Summary
Background
Physical activity (PA) has been reported to be reduced in severe chronic obstructive pulmonary disease (COPD). Studies in moderate COPD are currently scarce. The aim of the present study was to investigate physical activity in daily life in patients with COPD (n = 70) and controls (n = 30).
Methods
A multi-center controlled study was conducted. PA was assessed using a multisensor armband device (SenseWear, BodyMedia, Pittsburgh, PA) and is reported as the average number of steps per day, and the time spent in mild and moderate physical activity.
Results
Patients suffered from mild (n = 9), moderate (n = 28), severe (n = 23) and very severe (n = 10) COPD. The time spent in activities with mild (80 ± 69 min vs 160 ± 89 min, p < 0.0001) and moderate intensity (24 ± 29 min vs 65 ± 70 min; p < 0.0036) was reduced in patients compared to controls. The number of steps reached 87 ± 34%, 71 ± 32%, 49 ± 34% and 29 ± 20% of control values in GOLD-stages I to IV respectively. The time spent in activities at moderate intensity was 53 ± 47%, 41 ± 45%, 31 ± 47% and 22 ± 34% of the values obtained in controls respectively with increasing GOLD-stage. These differences reached statistical significance as of GOLD stage II (p < 0.05). No differences were observed among centers.
Conclusions
Physical activity is reduced early in the disease progression (as of GOLD-stage II). Reductions in physical activities at moderate intensity seem to precede the reduction in the amount of physical activities at lower intensity.
Keywords: Physical activity, COPD, Activity monitor, Steps, Energy expenditure
Introduction
Chronic obstructive pulmonary disease was recently defined as a preventable and treatable disease of the airways, with significant systemic consequences.1 Inactivity is believed to be crucial to the development of these systemic consequences of COPD2 such as skeletal muscle weakness, osteoporosis and cardiovascular disease.3 Recent data suggest that patients suffering from COPD with low levels of physical activity have increased risk for hospital admission and have significantly enhanced mortality.4 Epidemiological data suggest that this may directly or indirectly lead to more rapid decline in lung-function. 5 In the latter study, patients with COPD were followed for 20 years. Being moderately physically active resulted in a median survival benefit of roughly 7 years compared to patients with a very sedentary life style. Given the multiple health benefits of appropriate physical activity it is important to study levels of physical activity, particularly in mild to moderate disease. Identifying modifiable risk factors in early disease stages is particularly attractive as it offers the possibility to provide interventions with potential long term benefits. Recent guidelines of the American Heart Association have reinforced the importance of physical activity in maintaining health, including the elderly population and patients with chronic conditions.6
To date, only single centre studies have shown that patients with moderate to severe COPD have physical activity levels well below control subjects. Step or motion counts were 40 to 60 % lower7–9 and walking time was 55 % lower10 in patients with moderate to severe COPD compared to healthy control subjects. Patients on long term oxygen therapy were particularly inactive.8 To our knowledge, the only data available so far in mild to moderate COPD come from a cohort that has been followed in a single center in Germany.11,12 In these studies reduced daily physical activity was reported as of GOLD-stage II and a gradual decline of physical activity per GOLD-stage was found. A limitation of this study is that control subjects were all former smokers with symptoms of chronic bronchitis who were previously connected to the hospital. Hence it remains possible that physical activity levels could be even lower in comparison with a healthy control group with no prior connection to a hospital. Furthermore, only one study has so far compared physical activity levels from different centers and showed that patients from South America were more physically active than patients from Central Europe.13 This underlines the need to collect data in different stages of the disease and in different regions of the world in order to increase the generalizability of results.
The aim of the present pilot-study was therefore to investigate physical activity levels in patients across different disease stages in three different centers located in different regions of the world and compare the levels of physical activity with healthy, age-matched control subjects.
Materials and methods
A sample of 70 volunteers with COPD was actively recruited from three outpatient clinics in Palermo, Italy (n = 29), Leuven, Belgium (n = 20), and Pittsburgh, US (n = 21) by personal invitations from the pulmonary physician to participate in the study. In addition, 30 age-matched healthy control subjects were recruited in Leuven, Belgium. None of the volunteers had a previous connection with the hospital and all were relatives of students at the Department of Rehabilitation Sciences of the Katholieke Universiteit Leuven. Control subjects could participate if they were not involved in competitive sports activities and if they had normal lung function. In the healthy controls a clinical examination including a maximal cardiopulmonary exercise test was conducted to exclude any apparent chronic diseases or other morbidity. Subjects were instructed to report any current or past health problems. Resting ECG and lung function were normal in all control subjects. The appropriate institutional review boards approved the study and both patients and healthy controls gave informed consent.
Patients in the study had a known diagnosis of smoking related COPD and post bronchodilator FEV1 was used to classify the patients into the appropriate GOLD stage.14 Subjects could participate in the study if they had no other significant co-morbid conditions that would preclude the participants from having potentially normal physical activity levels (except for COPD in the patients). None of the participants used walking aids, as these may interfere with the assessment of physical activity levels with the activity monitor used in the current study. All patients had stable COPD without exacerbations in the past three months. None of the patients was referred for rehabilitation. Patients were distributed over the four GOLD stages. The characteristics of patients and control subjects are displayed in Table 1.
Table 1.
Group characteristics: age, anthropometry, forced expiratory volume in one second (FEV1 in liters and expressed as a percentage of the predicted value), and Forced Vital Capacity, expressed in % of the predicted value.
| Controls | COPD | P-value | |
|---|---|---|---|
| Age (yrs) | 65 ± 7 | 66 ± 9 | 0.65 |
| Gender (F/M) | 11/19 | 12/58 | 0.03 |
| Height (cm) | 168 ± 8 | 169 ± 8 | 0.58 |
| Weight (kg) | 74 ± 12 | 75 ± 15 | 0.66 |
| FEV1 (L) | 3.00 ± 0.73 | 1.48 ± 0.66 | <0.0001 |
| % Predicted | 114 ± 16 | 54 ± 23 | <0.0001 |
| GOLD I–II–III–IV (n) | – | 9–28–23–10 | |
| FVC (% predicted) | 125 ± 19 | 93 ± 22 | <0.0001 |
Study procedures
During an outpatient visit, spirometry was performed according to the guidelines of the American Thoracic Society and the European Respiratory Society.15 The best FEV1 and FVC of at least three acceptable maneuvers are reported. Body weight and height were assessed on the first study visit barefoot while wearing light clothing.
Assessment of physical activity
Subjects were instructed to wear a device able to assess physical activity (SenseWear Armband, Bodymedia, Pittsburgh, PA) continuously (day and night) for six to eight days. The light weight (80 g) activity monitor is worn on the back of the upper right arm at the level of the triceps. It assesses accelerations in two planes using a bi-axial accelerometer. Furthermore skin temperature, near body temperature, heat flux, and galvanic skin resistance are assessed and stored in one minute bins for further analysis. The use of sensors other than accelerometers and multisensor algorithms based on pattern recognition ensures that the device is relatively insensitive to motion artifacts such as driving a car or other artifacts due to external body movements. Total energy expenditure estimates of the activity monitor have recently been validated against the gold-standard of doubly labeled water by St-Onge and colleagues.16 Further promising results concerning validation of energy expenditure estimates during selected daily life activities in comparison with indirect calorimetry in patients with COPD have been reported recently.17,18 The device switches on automatically upon skin contact. Adherence with monitor use can be checked directly upon data retrieval.
The compliance with wearing the device, the number of steps, and time spent above pre-defined levels of energy expenditure were calculated and summarized per available day of assessment. The data reported, represent the mean values calculated over all days of assessment. Steps and time spent in at least moderate intense physical activities were regarded as co-primary endpoints since these parameters contain information on both the amount of daily activity and the intensity at which these activities are performed. Current guidelines stress the importance of engaging in activities of at least moderate intensity to achieve health benefits.6 Times spent in mild and high intensity physical activity were used as secondary outcomes.
Mildly active time was defined as the time spent in activities above an estimated energy expenditure of 2.5 Metabolic Equivalents (METs). This represents activities such as slow walking and light housework.19 The threshold for moderate physical activities was either set at 4.5 METs for people up to the age of 65 years or at 3.6 METs for subjects aged 65 years and older as proposed by Haskell and Pollock.20 Moderate intensity physical activity includes activities such as brisk walking and sweeping floors and vacuuming.19 Highly intense activity was defined as physical activity performed at an intensity above 6 METs. This includes activities such as jogging, shoveling, or carrying heavy loads.19
Statistical analysis
Statistical analysis was performed using the SAS statistical package (v8.1, SAS Institute, Cary, NC, USA). Data are presented using mean and standard deviation, unless specified otherwise. Baseline characteristics of patients and controls were compared using a t-test or a chi-square test. Linear trends for physical activity were compared in GOLD stages using ANOVA and Tukey’s multiple comparison tests. Outcomes were compared between centers using ANOVA testing with Duncan’s post-hoc testing. Correlation analysis was done using the Pearson’s correlation coefficient. In addition, a stepwise multiple regression analysis was performed to identify independent contributors to physical activity and steps. Age, anthropometric characteristics and FEV1, expressed as a percentage of the predicted value, were included in the model as possible covariates. Comparison of physical activity between centers was done using the general linear models procedure, adjusting for age, gender, body mass index, and predicted FEV1. A priori, a p-value of less than 0.05 was considered to be statistically significant.
Results
Patients and healthy controls were matched in terms of age and anthropometric characteristics except for gender distribution (Table 1). Distribution of patients across different GOLD stages is presented in Table 1. Patients in Pittsburgh were slightly younger (62 ± 10 years) than those in Leuven (68 ± 7 in Leuven p < 0.05), and slightly more overweight (BMI 28 ± 5 kg m−2) than those in Palermo (BMI 25 ± 5 kgm−2, p < 0.05). No other anthropometric differences were noted.
Physical activity in COPD compared to control
Study subjects wore the activity monitor for an average of 6.23 ± 0.66 days. Patients had significantly lower physical activity levels compared to control subjects. The number of steps per day was 5584 ± 3360 in patients, compared to 9372 ± 3574 in controls (p < 0.0001). In patients, the time spent in activities of moderate intensity reached 36 ± 45% of control values (24 ± 29 min/day vs. 65 ± 70 min/day; p = 0.004) and the time spent in activities of mild (above 2.5 METs) intensity reached 50 ± 43% of controls (80 ± 69 min/day vs. 160 ± 89 min/day, p < 0.0001). The time spent in activities of high intensity (above 6 METs) was only 34 ± 70% of control subjects (2 ± 5 min/day versus 7 ± 9 min/day; p = 0.01).
Comparison by GOLD stage
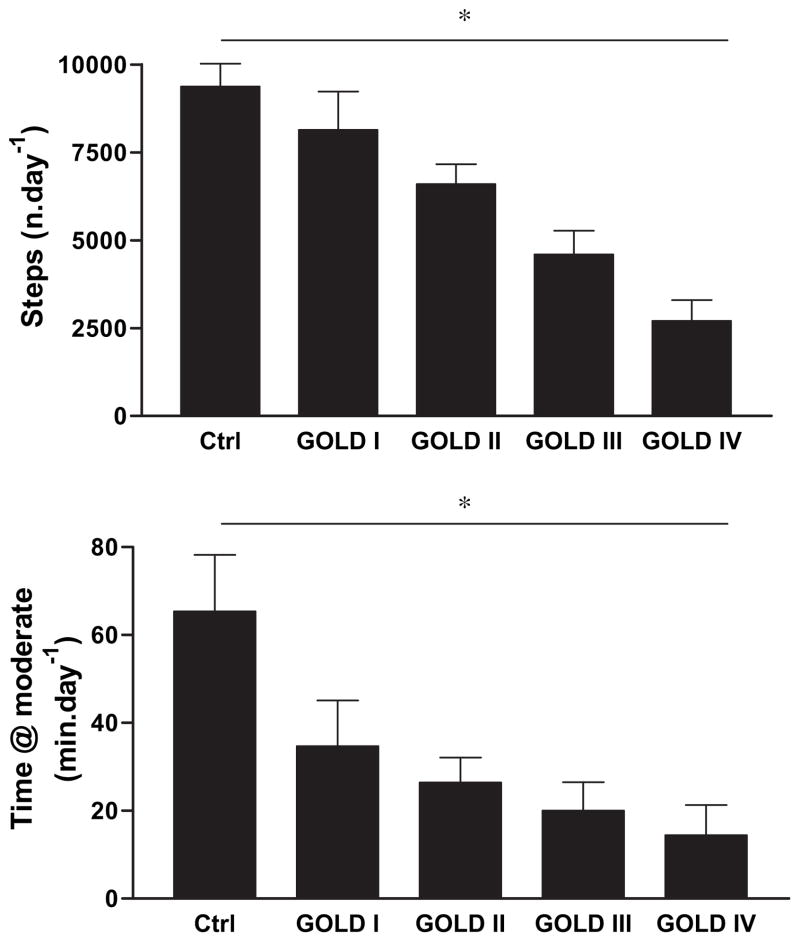
Fig. 1 illustrates the gradual reduction in physical activity levels across GOLD-stages. ANOVA for linear trends was significant for the number of steps per day and time spent at mild intensity (data not shown) and moderate intensity.
Figure 1.
The number of steps (upper panel) and the time spent in moderate physical activities (lower) in control subjects and patients with mild (GOLD I) to very severe (GOLD IV) COPD. Statistical significance of the ANOVA linear trend is indicated with *, post-hoc analysis indicated that physical activity was significantly different from controls as of GOLD-stage II.
As of GOLD stage II all physical activity related outcomes were statistically significantly reduced compared to controls. Compared to controls, the number of steps per day was reduced by −13 ± 34, −29 ± 32, −51 ± 34 and −71 ± 20% in GOLD stages I to IV respectively. The time spent in activities at moderate intensity was reduced by −47 ± 47, −59 ± 45, −69 ± 47 and −78 ± 34% respectively with increasing GOLD stage. These differences reached statistical significance as of GOLD stage II (p < 0.05). Patients in GOLD stage I and II had disproportionably larger reductions in time spent in moderate physical activities than in steps. In GOLD stage 1 for example we find a 13% reduction in steps in contrast with a 47% reduction in moderate intense physical activity in comparison with healthy controls (Table 2). In more severe stages the decline in steps and time spent in moderate physical activity became more proportional.
Table 2.
Reduction in the number of steps and the time spent in activities of at least moderate intensity (moderate PA) in patients with mild (GOLD I) to severe (GOLD IV) COPD. All data are expressed as a percentage of the healthy control subjects. The p-value represents the significance level of the difference in the proportional reduction of steps vs. time in moderate intense PA for each GOLD-stage.
| Steps | Time in moderate PA | P-value | |
|---|---|---|---|
| Gold I (% controls) | 87 ± 35 | 53 ± 48 | 0.0008 |
| Gold II (% controls) | 70 ± 32 | 41 ± 45 | 0.002 |
| Gold III (% controls) | 49 ± 34 | 31 ± 48 | 0.06 |
| Gold IV (% controls) | 29 ± 19 | 22 ± 33 | 0.31 |
Relation of physical activity with clinical and demographic parameters
In patients with COPD, a significant positive correlation was observed between FEV1 (%predicted) and the number of steps per day (R = 0.51, p < 0.0001), time spent in activities of mild intensity (R = 0.33, p = 0.006), but not time spent at moderate intensity (R = 0.18, p = 0.14). In addition a significant inverse relation was found between age and the number of steps (R = −0.33, p = 0.006) and time spent in activities at mild intensity (R = −0.23, p = 0.05). In a stepwise multiple regression analysis FEV1 (partial R2 = 0.26, p < 0.0001) was positively, and age (partial R2 = 0.11, p = 0.001) was negatively related to the number of steps per day (total R2 = 0.37). Similar results were also noted for mild physical activity in a stepwise multiple regression analysis with FEV1 (partial R2 = 0.11, p = 0.0058) being a positive predictor, and age (partial R2 = 0.05, p = 0.0428) as a negative predictor (total R2 = 0.16). No significant predictors of time spent in moderate physical activity were noted.
As expected, the different physical activity estimates from the armband were related. A correlation was found between the time at moderate intensity (R = 0.50, p < 0.0001), and the number of steps. A better relation was obtained between the time spent at intensities > 2.5 METs (mild) and the number of steps (R = 0.84, p < 0.0001). These relations are displayed in Fig. 2.
Figure 2.
Relation between the numbers of steps per day and the time spent in mild activities (Left panel) and the time spent in moderate intensity (Right panel) in patients (open symbols) and control subjects (closed symbols).
There were no significant differences in disease severity between centers (FEV1: 59%, 55%, and 50% of the predicted value in Pittsburgh, Palermo and Leuven respectively). After correcting for covariates age, BMI, FEV1 and gender, the mean number of steps were 6383 ± 643 (±SEM) in Leuven, 5115 ± 675 in Pittsburgh and 6610 ± 804 in Palermo, respectively. The time spent at moderate intensity was found to be 27 ± 7 min in Leuven, 21 ± 7 min in Pittsburgh, and 10 ± 8 min in Palermo. These estimates for time in mild physical activity were 93 ± 15 min in Leuven, 62 ± 15 min in Pittsburgh, and 64 ± 18 min in Palermo, respectively. None of these differences reached statistical significance. The ‘Center’ did not contribute in the multiple regression analysis to explain variability in physical activity.
Discussion
The main finding of this pilot-study is the significant reduction in physical activity observed at each of three centers as of GOLD-stage II. The study provides evidence for a gradual reduction in daily physical activity levels with increasing GOLD stage, although the correlation between physical activity and lung function is weak. Our data clearly show that inactivity starts early in the disease and this pattern was consistent across centers.
The present study was partly designed to investigate the feasibility of physical activity monitoring in larger multi-center trials. There are three noteworthy findings to be highlighted. First, the present study shows that is feasible to use activity monitors in multi-center studies. Patients showed good compliance wearing the activity monitor. For 93% of the days, the activity monitor was worn for more than 90% of the time. To our knowledge only one other study so far reported data on compliance and found that a tri-axial accelerometer was only worn for 60–70% of the instructed time (daytime hours).21 Whether these differences in adherence are caused by the measuring devices themselves or due to the instructions given to the patients needs to be further explored. Furthermore no large differences between centers were found for intensity and amount of daily activity. This is in contrast with recent data showing significantly different levels of physical activity in patients from Austria in comparison with patients from Brazil.13 Further studies in larger samples are needed to clarify whether systematic differences in physical activity levels exist between countries. The current conclusions are based on relatively small samples and could be influenced by selection bias.
Second, in the present study physical activity level was assessed using steps; an outcome which is conventionally assessed using pedometers or accelerometers. In addition, the time spent above pre-determined thresholds of intensity was calculated. We used an age dependent threshold to identify physical activity at moderate intensity and activities above 2.5 METs were classified as mild physical activity. In patients with moderate disease physical activity at moderate intensity was clearly more reduced (−60%) than the number of steps (−29%, Table 2). Time at moderate physical activity is important to maintain or improve physical fitness and health.22 Simply using steps as an outcome may therefore overestimate the level of physical activity probably because the walking speed of patients is rather low. Hence, despite what current knowledge might consider to be a reasonable step count24 (or pedometer count), patients may still fail to reach the recently updated recommendations to maintain health (i.e. more than 30 minutes of physical activity at moderate intensity on 5 out of 7 days).22
Lastly our study reinforces the importance of physical activity monitoring, rather than the use of questionnaires to accurately assess physical activity. In a large population based study conducted in Denmark, the proportion of patients with low physical activity levels in GOLD-stage I was 11%.4 In the whole cohort this proportion was 13%.5 In GOLD-stage II the proportion of inactive patients was 21%. Our data and that of others11 suggest more important levels of inactivity in these milder stages in comparison with healthy controls which is probably related to the method of measuring.23
Although the present study is one of the largest studies to investigate physical activity levels in patients across different stages of COPD and in healthy controls, a word of caution is needed when interpreting the data. Since healthy controls were only recruited from one Belgian centre it is possible that they are not completely representative for the general level of activity in this age cohort. Activity questionnaire data from large population samples suggest that Belgians are among the least active populations worldwide.25,26 Furthermore there was a slightly larger proportion of females in the control cohort. Both of these facts have probably resulted in a rather conservative estimate of normal activity in our study and may therefore have resulted in an underestimation of the difference between patients and controls.
We studied a substantially larger group of patients in GOLD-stage II compared to previous studies, which largely focused on GOLD-stages III and IV.7–10 Our data show remarkable similarity with two other recent studies that used similar methodology to assess physical activity levels.11,12 The number of steps in their patients in GOLD-stage I was 7990 ± 3370 (vs. 8141 in the present study). Pooling the results from both studies, patients in GOLD-stage I tended to be less active compared to the control subjects of the present study (p = 0.12). Steps of control subjects were also very similar (9110 ± 3857 vs. 9372 ± 3574 in the present study). The reduction of physical activity levels at moderate intensity by up to 40 to 50% in patients suffering from moderate COPD clearly emphasizes that treatment in these patients should include attention to enhance physical activity levels.
Although pulmonary rehabilitation should aim at enhancing physical activity2 surprisingly few studies have investigated the impact of pulmonary rehabilitation on physical activity in general. None of these were specifically directed at patients in GOLD-stage II. Some uncontrolled studies have on average shown modest improvements of physical activity levels after exercise training.25–32 One of these studies suggests that pulmonary rehabilitation is particularly effective to increase participation in daily physical activity in patients in early disease stages.29 The effect of drug therapy on physical activity levels is not studied. Several bronchodilators have been shown to enhance exercise tolerance.33–35 Whether this translates into more participation in daily physical activity is currently under investigation.
In COPD, pilot data suggest that physical activity levels could be enhanced by increasing awareness on the actual physical activity levels by using a simple pedometer.36 This strategy seems to be effective across different patient populations and also in healthy sedentary adults.37
Previous studies suggested that the association between physical activity and lung function was weak10,38 to moderate.39 In the present study the positive relation with the total number of steps (R = 0.51, p < 0.0001) and physical activity at mild intensity (R = 0.33, p = 0.006) are statistically significant. The relation with physical activity at moderate intensity, however, is not significant. In fact the reduction in moderate physical activity is large, even in mild disease. It is important to mention that these activities at moderate intensity are required to maintain optimal health.22
In conclusion, the present multi-center study confirms the impressive reduction in physical activity in a relatively large group of patients with moderate COPD (GOLD II) as well as those with more severe disease (GOLD Stage III & IV) across different centers in different countries. Further research should focus on interventions focused specifically on enhancing physical activity in these patients.
Footnotes
Conflict of interest statement
All authors declare that they have no financial or personal relationships with other people or organizations that have influenced the current work.
References
- 1.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Nici L, Donner C, Wouters E, et al. American thoracic society/european respiratory society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 3.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–87. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–8. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175:458–63. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 6.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Morgan MD. Activity monitors can detect brisk walking in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2001;21:143–8. doi: 10.1097/00008483-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Sandland CJ, Singh SJ, Curcio A, Jones PM, Morgan MD. A profile of daily activity in chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2005;25:181–3. doi: 10.1097/00008483-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Schonhofer B, Ardes P, Geibel M, Kohler D, Jones PW. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997;10:2814–9. doi: 10.1183/09031936.97.10122814. [DOI] [PubMed] [Google Scholar]
- 10.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–7. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 11.Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177:743–51. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- 12.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33:262–72. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 13.Pitta F, Breyer MK, Hernandes NA, et al. Comparison of daily physical activity between COPD patients from Central Europe and South America. Respir Med. 2009;103:421–6. doi: 10.1016/j.rmed.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 14. [accessed 1.03.07];Global strategy for diagnosis, management, and prevention of COPD. 2007 doi: 10.1183/09031936.03.00063703. www.goldcopd.com. [DOI] [PubMed]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85:742–9. doi: 10.1093/ajcn/85.3.742. [DOI] [PubMed] [Google Scholar]
- 17.Langer D, Gosselink R, Sena R, Burtin C, Decramer M, Troosters T. Validation of two activity monitors in patients with COPD. Thorax. 2009;64:641–2. doi: 10.1136/thx.2008.112102. [DOI] [PubMed] [Google Scholar]
- 18.Patel SA, Benzo RP, Slivka WA, Sciurba FC. Activity monitoring and energy expenditure in COPD patients: a validation study. COPD. 2007;4:107–12. doi: 10.1080/15412550701246658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1996;30:975–91. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Hecht A, Ma S, Porszasz J, Casaburi R. Methodology for using long-term accelerometry monitoring to describe daily activity patterns in COPD. COPD. 2009;6:121–9. doi: 10.1080/15412550902755044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 23.Thompson D, Batterham AM, Markovitch D, Dixon NC, Lund AJ, Walhin JP. Confusion and conflict in assessing the physical activity status of middle-aged men. PLoS One. 2009;4:e4337. doi: 10.1371/journal.pone.0004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Bauman A, Bull F, Chey T, et al. The International prevalence study on physical activity: results from 20 countries. Int J Behav Nutr Phys Act. 2009;6:21. doi: 10.1186/1479-5868-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varo JJ, Martinez-Gonzalez MA, De Irala-Estevez J, Kearney J, Gibney M, Martinez JA. Distribution and determinants of sedentary lifestyles in the European Union. Int J Epidemiol. 2003;32:138–46. doi: 10.1093/ije/dyg116. [DOI] [PubMed] [Google Scholar]
- 27.Steele BG, Belza B, Hunziker J, et al. Monitoring daily activity during pulmonary rehabilitation using a triaxial accelerometer. J Cardiopulm Rehabil. 2003;23:139–42. doi: 10.1097/00008483-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Steele BG, Belza B, Cain KC, et al. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Arch Phys Med Rehabil. 2008;89:404–12. doi: 10.1016/j.apmr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Walker PP, Burnett A, Flavahan PW, Calverley PM. Lower limb activity and its determinants in COPD. Thorax. 2008;63:683–9. doi: 10.1136/thx.2007.087130. [DOI] [PubMed] [Google Scholar]
- 30.Mercken EM, Hageman GJ, Schols AM, Akkermans MA, Bast A, Wouters EF. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:994–1001. doi: 10.1164/rccm.200411-1580OC. [DOI] [PubMed] [Google Scholar]
- 31.Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest. 2005;128:1194–200. doi: 10.1378/chest.128.3.1194. [DOI] [PubMed] [Google Scholar]
- 32.Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134:273–80. doi: 10.1378/chest.07-2655. [DOI] [PubMed] [Google Scholar]
- 33.Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128:1168–78. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 34.Casaburi R, Kukafka D, Cooper CB, Witek TJ, Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127:809–17. doi: 10.1378/chest.127.3.809. [DOI] [PubMed] [Google Scholar]
- 35.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med. 2006;119:21–31. doi: 10.1016/j.amjmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 36.de Blok BM, de Greef MH, ten Hacken NH, Sprenger SR, Postema K, Wempe JB. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns. 2006;61:48–55. doi: 10.1016/j.pec.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 38.Belza B, Steele BG, Hunziker J, Lakshminaryan S, Holt L, Buchner DM. Correlates of physical activity in chronic obstructive pulmonary disease. Nurs Res. 2001;50:195–202. doi: 10.1097/00006199-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Steele BG, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner DM. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000;117:1359–67. doi: 10.1378/chest.117.5.1359. [DOI] [PubMed] [Google Scholar]