Abstract
Evolution of unresponsiveness to homeostasis-promoting signals from the microenvironment is a hallmark of malignant tumor cells. In Dunning R3327 model rat prostate tumors that are comprised of distinct stromal and epithelial compartments, progression from non-malignant, androgen-responsive tumors to malignancy is characterized by loss of compartmentation coincident with a loss of resident epithelial cell FGFR2IIIb that receives instructive signals from stromal FGF7 and FGF10. Restoration of FGFR2IIIb to malignant tumor cells restores responsiveness to stromal cells, restores distinct stromal and epithelial compartments and retards malignant progression. Cultured stromal cells from two compartment tumors are comprised of smooth muscle α−actin-positive cells that express predominantly FGFR3 and fibroblast-like cells devoid of α−actin and FGFR3. Here we show that it is primarily the smooth muscle cell-like α−actin-expressing stromal cells that survive, morphologically differentiate and delay tumor incidence and size in the presence of malignant cells in which FGFR2IIIb has been restored. Expression of FGFR3 by transfection in the fibroblast-like stromal cells conferred ability to respond similar to the smooth muscle cell-like stromal cells in which FGFR3 is normally resident. These results highlight the importance of the two-way communication back and forth between stroma and epithelium that is mediated by signaling within the FGFR family during progression to malignancy.
Keywords: cell-cell communication, Dunning tumors, tumor microenvironment, prostate cancer, receptor tyrosine kinases, stromal-epithelial interactions, tissue homeostasis
Introduction
A hallmark of malignant cancer is its autonomy and independence on homeostasis-promoting influences of the microenvironment. Normal tissue homeostasis results from a precise and clear two way communication among different cellular compartments most commonly stromal and epithelial in the case of parenchymal organs as prostate (Hayward et al., 1997; Aboseif et al., 1999). Relatively benign early stage non-malignant tumors retain elements of homeostasis-promoting crosstalk between stroma and epithelium. The Dunning R3327 transplantable tumor that arose spontaneously in an aged rat prostate is a unique example of symbiosis between epithelium and stroma that can survive serial heterotopic passage (Isaacs et al., 1978; Yan et al., 1993). The parent tumor which is well-differentiated, slow-growing and non-malignant is comprised of a well-defined epithelium and stroma that travels together independent on the site of transplantation into syngenic hosts. Similar to the progression of human prostate cancer, the tumor eventually progresses to a rapidly growing, highly malignant single compartment adenocarcinoma after androgen-deprivation. When subjected to cell culture, the parent tumor gives rise to both epithelial and stromal cells that when mixed and implanted back into host animals give rise to differentiated, slow-growing and benign two compartment tumors in which the symbiosis between stroma and epithelium is re-established (Yan et al., 1993). The differentiated and slow-growing character of the epithelial cells that dominate the overall benign character of the resultant two-compartment tumors is dependent on the co-implanted stromal cells. The survival of the stromal cells is also dependent on the co-implanted epithelial cells. In contrast, the implanted cultured epithelial cells in absence of stromal cells progress to the highly malignant single compartment tumors similar to those that arise upon androgen-deprivation from the parent tumors.
A relatively homogenous, stable and androgen-unresponsive population of epithelial cells (DTE) arises in vitro from the androgen receptor-positive parent Dunning tumors. The epithelial cells are characterized by specific expression of FGFR2IIIb, a splice variant of the FGFR family that specifically responds to stromal cell-derived FGF7 and FGF10 and the complete absence of mesenchymal cell-associated FGFR1 (Yan et al., 1992; Yan et al., 1993; Feng et al., 1997; Lu et al., 1999). DTE cells are also characterized by expression of FGF9 which is very low in both normal prostate and the parent Dunning tumors (Jin et al., 2004). Stromal cell cultures that arise from the parent Dunning tumors express FGFR1, FGFR3, FGF7 and FGF10 (Yan et al., 1992; Lu et al., 1999; Jin et al., 2004). Expression of both FGF7 and FGF10 is responsive to androgen. The stromal cell cultures consist of two general cell types, one that is distinguished by expression of smooth muscle cell α-actin and only FGF7 (DTS2) and the other that is devoid of α-actin and expresses both FGF7 and FGF10 (DTS1) (Wu et al., 2003; Jin et al., 2004). DTS1 cells are characterized by a high level of expression of FGFR1 and are devoid of FGFR3. DTS2 cells are characterized by expression of FGFR3 in addition to low levels of FGFR1.
In contrast to the parent Dunning tumors, cultured cells from their highly malignant derivatives (AT3 tumors) that arise after androgen-deprivation or malignant tumors that arise from DT3 cells implanted alone give rise to heterogeneous mixtures of cells that have switched from exclusive expression of splice variant FGFR2IIIb to FGFR2IIIc, an isoform that does not recognize stromal FGF7 or FGF10 (Yan et al., 1993). Other tumor cells are completely devoid of FGFR2 (Feng et al., 1997). The AT3 cells are also characterized by high levels of expression of FGF9 and the normally stromal cell FGFR isotype, FGFR1 (Jin et al., 2004). FGFR1 is absent in normal prostate epithelial cells and DTE tumor epithelial cell precursors of the AT3 cells. Despite their extreme malignancy and unresponsiveness to stroma and the microenvironment, restoration of FGFR2IIIb to AT3 cells (AT3/R2IIIb) inhibits their growth rate and restores responsiveness to DTS stromal cells. This occurs concurrently with reduction in the rate of growth of tumors resulting from implantation of mixtures of the AT3/R2 and DTS cells.
In this report we determined the response of the two types of stromal cells to FGFR2IIIb expressing tumor epithelial cells and their effect on their tumorigenicity. The smooth muscle cell α−actin-positive FGFR3-expressing DTS2 cells dramatically reduced tumor incidence and size relative to fibroblast-like DTS1 cells coincident with a notable morphological organization of the α−actin-positive stromal cells in co-implanted mixtures with the AT3/R2IIIb cells. Forced expression of FGFR3 in DTS1 cells conferred the ability to express α−actin and to morphology differentiate similar to DTS2 cells in implants with the AT3/R2IIIb cells. The co-implanted FGFR3 transfected DTS1 cells delayed tumor progression to an extent exceeding that of DTS2 cells.
Materials and Methods
Implantation and tumor formation
Six to 7 week old host Copenhagen male rats (100–125 g) were purchased from Harlan Laboratories (Houston, Texas). Rats were observed for at least one week prior to experimentation. Experimental procedures were approved by the IBT Animal Care and Use Committee. Prior to implantation, the indicated cells were cultured for 24 to 48 hours and harvested at 80 percent confluence. Cells were harvested with pronase, washed with serum-free RD medium (1:1 RPMI:DMEM, Invitrogen Life Technologies, Gaithersburg, MD), and re-suspended in the same medium. AT3/R2 tumor cells (1 × 105) mixed with the indicated ratio of stromal cell types indicated in the text were implanted into each flank of host rats. Rats were observed daily after one week for palpable tumors at the site of implant. When control tumors reached animal protocol limits of 5 cm in any dimension, animals were euthanized and tumors were harvested for analysis. Tumor size in 3 dimensions was recorded. A portion of tumor tissues was fixed for histological analyses, and the remainder quick frozen for RNA and protein extraction and analysis (Wu et al., 2003). Tumor tissue was fixed with 4% paraformaldehyde-PBS for 4 hours, and then paraffin embedded.
Cell culture and transfections
AT3/R2 tumor cells were prepared as described (Feng et al., 1997). Prostate tumor stromal cells, DTS1 and DTS2, were cultured in RD medium containing 5% fetal bovine serum (FBS) as described previously (Wu et al., 2003). Diverse cell types whether native or stably transfected were periodically assessed for FGF and FGFR expression as well as relevant cytoskeletal markers. Recombinant cDNA encoding FGFR3IIIc was constructed as described (Jin et al., 2004) and subcloned into mammalian expression vector pcDNA3.1/Zeo (Strategene, La Jolla, CA) for overexpression experiments. Stable transfection of DTS1 cells with FGFR3IIIc-pcDNA3.1/Zeo was carried out with LipofectAMINE (Invitrogen Life Technologies, Gaithersburg, MD) according to the manufacturer’s suggestions. FGFR3 expression in transfected DTS1 cells was confirmed with RT-PCR and radioreceptor binding assay as described (Jin et al., 2004). Clones with highest FGFR3 expression were selected and pooled for mixing and implantation experiments.
Histochemistry
Tumor tissue sections of 5 µm were prepared with an Olympus Cut 4060 Microtome (Olympus America Inc., Melville, NY) and mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were re-hydrated, stained with hemotoxylin and eosin (H&E) or subjected to immunohistochemical analysis. α-smooth muscle actin (α-SMA) was detected using the α-SMA immunohistology kit (Product No. IMMH-2, Sigma, St. Louis, MO) according to the manufacturer’s protocol. The primary antibody was mouse monoclonal anti-α-smooth muscle actin. The bound primary antibody was visualized by application of the biotinylated secondary antibody (goat anti-mouse IgG). The substrate reaction was performed with 3-amino-9-ethylcarbazole (AEC) chromogen in 3% hydrogen peroxide to produce a red-brown precipitate in the cytoplasm of α-SMA–positive cells. The slides were counterstained with Mayer’s hematoxylin (Sigma). Cells exhibiting α-SMA were visualized with a microscope (Axioskop; Zeiss, Germany) connected to a digital imaging camera (DXM1200, Nikon, Japan).
Results and Discussion
α-actin- and FGFR3-expressing stromal cells survive, differentiate and restore compartmentation in tumors derived from prostate tumor cells expressing FGFR2IIIb
Unlike normal prostate or benign Dunning R3327 prostate tumors that exhibit distinctly differentiated epithelial and stromal compartments, malignant AT3 tumors that emerge after androgen-deprivation are comprised of a single compartment and give rise to only malignant epithelial-like cells in culture (Yan et al., 1993; Feng et al., 1997). Restoration of the lost FGFR2IIIb kinase to malignant AT3 cells (designated as AT3/R2) depressed their tumorigenicity and when mixed with DTS stromal cells from the parent tumor resulted in distinct epithelial and stromal compartments in the tumors. Two major subtypes, DTS1 and DTS2, have been identified in mixed cultures of stromal cells from the parent two compartment well-differentiated parent Dunning tumors (Wu et al., 2003). DTS1 cells have properties of undifferentiated fibroblast-like cells that express high levels of FGFR1IIIc and both stromal to epithelial cell signaling factors FGF7 and FGF10. DTS2 cells are SMC-like cells based on their expression of α-actin and characterized by expression of FGFR3 and only FGF7, but not FGF10 (Jin et al., 2004). DTS2 cells express low levels of FGFR1IIIc.
To determine the contribution of the two different stromal cell types on tumor formation by AT3/R2 cells, we implanted DTS1 and DTS2 cells at a ratio of 10 to 1 to AT3/R2 cells planted subcutaneously into flanks of syngenic hosts. Mice were examined daily for changes at the site of inoculation. A reduction in both the incidence and size of tumors due to co-implantation with the stromal cell subtypes was apparent after about a month (Fig. 1, Table 1). The SMC-like, FGFR3-expressing DTS2 cells reduced incidence to 1 in 6 trials and weight of the one resultant tumor to less than 2 percent of 6 control AT3/R2 tumors while DTS1 cells reduced tumor incidence to 4 in 6 and mean weight of tumors by 48 percent. This indicated an impact of specifically DTS2 cells on tumor formation beyond that of DTS1 cells. It is unclear whether the effect of DTS1 cells and part of that of DTS2 cells was due to simply dilution of the implanted tumorigenic AT3/R2 cells at inoculation or that DTS1 cells also exerted an inhibitory biological effect on progression of the tumors. Similar to mixed cultures of DTS cells (Yan et al., 1993; Feng et al., 1997), neither DTS1 nor DTS2 cells survived implantation without AT3/R2 tumor cells.
Fig. 1. Effect of DTS stromal cells on tumorigenicity of AT3 cells expressing FGFR2IIIb.
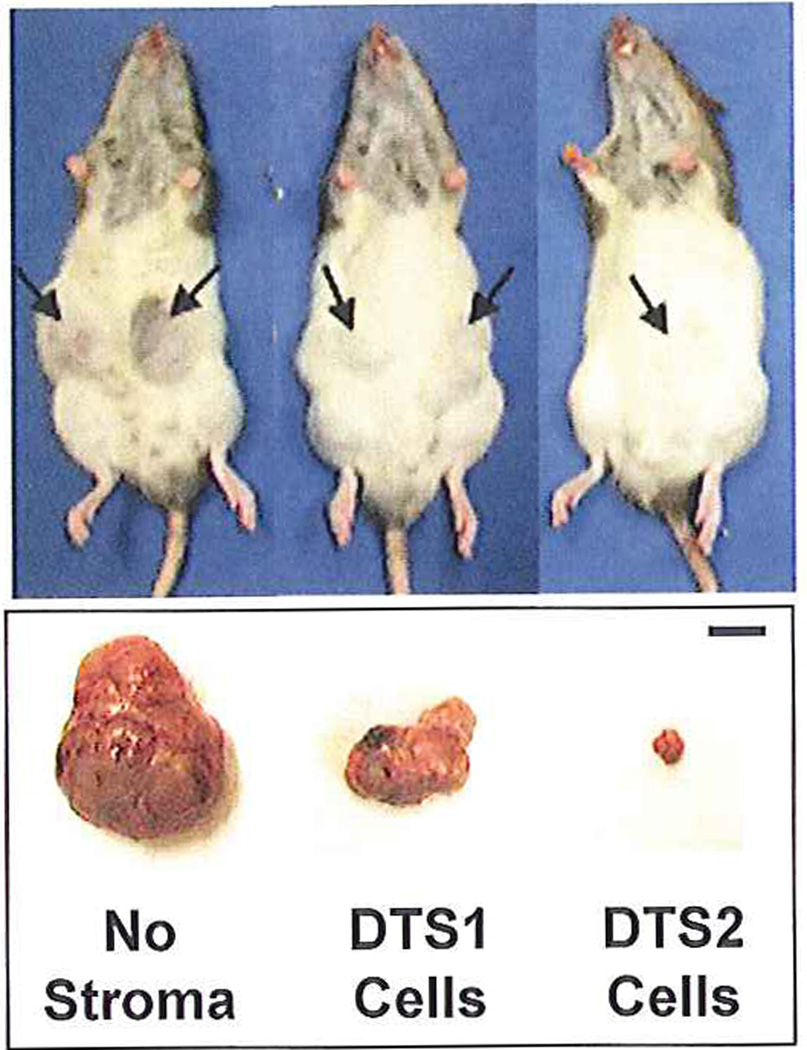
The indicated subtype of DTS stromal cells and AT3/R2 cells at a ratio of 10:1 were co-implanted subcutaneously into each flank of hosts (3 rats per group) and observed daily after one week as described in Methods. Representative hosts bearing tumors described in Table I at 26 days are shown. Tumors are indicated by arrow. The bar represents 1 cm.
Table I.
Effect of DTS1 and DTS2 stromal cells on incidence and size of tumors from AT3 tumor cells expressing FGFR2IIIba
| Cell mixture | Tumor incidence | Tumor weight (g) |
|---|---|---|
| AT3/R2 | 6/6 | 7.26 ± 1.16 |
| DTS1 + AT3/R2 | 4/6 | 3.77 ± 0.93 |
| DTS2 + AT3/R2 | 1/6 | 0.13 |
Incidence and weight (mean ± SD) of tumors resulting from mixtures of the two types of DTS stromal cells and FGFR2IIIb-expressing AT3 cells (AT3/R2) at a 10 to 1 ratio. On day 26 after implantation, animals were euthanized and tumors were harvested for determination of wet weight and histochemical analysis. Weights are the mean ± SD of the incident tumors. Weight of rat hosts was an average of 210g with a variation of less than 15g.
Tumors were examined histochemically to derive clues that might underlie the significantly different effects of the two types of stromal cells on AT3/R2 tumors (Fig. 2). As reported previously (Yan et al., 1993; Feng et al., 1997), AT3/R2 cells, despite a reduced growth rate both in vitro and in vivo induced by restoration of FGFR2IIIb, gave rise to relative homogenous epithelial cell tumors similar to those of the parent AT3 cells devoid of FGFR2IIIb. Tumors from the mixture of AT3/R2 and DTS1 cells appeared similar to the tumors resulting from AT3/R2 alone. No evidence of organized stromal cells could be distinguished.in the tumors. In contrast, morphological organization of both epithelial cells and α-actin-expressing stromal cells was apparent in the tumors resulting from the mixture of AT3/R2 cells and SMC-like DTS2 cells (Fig. 2). α-actin-expressing stromal cells were organized into apparent lumenal-like structures in intimate contact with the tumor cells both inside and outside the structures. This indicates that restoration of FGFR2IIIb to malignant AT3 cells restores the ability of the otherwise extremely malignant AT3 cells to communicate specifically the SMC-like DTS2 fraction of stromal cells. This interaction not only supported survival of the implanted stromal cells in vivo, but also resulted in induction of morphological differentiation of the DTS2 cells. The response was coincident with a significant inhibition of overall tumor progression.
Fig. 2. Morphological differentiation and compartmentation in mixtures of stromal cells expressing FGFR3 and tumor cells expressing FGFR2IIIb.
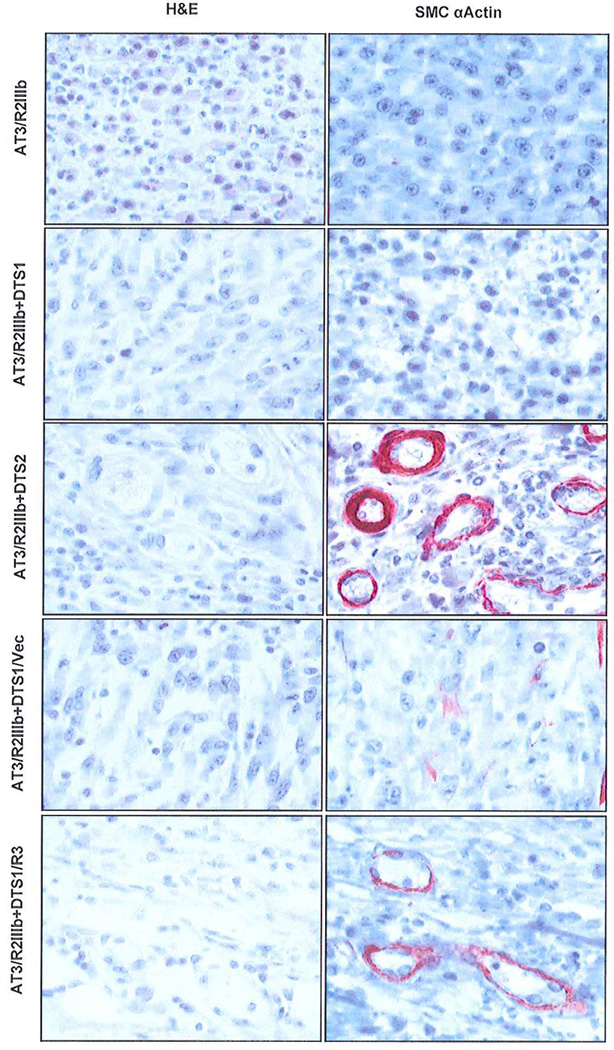
Sections from paraffin-embedded tumor tissue derived from the indicated combinations of stromal and AT3/R2 tumor cells at a ratio of 5:1 were stained with H&E and analyzed by immunostaining with anti-α-smooth muscle actin antibody as described in Methods. α-actin positive cells appear as reddish brown.
Expression of FGFR3 in α-actin-negative fibroblast-like stromal cells confers ability to exhibit SMC-like differentiation and suppress tumors in response to FGFR2IIIb-expressing tumor cells
To test for a causal role of FGFR3 in behavior of stromal cells in response to FGFR2IIIb-expressing tumor cells, we expressed FGFR3 in DTS1 cells by transfection. At a ratio of 10 FGFR3-transfected DTS1 cells to 1 AT3/R2 cells, no palpable tumors could be observed for up to five weeks at which time control tumors were necessarily terminated (Table 2). DTS1 cells transfected with a control vector devoid of FGFR3 coding sequence inhibited tumor progression by about 50 percent at three weeks (not shown) and 80 percent at five weeks similar to untransfected DTS1 cells (Table 2). We reduced the ratio of DTS1/R3 cells to AT3/R2 cells to generate tumors of sufficient size for histochemical analysis. No significant difference in incidence and size of tumors from mixtures of DTS1/R3 and control vector-transfected cells and the AT3/R2 cells was apparent at ratios of 1:1 and 5:1. However, histochemical analysis of tumors from the 5 DTS1/R3 to 1 AT3/R2 cell mixture revealed the presence of the characteristic α-actin-staining structures observed in mixtures of DTS2 and AT3/R2 cells (Fig. 2). No evidence of the structures or differentiation of compartments was observed in tumors from the 1:1 mixtures (not shown). These results indicate that fibroblast-like DTS1 cells remain competent to express α-actin and differentiate in vivo into SMC-like cells under the influence of epithelial cells expressing FGFR2IIIb if FGFR3 is expressed in them.
Table II.
Effect of DTS1 stromal cells expressing FGFR3 on tumorigenicity of AT3 tumor cells expressing FGFR2IIIba
| Cell mixture | Tumor incidence | Tumor weight (g) |
|---|---|---|
| AT3/R2 | 6/6 | 20.99 ± 0.46 |
| DTS1/vector + AT3/R2 | 2/6 | 4.09 ± 0.10 |
| DTS1/R3 + AT3/R2 | 0/0 | NA |
DTS1 stromal cells transfected with FGFR3 (DTS1/R3) and AT3/R2 cells were co-implanted at a ratio of 10:1 and observed daily until AT3/R2 controls were required to be euthanized by institutional animal protocol (5 weeks).
Conclusions
Differentiated SMC-like stromal cells appear to be the major stromal cell type that maintains distinct compartmentation between stromal and epithelium and thus restrain malignant progression of epithelial cells that express epithelial cell resident FGFR2IIIb. The SMC-like stromal cell response to FGFR2IIIb-expressing epithelial cells is dependent on specifically FGFR3. FGF9 which is overexpressed in tumor epithelial cells (Jin et al., 2004; Li et al., 2008) is the candidate tumor epithelial cell signal that acts specifically on FGFR3 in SMC-like stromal cells to promote their growth (Wu et al., 2003) and survival and morphological differentiation in vivo. The predominance of differentiated SMC-like, FGFR3-expressing stromal cells that express exclusively FGF7 (Wu et al., 2003; Jin et al., 2004) insures that FGFR2IIIb-expressing epithelial cells are instructed by only stromal FGF7 that is specific for epithelial cell FGFR2IIIb resulting in predominantly a growth limiting effect (Feng et al., 1997; Matsubara et al., 1998). In contrast, undifferentiated fibroblast-like stromal cells express FGF10 in addition to FGF7. Although FGF10 shares with FGF7 the ability to bind and activate FGFR2IIIb, it also binds FGFR1IIIb (Lu et al., 1999). Thus the fibroblast-like stromal cells have both potential to delay progression via FGFR2IIIb, but when they are predominant may drive tumor progression through FGFR1IIIb when it appears ectopically in tumor epithelial cells (Memarzadeh et al., 2007). It remains to be determined whether FGFR3 represses expression of FGF10 to contribute to the apparent reduced tumorigenicity in presence of FGFR3-positive smooth muscle cell-like stromal cells.
Acknowledgements
This work was supported by Public Health Service grant P50CA140388 (FW/WLM), the Susan Komen Foundation (WLM) and aid from the John S. Dunn Research Foundation (WLM).
References
- Aboseif S, El-Sakka A, Young P, Cunha G. Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation. 1999;65:113–118. doi: 10.1046/j.1432-0436.1999.6520113.x. [DOI] [PubMed] [Google Scholar]
- Feng S, Wang F, Matsubara A, Kan M, McKeehan WL. Fibroblast growth factor receptor 2 limits and receptor 1 accelerates tumorigenicity of prostate epithelial cells. Cancer Res. 1997;57:5369–5378. [PubMed] [Google Scholar]
- Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79(Suppl 2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- Isaacs JT, Heston WD, Weissman RM, Coffey DS. Animal models of the hormone-sensitive and -insensitive prostatic adenocarcinomas, Dunning R-3327-H, R-3327-HI, and R-3327-AT. Cancer Res. 1978;38:4353–4359. [PubMed] [Google Scholar]
- Jin C, Wang F, Wu X, Yu C, Luo Y, McKeehan WL. Directionally specific paracrine communication mediated by epithelial FGF9 to stromal FGFR3 in two-compartment premalignant prostate tumors. Cancer Res. 2004;64:4555–4562. doi: 10.1158/0008-5472.CAN-03-3752. [DOI] [PubMed] [Google Scholar]
- Li ZG, Mathew P, Yang J, Starbuck MW, Zurita AJ, Liu J, Sikes C, Multani AS, Efstathiou E, Lopez A, Wang J, Fanning TV, Prieto VG, Kundra V, Vazquez ES, Troncoso P, Raymond AK, Logothetis CJ, Lin SH, Maity S, Navone NM. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. The Journal of clinical investigation. 2008;118:2697–2710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274:12827–12834. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Kan M, Feng S, McKeehan WL. Inhibition of growth of malignant rat prostate tumor cells by restoration of fibroblast growth factor receptor 2. Cancer Res. 1998;58:1509–1514. [PubMed] [Google Scholar]
- Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA, Witte ON. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Jin C, Wang F, Yu C, McKeehan WL. Stromal cell heterogeneity in fibroblast growth factor-mediated stromal-epithelial cell cross-talk in premalignant prostate tumors. Cancer Res. 2003;63:4936–4944. [PubMed] [Google Scholar]
- Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL. Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol Cell Biol. 1993;13:4513–4522. doi: 10.1128/mcb.13.8.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to- epithelial-cell andromedin. Molecular endocrinology (Baltimore, Md. 1992;6:2123–2128. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]


