|---|---|---|
| Pre-Ischemia | ||||||||
| Transgenic TNFα (n = 14) | 298.4 ± 28.7* | 7.6 ± 1.0 | 46.4 ± 1.1 | 351 ± 14 | 90 ± 5 | 7.31 ± 0.02 | 48.9 ± 4.2 | 72.4 ± 2.9 |
| Non-transgenic (n = 17) | 317.5 ± 28.7 | 7.3 ± 1.0 | 45.8 ± 1.1 | 357 ± 15 | 92 ± 8 | 7.31 ± 0.01 | 50.2 ± 5.6 | 71.1 ± 2.4 |
| Ischemia | ||||||||
| Transgenic TNFα (n = 14) | 360 ± 0.0 | 91 ± 7 | 7.31 ± 0.02 | 46 ± 2.6 | 74.6 ± 3.6 | |||
| Non-transgenic (n = 17) | 357 ± 10 | 89 ± 10 | 7.31 ± 0.01 | 48.1 ± 3.2 | 73 ± 2.9 | |||
| Post-ischemic Reperfusion | ||||||||
| Transgenic TNFα (n = 14) | 360 ± 0.0 | 93 ± 9 | 7.31 ± 0.01 | 45.3 ± 3.5 | 75 ± 4.2 | |||
| Non-transgenic (n = 17) | 358 ± 7 | 92 ± 8 | 7.32 ± 0.01 | 45.5 ± 3.7 | 76.4 ± 3.8 |
p ≤ 0.05
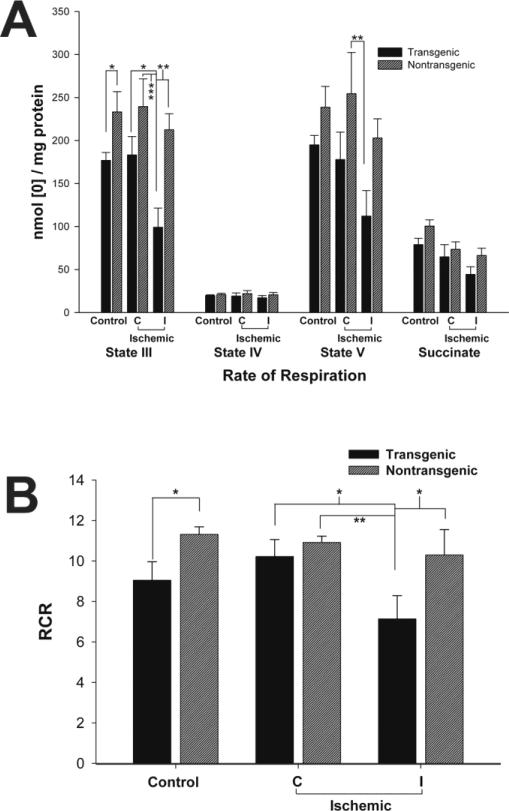
We characterized brain mitochondrial bioenergetics in TNFα-Tg and non-Tg rats by measuring oxygen consumption rates of different mitochondrial substrates and calculating the Respiratory Control Ratio (RCR; Figure 1), quantifying ROS production (Figure 2) and content of nicotinamide adenine dinucleotide (hydride) (NADH; Figure 3), and determining maximum mitochondrial calcium uptake capacity before mPT formation (Figure 4). The measured rates of oxygen consumption of different mitochondrial substrates and the derived RCR serve as important indicators of mitochondrial respiratory capacity and functional homeostasis. In the TNFα-Tg animal, altered mitochondrial respiration was observed in the control, non-ischemic state and in response to MCAO, shown in Figure 1. In Panel A of this Figure, ATP phosphorylation (State III) rates were significantly decreased in mitochondria sampled from TNFα-Tg rats, both in the control state (p ≤ 0.05; unpaired t-test; n = 6 animals per group) and after MCAO (p ≤ 0.05, 0.01, or 0.001; ANOVA/Fisher's test; n = 5 animals per group), when compared to non-Tg littermates. The most significant difference was in State III calculated for ischemic tissue within the ipsilateral hemisphere of TNFα-Tg animals, 99.1 ± 22.4 nmol [O]/mg protein, compared to mitochondria sampled from the unaffected, contralateral hemisphere in non-Tg rats, 239.5 ± 32.4 (p ≤ 0.001). There were no statistically significant differences observed between TNFα-Tg and non-Tg animals in respiration rates of State IV and succinate. Inter-group differences in State V were insignificant in control rats, although this variable was significantly decreased in mitochondria sampled from within the ischemic core in TNFα-Tg animals compared to unaffected cortex in non-Tg rats (p ≤ 0.01; ANOVA/Fisher's test; n = 5 animals per group). In Panel B of Figure 1, control TNFα-Tg rats had significantly lower RCR than non-Tg littermates (p ≤ 0.05; unpaired t-test; n = 6 animals per group). After MCAO, RCR was significantly lower in ischemic cortex sampled from TNFα-Tg rats compared with all other ischemic groups (p ≤ 0.05; ANOVA/Fisher's test; n = 5 animals per group). Again, the most significant difference was between reduced RCR in ischemic tissue from the ipsilateral hemisphere of TNFα-Tg animals, 7.1 ± 1.3, compared to mitochondria isolated from the unaffected, contralateral hemisphere in non-Tg rats, 10.9 ± 0.8 (p ≤ 0.01). From the data shown in Figure 1, we conclude that there is physiologically relevant compromise of bioenergetics in mitochondria sampled from TNFα-Tg rat brain, even without the physiological stress of cerebral ischemia. We observed that cerebral ischemia further impaired mitochondrial bioenergetics in TNFα-Tg rats to reduce ATP production in affected neural cells. These results substantiate the fundamentally important observation that pro-inflammatory TNFα signaling has negative effects on mitochondrial bioenergetics and function in cerebral ischemia.
Figure 1. Mitochondrial dysfunction observed in TNFα-transgenic and non-transgenic rat brain in the control state and after focal cerebral ischemia.
Mitochondrial function was assessed by measurement of respiration rate (nmoles [O]/mg protein; Panel A) and by calculation of respiratory control ratio (RCR; respiration in the presence of ADP as State III [ATP synthesis capacity] divided by respiration in absence of ADP as State IV; Panel B). In Panel A, control TNFα-Tg rats had significantly lower State III rates as compared with non-Tg littermates (n = 6 per group; p ≤ 0.05; unpaired t-test). After MCAO and 1 hour of reperfusion, mitochondria sampled from the ischemic zone (ipsilateral or I) in TNFα-Tg rat brain showed a significant decrease in State III as compared with the unaffected (contralateral or C) cortex in non-Tg rats (n = 5 per group; p ≤ 0.001; ANOVA/Fisher's test). Likewise, there were no significant between-group differences in control animals for State V but mitochondria isolated from the ischemic zone in TNFα-Tg brain again showed suppression of this respiration rate in comparison to unaffected cortex in non-Tg rats (p ≤ 0.01; ANOVA/Fisher's test). In Panel B, TNFα-Tg control rats had a significant decrease in mitochondrial RCR (State III/State IV) as compared with non-Tg animals (n = 6 per group; p ≤ 0.05; unpaired t-test). After MCAO, a significant decrease in RCR was observed within the ischemic zone in TNFα-Tg rat brain compared to the comparable volume in non-Tg brain (n = 5 per group; p ≤ 0.05; ANOVA/Fisher's test). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
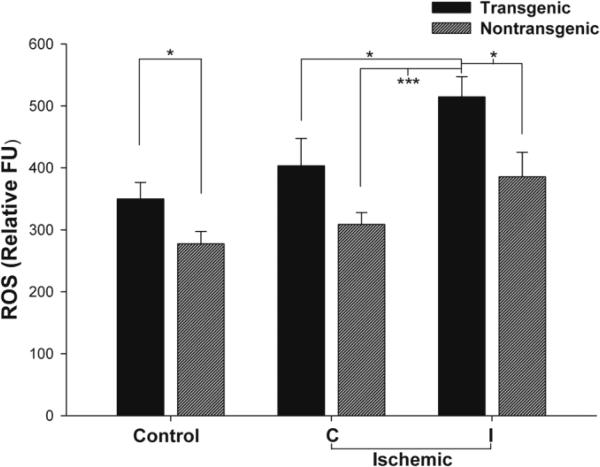
Figure 2. ROS production in cortical mitochondria isolated from TNFα-transgenic and non-transgenic rat brain in the control state and after focal cerebral ischemia.
Production of ROS was significantly greater in mitochondria isolated from control TNFα-Tg rat brain compared with non-Tg littermates (n = 7 - 8 per group; p ≤ 0.05; unpaired t-test). After MCAO and 1 hour of reperfusion, the ROS level in mitochondria isolated from the ischemic zone (ipsilateral or I) in TNFα-Tg rat brain was significantly greater than in the unaffected (contralateral or C) cortex in non-Tg rats (n = 5 per group; p ≤ 0.001; ANOVA/Fisher's test). *p ≤ 0.05; ***p ≤ 0.001
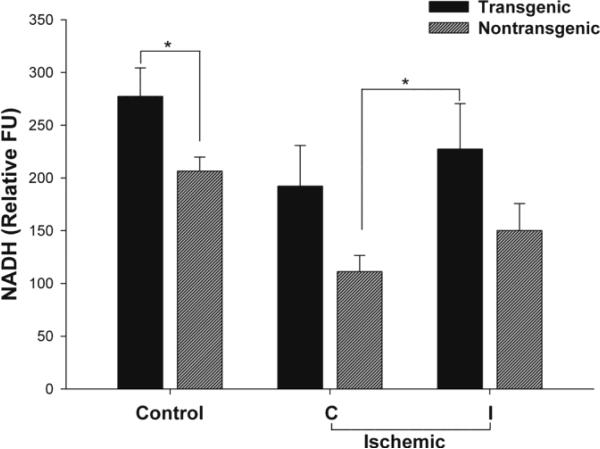
Figure 3. NADH Levels in cortical mitochondria isolated from TNFα-transgenic and non-transgenic rat brain in the control state and after focal cerebral ischemia.
Cortical mitochondrial NADH content was significantly higher in control TNFα-Tg rat brain than in non-Tg littermates (n = 4 - 6 per group; p ≤ 0.05; unpaired t-test). After MCAO and 1 hour of reperfusion, the NADH level in mitochondria isolated from the ischemic zone (ipsilateral or I) in TNFα-Tg rat brain was significantly greater than in the unaffected (contralateral or C) cortex in non-Tg rats (n = 4 - 5 per group; p ≤ 0.05; ANOVA/Fisher's test). *p ≤ 0.05
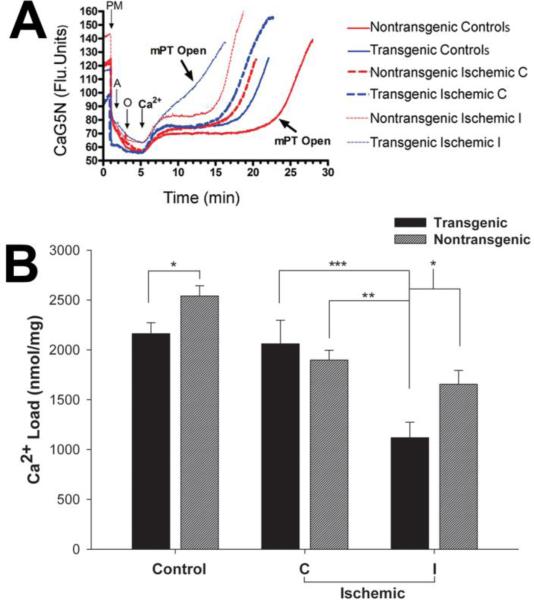
Figure 4. Measurement of calcium loading capacity in cortical mitochondria isolated from TNFα-transgenic and non-transgenic rat brain in the control state and after focal cerebral ischemia.
In Panel A, representative traces are shown to depict calcium storage capacity in regard to opening of the mitochondrial permeability transition (mPT) pore. Calcium was infused at the rate of 160 nmol/mg protein/min after isolated mitochondria were energized with the substrates pyruvate + malate, ADP, and oligomycin at 1,2 and 3 minutes, respectively. The pump was set for continuous infusion of Ca2+ starting at 5 minutes before anticipated opening of mPT to demonstrate immediate increase in extracellular Ca2+. Mitochondria isolated from control TNFα-Tg rat brain had attenuated calcium loading capacity, as represented by earlier opening of mPT that was not observed in non-Tg mitochondria. After MCAO and 1 hour of reperfusion, the most attenuated calcium loading capacity was observed in mitochondria isolated from the ischemic zone (ipsilateral or I) in TNFα-Tg rat brain. Quantitative measurements of maximal calcium loading capacity for mitochondria isolated from control and ischemic TNFα-Tg and non-Tg rat brain are shown in Panel B. Calcium loading capacity was significantly lower in control TNFα-Tg rat brain than in non-Tg littermates (n = 6 - 8 per group; p ≤ 0.05; unpaired t-test). After MCAO and 1 hour of reperfusion, mitochondria isolated from the ischemic zone of TNFα-Tg rat brain had significantly lower calcium loading capacity than in the same region in non-Tg cortex (n = 5 per group; p ≤ 0.05; ANOVA/Fisher's test). Significant differences were also observed between calcium loading capacity within the ischemic zone (ipsilateral or I) in TNFα-Tg brain and unaffected (contralateral or C) cortex in non-Tg animals (p ≤ 0.01) and between the ischemic zone and contralateral cortex in TNFα-Tg rats (p ≤ 0.001). *p ≤ 0.05; **p ≤ 0.01; ***p 0.001
Increased synthesis of mitochondrial ROS is an important contributor to acute neuronal degeneration observed in ischemic brain. We measured real-time synthesis of ROS in neural mitochondria sampled from TNFα-Tg and non-Tg littermates under control and ischemic conditions. As shown in Figure 2, control TNFα-Tg rats had significantly higher ROS production as compared to non-Tg littermates (p ≤ 0.05; unpaired t-test; n = 6 animals per group). After MCAO, ROS production was most significantly elevated in ischemic cortex sampled from TNFα-Tg rats compared with all other ischemic groups (p ≤ 0.05; ANOVA/Fisher's test; n = 5 animals per group). The most significant increase in ROS was in ischemic tissue sampled from the ipsilateral hemisphere of TNFα-Tg animals, 514.6 ± 32.7 relative FU, compared to mitochondria isolated from the unaffected contralateral hemisphere in non-Tg rats, 308.8 ± 19.0 (p ≤ 0.001).
The findings shown in Figure 2 demonstrate that mitochondrial ROS production was increased in association with constitutive upregulation of TNFα synthesis in the transgenic animal, whether under control or ischemic conditions. We cannot exclude that upregulated synthesis of TNFα was largely responsible for these effects. However, the occurrence of the peak level of ROS production in the ischemic brain of the TNFα-Tg rat, as it was significantly greater than in ischemic non-Tg animals, argues for an additive effect of the physiological stress of ischemia to the upregulation of TNFα synthesis.
We quantified mitochondrial content of NADH as a metabolic intermediate of the electron transport chain (ETC), as shown in Figure 3. In control TNFα-Tg rats, the mitochondrial content of NADH was significantly higher than in non-Tg littermates (p ≤ 0.05; unpaired t-test; n = 6 animals per group). At 1 hour of post-ischemic reperfusion, the NADH content declined in both TNFα-Tg and non-Tg rats, by respective comparison to control, non-ischemic animals. In both TNFα-Tg and non-Tg ischemic rats, the cortical mitochondrial NADH content was higher in the ischemic core than in a comparable region within the contralateral, unaffected hemisphere. In TNFα-Tg rats, the cortical mitochondrial NADH content within the ischemic core was significantly greater than in the unaffected hemisphere of the ischemic non-Tg group (p ≤ 0.05; ANOVA/Fisher's test; n = 4 - 5 animals per group). As depicted, there was no significant difference in NADH levels quantified within the ischemic core sampled from TNFα-Tg and non-Tg rat brain. However, there appeared to be relatively greater accumulation of NADH in non-Tg animals (approximately 35% increase in NADH above the level in well-perfused, contralateral hemisphere, compared to 20% in TNFα-Tg rats). These results suggest a differential effect of elevated TNFα on NADH utilization or on the activity of the tricarboxylic acid cycle, within mitochondria.
We measured mitochondrial calcium loading capacity in control and ischemic TNFα-Tg and non-Tg brain, as depicted in Figure 4. In Panel A of this Figure, we show representative traces depicting calcium storage capacity in mitochondria sampled from control and ischemic transgenic and non-Tg brain. These traces indicate that mitochondrial calcium storage capacity was lower in control, non-ischemic TNFα-Tg rat brain compared to non-Tg littermates. At 1 hour of post-ischemic reperfusion after MCAO, calcium load capacity was reduced even further in ischemic tissue within both TNFα-Tg and non-Tg brain. Importantly, the most severely affected mitochondrial calcium load capacity was observed in tissue sampled from the ischemic zone in TNFα-Tg rats. Also demonstrated in Panel A is premature formation and opening of the mPT pore in mitochondria sampled from ischemic TNFα-Tg brain. The data in Panel B of Figure 4 were prepared from calcium load traces calculated from individual samples, for quantitative assessment of calcium load capacity in TNFα-Tg and non-Tg brain. Control, non-ischemic TNFα-Tg rats had significantly lower mitochondrial calcium uptake compared with non-Tg littermates (p ≤ 0.05; unpaired t-test; n = 6 - 8 animals per group). We also noted that calcium uptake appeared to be less robust in the contralateral, normally perfused hemisphere in ischemic non-Tg animals, compared to non-ischemic controls, than by the same comparison in TNFα-Tg rats. After MCAO, the lowest calcium storage capacity was observed in mitochondria sampled from ischemic TNFα-Tg cortex, due to rapid opening of the mPT pore. This result indicates that mitochondria in TNFα-Tg brain are uniquely susceptible to ischemic injury, due to premature opening of the mPT pore.
3. Discussion
Our results show that TNFα-Tg rats have altered mitochondrial homeostasis as shown by higher basal ROS production that may be exacerbated during cerebral ischemia. We also noted less robust ATP phosphorylation, suppressed RCR, and greater susceptibility to calcium-induced mPT pore formation, all of which may predispose to neural cell death after ischemic stress. Mitochondria in control, non-ischemic TNFα-Tg rat brain appeared to retain unaltered ETC function but had impaired capacity for ATP synthesis. Overall, our findings show that chronic elevation of TNFα protein in brain undermines synthesis of ATP in neural mitochondria, an effect that may be aggravated by ischemic stress. When comparing bioenergetic capacity in ischemic TNFα-Tg and non-Tg brain, we observed that mitochondria sampled from transgenic rats had elevated NADH levels, more pronounced attenuation of ATP synthesis, and increased ROS production. These associated effects provide strong evidence of basally attenuated ETC cycling in neural mitochondria from TNFα-Tg brain tissue. Observed changes in mitochondrial bioenergetics in ischemic TNFα-Tg and non-Tg rat brain corroborated with larger 24-hour infarct size noted in transgenic animals, as described in our previous work (Pettigrew et al., 2008).
Among ischemic rats, we observed that some of the most significant differences in assayed mitochondrial function occurred between the ischemic zone in TNFα-Tg brain and the unaffected cortex in non-Tg littermates. We noted this effect in ATP phosphorylation (States III and V), RCR, ROS synthesis, NADH, and calcium buffering capacity. In all cases, these inter-regional differences in ischemic TNFα-Tg and non-Tg rat brain were associated with more fundamental differences in the same function, but in control, non-ischemic brain. We speculate that constitutive upregulation of TNFα synthesis in the transgenic rat compromises mitochondrial function even in the absence of ischemic stress. Therefore, the most significant differences in mitochondrial function could arise from comparison of maximally compromised tissue in the ischemic zone of TNFα-Tg brain to unaffected cortex in ischemic, non-Tg animals with normal mitochondria. We also noted that mitochondrial calcium buffering capacity was reduced more prominently in the unaffected, contralateral hemisphere of non-Tg rats than in transgenic animals, when compared to respective, non-ischemic controls. This observation echoes the findings of Clarkson and coworkers (2007) who reported disruptions of mitochondrial oxidative phosphorylation and ETC complexes within contralateral cortex in a neonatal rat model of unilateral, hemispheric hypoxia-ischemia. Those investigators attributed the observed, within-subject difference in mitochondrial function to trans-hemispheric diaschisis, which may also be active in our experimental preparation.
We measured mitochondrial content of NADH, an intermediate metabolite of oxidative phosphorylation, to better understand ETC regulation in ischemic brain subjected to chronic over-expression of TNFα protein. Our discovery of an increased level of NADH in non-ischemic TNFα-Tg brain suggests that constitutive upregulation of TNFα synthesis attenuates ETC cycling in the mitochondrial membrane, even without the added physiological stress of ischemia. These data fit well with our results assessing mitochondrial NADH-driven respiration and indicate that TNFα directly affects complex-I NADH utilization or alters components of the tricarboxylic acid cycle associated with NADH production. In animals that have not been rendered ischemic, the former possibility is favored by our observation that mitochondrial NADH is elevated in transgenic rats compared to non-Tg littermates. Based on this conclusion, we speculate that a lack of NADH was not the underlying cause of changes in mitochondrial respiration observed between these genotypes. In tissue sampled from the ischemic core, we observed reductions of mitochondrial NADH levels that were most prominent in non-Tg rats. During post-ischemic reperfusion, energy demands are increased uniformly in affected brain tissue. NADH would be consumed more rapidly than under control conditions as neural mitochondria increase respiration and utilize NADH to compensate for declining ATP levels. If TNFα-Tg animals have reduced State III mitochondrial respiration due to a direct modulation in TNFα , this effect could be exacerbated during cerebral ischemia and NADH would be relatively preserved. Therefore, reduction of mitochondrial NADH would be more pronounced in ischemic non-Tg rat brain than in TNFα-Tg animals, as we noted.
Our findings of altered mitochondrial bioenergetics in ischemic TNFα-Tg rat brain are extended from those reported previously in non-transgenic species. Ischemic neuronal death that is mediated by perturbation of mitochondrial function begins with excitotoxic neurotransmission that induces calcium influx to elevate intracellular Ca2+ content (Dugan et al., 1995; Dykens, 1994; Starkov and Fiskum, 2003; Sullivan et al., 2005). In stressed neurons, mitochondria will attempt to buffer elevated intracellular Ca2+ through upregulated absorption capacity. Increased mitochondrial Ca2+ has been shown to negatively affect organelle homeostasis by increasing ROS production, accelerating lipid and protein oxidation, and inhibiting ATP synthesis. The effect of Ca2+ cycling on induction of mPT and neuronal death is well recognized (Dugan et al., 1995; Dykens, 1994; Lipton, 1999; Sullivan et al., 2005). Mitochondrial swelling will occur in neurons within the first few hours of post-ischemic reperfusion (Petito and Pulsinelli, 1984) but can be prevented by mPT inhibitors (Kristal and Dubinsky, 1997). Impairment of mitochondrial respiration has been reported during 1 to 24 hours of post-ischemic reperfusion (Dave et al., 2001; Sims and Pulsinelli, 1987) and in hypoxic, immature brain (Puka-Sundvall et al., 2000). Also, disruptions of mitochondrial oxidative phosphorylation and ETC complexes (States I – IV) have been attributed to trans-hemispheric diaschisis after neonatal hypoxia-ischemia, as causal factors in post-ischemic apoptosis and neurodegeneration (Clarkson et al., 2007).
Our results have important implications for understanding the pathogenesis of cerebral ischemia, as influenced by a deleterious synergism between TNFα and dysfunctional mitochondria. Brain levels of TNFα rise within hours of ischemic cerebral injury, as inflammatory cytokines are released from activated microglia (Allan and Rothwell, 2001; Feuerstein et al., 1998). Cellular apoptosis induced by TNFα is mediated through the generation of ROS (Shakibaei et al., 2005). Lacerda and colleagues showed that TNFα stimulates ROS production in mitochondria (Lacerda et al., 2006). During ischemia, the hypoxia-dependent activation of the ROS-mediated apoptosis pathway is stimulated by TNFα (Haddad and Land, 2001). In animal models of hypoxic-ischemic cerebral injury in neonates and of ischemic stroke in the developed human brain, a complex feedback cycle has been demonstrated to involve synergism between release of pro-inflammatory cytokines and ROS synthesis in mitochondria (Ali et al., 1999; Floyd, 1999; Ginsberg, 1998). Prior to this report, there has been no demonstration of a relationship between constitutive elevation of TNFα and susceptibility of affected mitochondria manifested as premature mPT formation and loss of calcium buffering capacity. The present work is the first description of TNFα-mediated, time-dependent mitochondrial dysfunction after cerebral ischemia.
Our observations also provide support for the role of the TNFα-Tg rat as a clinically relevant model of cerebral ischemic injury in human stroke victims. Our transgenic animal shows robust elevation of TNFα as an inflammatory response to focal cerebral ischemia (Pettigrew et al., 2008), replicating the same phenomenon in human brain (Tomimoto et al., 1996; Vila et al., 2000). The increased synthesis of TNFα in the ischemic brain of the transgenic rat, compared to non-Tg controls, causes proportionately larger infarct volume, as was observed in human brain injured by stroke (Montaner et al., 2003; Zaremba et al., 2001). Our summary findings were of baseline alterations in mitochondrial bioenergetics that became exacerbated during ischemic stress in the brain of the TNFα-Tg rat. It may be argued that our observations are unique to the TNFα-Tg rat, are not unexpected with constitutive upregulation of TNFα protein synthesis, and are of limited translation to a non-transgenic or wild type phenotype. We counter that our goal was to use the TNFα-Tg rat in a practical manner to examine the coincident effects of elevated TNFα and mitochondrial impairment, such as is described in ischemic human brain. Among our accumulated findings, the impact of these synergistic effects may be represented most obviously by incremental worsening of mitochondrial calcium buffering in ischemic, compared to non-ischemic TNFα-Tg rats. Our observations provide rationale for exploratory studies of therapeutic benefit in using mitochondrial protectants or modulators of TNFα activity for the treatment of ischemic brain injury.
In conclusion, we demonstrated that perturbation of mitochondrial bioenergetic function can be observed in non-ischemic TNFα-Tg rat brain that is subject to chronic elevation of biologically active TNFα protein. We found that this mitochondrial dysfunction was exacerbated during focal cerebral ischemia, due to lessened calcium buffering capacity and premature opening of the mPT pore. We showed that the resulting cellular injury cascade will increase synthesis of oxidation products such as ROS and will impair synthesis of ATP. Terminal events in this cascade would be mitochondrial release of apoptosis-inducing regulators, culminating in activation of the executioner caspase 3 and ischemic neuronal apoptosis. Our findings suggest that TNFα , an inflammatory cytokine that rises to injurious levels in ischemic, reperfused brain, has secondary effects on mitochondrial function that magnify the risk of ischemic neuronal death.
4. Experimental Procedure
4. 1. Reagents
Mannitol, sucrose, bovine serum albumin (BSA), ethylene glycol-bis (2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA), hydroxyethyl piperazine-1-ethanesulfonic acid potassium salt (HEPES), potassium phosphate monobasic anhydrous (KH2PO4), magnesium chloride (MgCl2), malate, pyruvate, adenosine 5’-diphosphate (ADP), succinate, calcium chloride (CaCl2), potassium chloride (KCl), and horseradish peroxidase (HRP) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tetramethylrhodamine ethyl ester (TMRE) and 2’,7’-dihydro-dichlorofluorescein diacetate (DCFH2) were purchased from Molecular Probes (Eugene, OR). Oligomycin A, rotenone, and carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) were purchased from Biomol (Plymouth Meeting, PA).Bicinchoninic acid (BCA) protein assay kit was purchased from Pierce (Rockford, IL).
4.2. Focal Cerebral Ischemia
The construction of the TNFα-Tg rat and its detailed characterization have been published previously (Pettigrew et al., 2008). All experimental methods using animal subjects were approved by the Institutional Animal Care & Use Committee of the University of Kentucky in Lexington, Kentucky. Every effort was made to provide ethical, humane and compassionate care for animals during experimental procedures, in full observance of federal guidelines and of recommendations issued by the American Veterinary Medical Association.
All rat pups underwent genotyping from tail-snip tissue at 21 post-natal days, to confirm the presence or absence of the murine TNFα transgene construct (Pettigrew et al., 2008). In preparation for suture-occlusion of the MCA, adult (eight-week post-natal age) male TNFα-Tg rats and non-Tg littermates of 275 – 350 g body weight were fasted overnight and were anesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg) and xylazine (4 mg/kg). Rectal and temporalis muscle temperatures were maintained at 36.5 – 37.5°C through the use of a thermistor-controlled surgical platform (Harvard Apparatus). Suture-occlusion of the MCA was performed for 1 hour by our modification of the Zea Longa technique (Pettigrew et al., 2008). In animals prepared to undergo MCAO, a femoral artery catheter was inserted for continuous recording of mean arterial blood pressure (MABP) and for sampling of arterial blood gases. During post-ischemic reperfusion, animals were kept normothermic until they were euthanatized to undergo brain sampling for mitochondrial isolation. Transcardiac perfusion with India ink was used to identify the infarct core and the peri-infarct border zone that included the ischemic penumbra, by our published method (Pettigrew et al., 1996; Pettigrew et al., 2008).
4.3. Assays of Mitochondrial Bioenergetics
Our techniques for mitochondrial isolation and all assays described below are standardized and published (Pandya et al., 2007; Pandya et al., 2009; Sullivan et al., 2007). Male adult (8-10 week) TNFα-Tg rats and non-Tg littermates were decapitated under anesthesia and the brains were rapidly removed. Brain mitochondria were isolated from control (non-ischemic) TNFα-Tg rats and non-Tg littermates and from the same types of animals that were subjected to 1 hour of MCAO, followed by 1 hour of post-ischemic reperfusion. In control rats, cortical mitochondria were isolated from both cerebral hemispheres. In ischemic animals, mitochondria were isolated by sampling cortex within the ischemic injury zone (containing the developing infarct core and the peri-infarct region) and from a comparable region within the unaffected, contralateral hemisphere.
4.4. Mitochondrial Isolation
All procedures for mitochondrial isolation and for subsequently performed assays were performed on ice. Samples were obtained of cortical tissue comprised of all neural cell groups (neurons, glia, supporting cells). These samples were minced in an all-glass dounce homogenizer containing isolation buffer with 1 mM EGTA (215 mM mannitol, 75 mM sucrose, 0.1 % BSA, 20 mM HEPES, 1 mM EGTA and pH is adjusted to 7.2 with KOH). Mitochondria were isolated by differential centrifugation and Ficoll density gradient separation (Brown et al., 2006; Pandya et al., 2009). Tissue homogenates were centrifuged at 1300 × g for 3 minutes twice in an Eppendorf microcentrifuge at 4°C and the supernatant was transferred to fresh tubes. The resulting supernatant was transferred and topped off with isolation buffer with EGTA and centrifuged at 13,000 × g for 10 minutes. The supernatant was discarded and the pellet was resuspended in 500 μl of isolation buffer with EGTA. Microsomal and synaptosomal disruption was performed in a nitrogen cell bomb incubated at 1200 psi for 10 minutes to maximize the yield of total (synaptic and non-synaptic) mitochondria. The mitochondrial sample was further purified by discontinuous Ficoll density gradient (layered 2 ml of 7.5% Ficoll solution on top of 2 ml of 10% Ficoll solution) and ultracentrifuged at 100,000g for 30 minutes using Beckman ultracentrifuge with SW55Ti rotor. The pellet was carefully removed and further resuspended in isolation buffer (without 1mM EGTA) and washed once at 10,000g for 10 minutes. The final pellet contained mitochondria of >99% purity and was resuspended in isolation buffer without EGTA to achieve concentration of ~ 10mg/ml. The protein concentration was determined using the BCA protein assay kit measuring absorbance at 562 nm with a BioTek Synergy HT plate reader (Winooski, Vermont).
4.5. Mitochondrial Respiration
Mitochondrial respiration was assessed with a Clark-type oxygen electrode in a continuously stirred, sealed chamber thermostatically maintained at 37°C (Oxytherm System, Hansatech Instruments Ltd), as described previously (Pandya et al., 2009). Approximately ~50-100 μg of mitochondrial protein were added into the chamber containing 250 μl of KCl-based respiration buffer (125 mM KCl, 2 mM MgCl2, 2.5 mM KH2PO4, 0.1% BSA, 20 mM HEPES, pH 7.2). After 1 minute equilibration, the rate of respiration was started by the addition of complex I substrates, 5 mM pyruvate and 2.5 mM malate to monitor State II respiratory rates. State III respiration rates were measured subsequently after addition of two boluses of 150 μM ADP for 2 minutes followed by state IV respiration rates that were measured by the addition of 1 μM oligomycin. The mitochondrial uncoupler FCCP (1 μM) was added for measurement of complex I-driven maximum electron transport (state V) and was followed by addition of 0.8 μM rotenone to inhibit complex I in the chamber. After inhibition of complex I respiration, 10 mM succinate was added to the chamber to measure complex II-driven electron transport. The rate of respiration for each of the substrates added was calculated based on the slope of oxygen consumption. Respiratory Control Ratios (RCRs) were calculated by dividing state III oxygen consumption (defined as rate of respiration in the presence of ADP) by state IV oxygen consumption (rate of respiration obtained in the presence of oligomycin).
4.6. Mitochondrial ROS and NADH Production
Mitochondrial ROS production was assessed with 2’,7’-dichlorofluorescein diacetate (DCF), as described previously (Pandya et al., 2007). For this assay, DCF served as an indicator of the ROS pool contributed by H2O2, the stable reaction product of highly volatile superoxide anion. The reaction was carried out at 37°C by incubating 50 μg of isolated mitochondria in a total volume of 100 μl of reaction mixture containing 125 mM KCl respiration buffer, 5 mM pyruvate, 2.5 mM malate, 1 μM oligomycin, 1 μg/ml horseradish peroxidase (HRP), and 10 μM DCFH2 as oxidative substrates and indicators. ROS production was measured for 30 minutes using a Biotek Synergy HT fluorometric plate reader (excitation 485 nm, emission 528 nm), as described in our published methods (Pandya et al., 2007; Sensi et al., 2003; Sullivan et al., 2000). Mitochondrial NADH content was measured in the same plate using different fluorescence wavelength settings (excitation 360 nm, emission 460 nm) during ROS production. NADH content was measured at a single time point immediately after incubation of the solution within the plate for 5 minutes. Mitochondrial NADH content was measured as relative fluorescence units and expressed as arbitrary fluorescence units.
4.7. Mitochondrial Calcium Load Capacity and mPT Formation
Intramitochondrial calcium uptake was measured as per our published methods (Brown et al., 2006; Naga et al., 2007). Briefly, 100 μg of mitochondrial protein was incubated in 125 mM KCl buffer within a thermostatically-controlled (37°C), constantly-stirred cuvette. The 2-ml reaction mixture in the cuvette was read in a Shimadzu spectrofluorophotometer (Model RF-5301; Holliston, MA) after the following fluorescence indicators were added: 100 mM Calcium Green 5-N (for measurement of calcium signal; excitation 506 nm, emission 532 nm) and 100 nM TMRE (for measurement of membrane potential; excitation 550 nm, emission 575 nm). Mitochondrial substrates were added at 1-minute intervals by the following schedule: 1 minute - 5mM pyruvate and 2.5mM malate, 2 minutes - 150 μM ADP, and 3 minutes - 1 μM of oligomycin at 3 minutes. At 5 minutes, the real time Ca2+ infusion within the incubated cuvette was started, using an infusion pump (delivery rate of 160 nmol calcium/mg protein/min). Calcium infusion proceeded until mPT pore formation occurred and mitochondria were no longer able to absorb calcium within the matrix (until 15-20 minutes). For individual mitochondrial samples, we calculated nmol of calcium infused/mg protein as calcium load capacity, defined from the start of calcium infusion until mPT formation.
4.8. Statistical Analysis
Endpoint data are presented as means ± SEM. Group means sampled from control, non-ischemic animals were compared by unpaired t-tests. For comparison of ischemic group means, we used analysis of variance (ANOVA) followed by the Fisher post-hoc test (Statview 5.0 statistical package; SAS Institute, Cary, NC). For comparison of serially-acquired physiological monitoring data in ischemic animals, repeated measures ANOVA was used. Statistical significance was defined at p<0.05.
Research Highlights.
Tumor necrosis factor-alpha (TNFα) is synthesized at high levels in ischemic brain.
The TNFα-transgenic (TNFα-Tg) rat over-expresses active TNFα in its brain.
The electron transport chain (ETC) is weakened in mitochondria from TNFα-Tg brain.
Focal cerebral ischemia exacerbates ETC dysfunction in TNFα-Tg mitochondria.
Coincident ischemic injury and TNFα synthesis will hasten neural cell death.
Acknowledgements
This work was supported by NIH/NINDS grants awarded to the University of Kentucky (R01s NS048191 [PGS] and NS047375 [LCP]; P30 NS051220) and a Merit Review Award from the Medical Research Service, Department of Veterans Affairs (LCP). We thank Susan D. Craddock for her expert technical assistance. Sherry Chandler Williams, ELS, edited the manuscript and prepared the figures.
Abbreviations
- ADP
adenosine 5’-diphosphate
- BSA
bovine serum albumin
- CaCl2
calcium chloride
- DCF
2’,7’-dichlorofluorescein diacetate
- DCFH2
2’,7’-dihydro-dichlorofluorescein diacetate
- EGTA
ethylene glycol-bis (2-aminoethylether)-N,N,N',N'-tetraacetic acid
- ETC
electron transport chain
- FCCP
carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- HEPES
hydroxyethyl piperazine-1-ethanesulfonic acid potassium salt
- HRP
horseradish peroxidase
- KCl
potassium chloride
- KH2PO4
potassium phosphate monobasic anhydrous
- MABP
mean arterial blood pressure
- MCAO
middle cerebral artery occlusion
- MgCl2
magnesium chloride
- mPT
membrane permeability transition
- NADH
nicotinamide adenine dinucleotide (hydride)
- RCR
respiratory control ratio; mitochondrial respiration in the presence of ADP as State III [ATP synthesis capacity] divided by respiration in absence of ADP as State IV
- ROS
reactive oxygen species
- SVZ
subventricular zone
- Tg
transgenic
- TMRE
tetramethylrhodamine ethyl ester
- TNF
tumor necrosis factor
- TNF-R1
p55/tumor necrosis factor-receptor 1
- TNF-R2
p75/tumor necrosis factor-receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol. 1999;277:L1057–65. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–44. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha: a mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Clarkson J, Jackson DM, Sammut IA. Mitochondrial involvement in transhemispheric diaschisis following hypoxia-ischemia: Clomethiazole-mediated amelioration. Neuroscience. 2007;144:547–561. doi: 10.1016/j.neuroscience.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, Perez-Pinzon MA. Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cereb Blood Flow Metab. 2001;21:1401–1410. doi: 10.1097/00004647-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated CA2+ and Na+: implications for neurodegeneration. J Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5:143–159. doi: 10.1159/000026331. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Neuroinflammatory processes are important in neurodegenerative diseases: an hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic Biol Med. 1999;26:1346–55. doi: 10.1016/s0891-5849(98)00293-7. [DOI] [PubMed] [Google Scholar]
- Ginsberg I. Could synergistic interactions among reactive oxygen species, proteinases, membrane-perforating enzymes, hydrolases, microbial hemolysins and cytokines be the main cause of damage in infectious and inflammatory conditions? Med Hypotheses. 1998;51:337–346. doi: 10.1016/s0306-9877(98)90059-7. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Land SC. A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1alpha. FEBS Lett. 2001;505:269–274. doi: 10.1016/s0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. Journal of Neuroscience. 2006;26:9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal BS, Dubinsky JM. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and -independent pathways. J Neurochem. 1997;69:524–538. doi: 10.1046/j.1471-4159.1997.69020524.x. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lacerda L, Smith RM, Opie L, Lecour S. TNFalpha-induced cytoprotection requires the production of free radicals within mitochondria in C2C12 myotubes. Life Sci. 2006;79:2194–2201. doi: 10.1016/j.lfs.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jacobowitz DM, Barone F, McCarron R, Spatz M, Feuerstein G, Hallenbeck JM, Siren AL. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. Journal of Cerebral Blood Flow and Metabolism. 1994;14:348–352. doi: 10.1038/jcbfm.1994.43. [DOI] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. Journal of Biological Chemistry. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Montaner J, Rovira A, Molina CA, Arenillas JF, Ribo M, Chacon P, Monasterio J, Alvarez-Sabin J. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J Cereb Blood Flow Metab. 2003;23:1403–7. doi: 10.1097/01.WCB.0000100044.07481.97. [DOI] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiological Reviews. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Sullivan PG. The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp Neurol. 2009;218:381–389. doi: 10.1016/j.expneurol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Petito CK, Pulsinelli WA. Sequential development of reversible and irreversible neuronal damage following cerebral ischemia. J Neuropathol Exp Neurol. 1984;43:141–153. doi: 10.1097/00005072-198403000-00004. [DOI] [PubMed] [Google Scholar]
- Pettigrew LC, Holtz ML, Craddock SD, Minger SL, Hall N, Geddes JW. Microtubular proteolysis in focal cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism. 1996;16:1189–1202. doi: 10.1097/00004647-199611000-00013. [DOI] [PubMed] [Google Scholar]
- Pettigrew LC, Kindy MS, Scheff S, Springer JE, Kryscio RJ, Li Y, Grass DS. Focal cerebral ischemia in the TNFalpha-transgenic rat. J Neuroinflammation. 2008;5:47. doi: 10.1186/1742-2094-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puka-Sundvall M, Wallin C, Gilland E, Hallin U, Wang X, Sandberg M, Karlsson J, Blomgren K, Hagberg H. Impairment of mitochondrial respiration after cerebral hypoxia-ischemia in immature rats: relationship to activation of caspase-3 and neuronal injury. Brain Res Dev Brain Res. 2000;125:43–50. doi: 10.1016/s0165-3806(00)00111-5. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M, Schulze-Tanzil G, Takada Y, Aggarwal BB. Redox regulation of apoptosis by members of the TNF superfamily. Antioxid Redox Signal. 2005;7:482–496. doi: 10.1089/ars.2005.7.482. [DOI] [PubMed] [Google Scholar]
- Sims NR, Pulsinelli WA. Altered mitochondrial respiration in selectively vulnerable brain subregions following transient forebrain ischemia in the rat. J Neurochem. 1987;49:1367–1374. doi: 10.1111/j.1471-4159.1987.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, Alnemri ES. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem. 2000;275:36152–36157. doi: 10.1074/jbc.C000533200. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma. 2007;24:991–999. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Akiguchi I, Wakita H, Kinoshita A, Ikemoto A, Nakamura S, Kimura J. Glial expression of cytokines in the brains of cerebrovascular disease patients. Acta Neuropathologica. 1996;92:281–287. doi: 10.1007/s004010050519. [DOI] [PubMed] [Google Scholar]
- Vila N, Castillo J, Dávalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death and Differentiation. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Skrobanski P, Losy J. Tumor necrosis factor-alpha is increased in the cerebrospinal fluid and serum of ischaemic stroke patients and correlates with the volume of evolving brain infarct. Biomedicine and Pharmacotherapy. 2001;55:258–263. doi: 10.1016/s0753-3322(01)00058-0. [DOI] [PubMed] [Google Scholar]