Abstract
Transduction of energetic signals into membrane electrical events governs vital cellular functions, ranging from hormone secretion and cytoprotection to appetite control and hair growth. Central to the regulation of such diverse cellular processes are the metabolism sensing ATP-sensitive K+ (KATP) channels. However, the mechanism that communicates metabolic signals and integrates cellular energetics with KATP channel-dependent membrane excitability remains elusive. Here, we identify that the response of KATP channels to metabolic challenge is regulated by adenylate kinase phosphotransfer. Adenylate kinase associates with the KATP channel complex, anchoring cellular phosphotransfer networks and facilitating delivery of mitochondrial signals to the membrane environment. Deletion of the adenylate kinase gene compromised nucleotide exchange at the channel site and impeded communication between mitochondria and KATP channels, rendering cellular metabolic sensing defective. Assigning a signal processing role to adenylate kinase identifies a phosphorelay mechanism essential for efficient coupling of cellular energetics with KATP channels and associated functions.
Delivery of metabolic signals to intracellular compartments is a critical determinant of cellular homeostasis. In particular, efficient communication between cellular energetics and membrane metabolic sensors is required for regulation of cell excitability and associated functions (1, 2). Plasmalemmal ATP-sensitive K+ (KATP) channels, formed by the Kir6.2 potassium channel and the sulfonylurea receptor (SUR), are unique nucleotide sensors that adjust membrane potential in response to intracellular metabolic oscillations (2–5). Transition of the SUR subunit from the ATP to the ADP-liganded state promotes K+ permeation through Kir6.2 and defines KATP channel activity (5–7). However, the mechanism that facilitates nucleotide exchange in the KATP channel environment and promotes coupling of membrane electrical events with cellular metabolic pathways remains unknown.
Cellular phosphotransfer reactions catalyze nucleotide exchange facilitating communication between sites of ATP generation and ATP utilization (8–11). In this way, the phosphotransfer enzyme adenylate kinase (AK) amplifies metabolic signals and promotes intracellular phosphoryl transfer by catalyzing the reaction ATP + AMP ↔ 2ADP (12, 13). Adenylate kinase has a distinct signaling role in setting the cellular response to stress through activation of AMP-dependent processes (12–15). Deletion of the major AK isoform (AK1) results in disturbed muscle energetic economy and decreased tolerance to metabolic stress (14, 15). Mutations in AK compromise nucleotide export from mitochondria (16), as well as the function of ATP-binding cassette transporters (17). Conversely, stimulation of AK phosphotransfer promotes nucleotide-dependent membrane functions (18, 19). However, the actual significance of AK phosphotransfer in communicating energetic signals to membrane metabolic sensors, such as the KATP channel, has not been established.
In this study, we demonstrate that AK phosphotransfer regulates the KATP channel response to metabolic challenge, promoting delivery of mitochondria-generated signals to the channel site. Such a signal processing function for AK was lost in cells with a null mutation of the AK1 gene, disrupting KATP channel coupling from cellular metabolism. Thus, the AK phosphorelay provides a novel pathway for integration of cellular energetics with membrane electrical events.
Materials and Methods
AK Knockout (KO) Mice.
AK1-KO mice were derived from embryonic stem cells carrying a replacement mutation in the AK1 gene (14, 15). Complete inactivation of AK1 expression was achieved by homologous DNA recombination, with a HygroB cassette vector used to replace the entire exon 3–5 region in the AK1 gene. Homozygous AK1-deficient mice lack the AK1 protein (14) and were compared with age-matched wild-type controls.
Channel Recording.
Electrophysiological recordings were performed in cardiomyocytes (20) from guinea pig or mice hearts and in COS-7 cells (21) transfected with Kir6.2 and SUR2A cDNA that encode cardiac KATP channel subunits (22). Pipettes (≈7–10 MΩ) were filled with 140 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes-KOH (pH 7.3), and cells were superfused with 140 mM KCl, 1 mM MgCl2, 5 mM EGTA, 5 mM Hepes-KOH (pH 7.3), 5 mM malic acid, 5 mM pyruvic acid, and 1 g⋅l−1 glucose. Membrane was permeabilized with digitonin to obtain the open cell-attached configuration (7). Channel activity, measured at −60 mV, was expressed as NP0, where N represents the number of channels and P0 the open channel probability.
Enzymatic Activity.
The ATP ↔ ADP exchange rate catalyzed by AK was measured in sarcolemmal membrane preparations using an enzyme-coupled spectrophotometric assay (13). AK activity was also determined in KATP channel immunoprecipitates or preimmune controls incubated with 2 mM ADP and 2 mM MgCl2 (20 h, 37°C; shaken at 170 rpm). The reaction was stopped by 2 mM HClO4 and kept on ice for 5 min. Proteins were precipitated by centrifugation at 15,000 × g (4°C, 5 min). Supernatant was neutralized with 2 M K2CO3, and on removal of potassium perchlorate precipitate adenine nucleotides were determined by HPLC (7).
Immunoprecipitation.
KATP channels were immunoprecipitated from sarcolemma with an anti-Kir6.2-specific Ab or preimmune serum (7, 23, 24). Membranes (400 μg) were solubilized in an immunoprecipitation buffer (50 mM Tris⋅HCl, 150 mM NaCl, 5 mM EDTA, and 50 mM NaF, pH 8.3) and incubated with an anti-Kir6.2 Ab raised in rabbit against amino acids N19–32C of rat Kir6.2. The resulting Ab-KATP channel complex was precipitated with protein A-Sepharose. Samples were centrifuged and resuspended in the immunoprecipitation buffer with 1% Nonidet P-40, 1 mM PMSF, 10 mg⋅ml−1 leupeptin, and PBS. Immunoprecipitates were probed by Western blotting using an anti-AK1-specific Ab (14). The anti-AK1 Ab was also used to immunoprecipitate AK with recombinant KATP channel subunits. The amount of channel proteins was calculated assuming an immunoprecipitation efficiency of 10% (7).
18O/31P NMR Spectroscopy.
AK phosphotransfer was measured using the 18O-phosphoryl-labeling technique (15) in guinea pig and mice hearts perfused (at 37°C, 40 min) with a 95% O2/5% CO2-saturated solution (123 mM NaCl, 6.0 mM KCl, 2.5 mM CaCl2, 19 mM NaHCO3, 1.2 mM MgSO4, 11.0 mM glucose, 0.5 mM EDTA, and 20 U⋅l−1 insulin). Hypoxia was induced with a 95% N2/5% CO2-gassed solution (at 37°C, 7 min) used to reduce partial oxygen pressure to 20–30 mmHg. Regular water in the perfusion solution was replaced for 30 s with 40% 18O water, and hearts were freeze-clamped in liquid nitrogen. Frozen hearts were extracted in 600 mM HClO4 and 1 mM EDTA, and proteins were pelleted by centrifugation. Supernatant was neutralized with 2 M KHCO3, precleaned with Chelex 100 resin, and supplemented with EDTA to remove metal ions. Samples were concentrated by vacuum centrifugation and supplemented with 10% deuterium water. 18O-induced shift in 31P NMR spectra was recorded at 242.9 MHz in a Bruker 14T spectrometer (Billerica, MA). The cumulative percentage of phosphoryl oxygens replaced by 18O in β-ADP was calculated as [%18O1 + 2(%18O2) + 3(%18O3)]/[3(% 18O in H2O)].
Metabolite Levels.
Tissue levels of AMP were quantified with HPLC (13).
Statistical Analysis.
Data are expressed as mean ± SE. Student's t test for unpaired samples was used for statistical analysis, and a difference at P < 0.05 was considered to be significant.
Results and Discussion
AMP-Driven KATP Channel Activation, Lost in the AK1-KO.
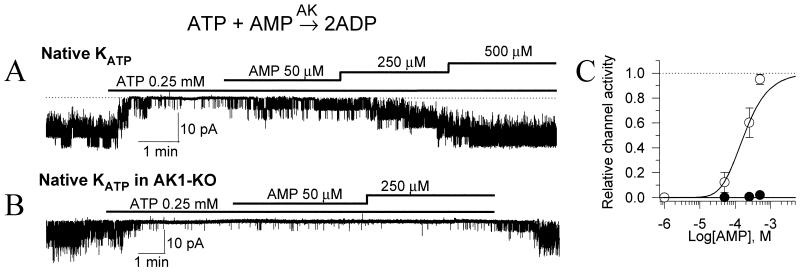
AK is highly expressed in tissues that require a rapid response to metabolic challenge, where it efficiently regulates adenine nucleotide exchange between cellular microdomains (12–15). The direction of the AK reaction within a cellular compartment depends on the availability of AMP, the pivotal signaling molecule driving the generation of ADP (10, 12). Local conversion of ATP to ADP could, in turn, promote KATP channel opening despite high intracellular ATP levels (19). Here, regulation of KATP channels by AMP was probed in cardiac cells expressing or lacking the AK1 gene (Fig. 1). In the open cell-attached patch configuration, which preserves the structural and metabolic integrity of a cell (7), KATP channels were kept closed by ATP in both normal and AK1-deficient cardiomyocytes (Fig. 1 A and B). Acceleration of the AK1 reaction by AMP promoted opening of ATP-inhibited KATP channels in wild-type (Fig. 1A), but not AK1-KO (Fig. 1B) cells. In the presence of intact AK1, the effect of AMP was concentration dependent, reaching half-maximal activation of KATP channels at 182 ± 30 μM (n = 7; Fig. 1C). In the absence of AK1, KATP channels were kept closed by ATP even at 500 μM AMP (n = 4; Fig. 1C). This suggests that AMP-dependent KATP channel opening was mediated through AK catalysis. Thus, each AMP-activated AK catalytic cycle would remove one ATP molecule from the channel site while generating two molecules of the channel activator ADP, thereby increasing the probability for KATP channel opening.
Figure 1.
AMP-dependent regulation of KATP channels is lost in AK1-KO cardiac cells. (A) Recording of native cardiac KATP channels demonstrate activation of ATP-inhibited channels following application of AMP, a pivotal AK substrate. (B) Absence of the AMP effect in cardiomyocytes from AK1-KO mice. (C) Concentration dependence of AMP-induced activation on 250 μM ATP-inhibited KATP channels in control (○) and AK1-KO (●) cardiomyocytes.
AK-Dependent KATP Channel Activation, Reversed by Creatine Kinase (CK).
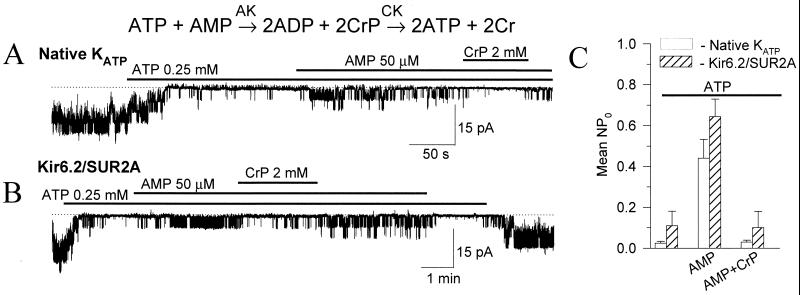
In the heart, CK is a major phosphotransfer system (8, 11, 13, 25) promoting ATP regeneration at ATP consumption sites (ADP + CrP ↔ ATP + Cr, where CrP is creatine phosphate and Cr is creatine). In fact, there is a close relationship between AK and CK phosphotransfer defining the directionality of nucleotide exchange in cellular compartments (8). Indeed, AMP-induced opening of native (Fig. 2A) or recombinant Kir6.2/SUR2A (Fig. 2B) cardiac KATP channels was reversed by removal of the product of the AK reaction, ADP, through activation of CK using CrP. On average, the efficacy of AMP to activate ATP-inhibited KATP channels was similar in native and recombinant channels and was dramatically reduced in the presence of the competing CK system (Fig. 2C). Thus, in the cell activation of KATP channels by AK could be counteracted by systems scavenging the product of AK catalysis, thereby keeping channels closed.
Figure 2.
CK regulates AK-mediated KATP channel activation. Although AMP activated both native cardiac (A) and recombinant Kir6.2/SUR2A (B) KATP channels, CrP, which promotes ADP scavenging by CK, antagonized AMP-induced KATP channel activation. (C) Average KATP channel activity (mean ± SE) in cardiomyocytes (n = 4; □) and in COS-7 cells expressing recombinant KATP channels (Kir6.2/SUR2A, n = 4; ▨), in the presence of 250 μM ATP, following addition of 50 μM AMP or following addition of AMP plus 2 mM CrP.
AK Associates with Sarcolemmal KATP Channels.
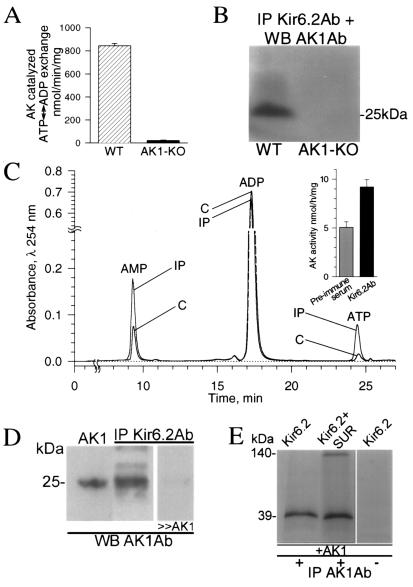
AK is localized throughout the cell, including mitochondria, cytosol, and membranes (14, 19). The close proximity and/or association of AK with an ATP consuming or ATP sensing site would assure targeted delivery of nucleotide-derived signals enhancing the efficiency of cellular energetics and metabolic signal transduction (13). Here, sarcolemmal preparations possessed high AK activity (844 ± 20 nmol⋅min−1⋅mg−1, n = 4) promoting ATP ↔ ADP exchange in the membrane environment (Fig. 3A). In contrast, sarcolemma from AK1-KO cells essentially lacked AK activity (21 ± 3 nmol⋅min−1⋅mg−1, n = 4), compromising nucleotide exchange (Fig. 3A). The marginal AK activity remaining could be attributed to minor AK isoforms and/or proteins possessing AK-like activity still present in the AK1-KO mice (14, 15). The localization of AK1 in the cell membrane raises the possibility of direct interaction with KATP channel subunits. In KATP channel immunoprecipitates, obtained using an anti-Kir6.2 Ab, a 25-kDa protein, corresponding to the molecular mass of AK1, was recognized by the anti-AK1 Ab (Fig. 3B). This protein was absent in channel immunoprecipitates from sarcolemma of AK1-KO mice (Fig. 3B). Thus, AK1 associates with KATP channels in the sarcolemma, an interaction lost following deletion of the AK1 gene.
Figure 3.
AK associates with sarcolemmal KATP channels. (A) Vigorous AK ATP ↔ ADP exchange activity in sarcolemma from wild-type (WT, n = 4; ▨), but not AK1-KO (n = 4; ■) mice. (B) Association of KATP channel protein with AK1 in wild-type, but not AK1-KO. Sarcolemmal KATP channels were immunoprecipitated (IP) with the anti-Kir6.2 Ab (Kir6.2Ab) and probed for AK1 by Western blot (WB) with the anti-AK1 Ab (AK1Ab). (C) HPLC chromatogram shows increased AK activity in KATP channel immunoprecipitates (IP) compared with control (C). The anti-Kir6.2 Ab was used to immunoprecipitate the channel complex from cardiac sarcolemma, while preimmune serum served as control. (D) Anti-AK1 Ab recognized, by Western blot, recombinant AK1 (left lane) and a protein of identical molecular mass in sarcolemmal Kir6.2 immunoprecipitates (center lane), which was competed off by excess of purified AK1 (right lane). (E) Channel proteins were in vitro translated and labeled with [35S]methionine. In the presence of nonlabeled recombinant AK1, the anti-AK1 Ab immunoprecipitated Kir6.2 (left lane) or Kir6.2/SUR subunits (center lane). Channel proteins were not immunoprecipitated by a control Ab (right lane). Proteins were run on denaturing SDS/PAGE, and visualized by Western blotting (B and D) or autoradiography (E).
Moreover, AK activity was detected in immunoprecipitates of KATP channel proteins (Fig. 3C). Cardiac KATP channel immunocomplexes catalyzed ATP ↔ ADP exchange at a rate significantly higher than that of preimmune controls (n = 9; Fig. 3C Inset). Such catalytic activity in channel immunoprecipitates was related to the 25-kDa AK1 protein revealed by Western blot analysis using the anti-AK1-specific Ab and competed off with purified AK1 protein (Fig. 3D). Conversely, the anti-AK1 Ab coimmunoprecipitated recombinant KATP channel subunits, Kir6.2 and SUR, in the presence of the AK1 protein (Fig. 3E). This was not observed in the absence of the AK1 protein, excluding a nonspecific interaction with channel proteins (Fig. 3E). Physical interaction of AK with KATP channels would facilitate nucleotide exchange in the channel's intimate environment. Therefore, AK associated with KATP channels can serve as an anchoring site for the cellular AK phosphotransfer network, accommodating metabolic signals from other intracellular compartments.
AK Phosphotransfer Response to Metabolic Challenge, Compromised in the AK1-KO.
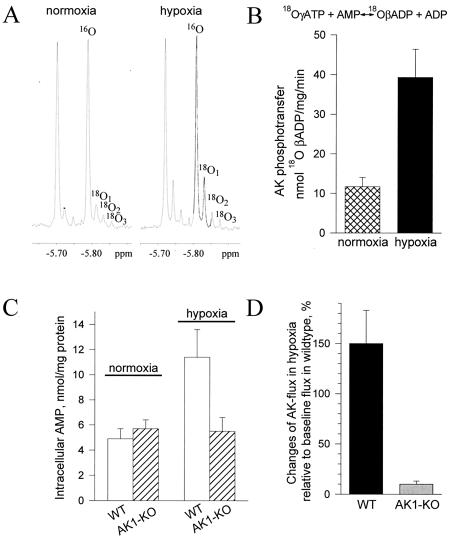
Distributed throughout the cell, sequential phosphotransfer reactions catalyzed by AK form a relay for propagation of energetic signals (10, 12, 18). The near equilibrium nature of the AK reaction ensures rapid adjustment of AK-mediated flux in response to changes in the cellular metabolic state (12). However, the response of AK phosphotransfer under conditions of metabolic stress, when KATP channels do open, is largely unknown. In the intact heart, the AK phosphotransfer rate was followed by 18O incorporation into β-phosphoryls of ADP revealed by the 18O-induced isotopic shift in the 31P NMR spectrum (Fig. 4A). In 18O-containing medium, AK-catalyzed incorporation of 18O into ADP results in the appearance of 18O1, 18O2, and 18O3 phosphoryl species (Fig. 4A). Under normoxic conditions, when KATP channels are closed, basal incorporation of 18O atoms into β-phosphoryls of ADP reflected a low AK phosphotransfer rate in the intact myocardium (Fig. 4A Left). Incorporation of 18O was significantly increased under hypoxia, indicating an augmented AK phosphotransfer rate following metabolic challenge (Fig. 4A Right). On average, AK-mediated phosphoryl flux increased >3-fold, from 11.7 ± 2.3 to 39.3 ± 7.1 nmol 18O-βADP per min per mg protein (n = 5; P < 0.05), following metabolic insult induced by hypoxia (Fig. 4B). The myocardial levels of AMP, the critical substrate in the AK reaction, also significantly increased under hypoxia (Fig. 4C). On average, the intracellular AMP concentration was 4.9 ± 0.8 and 11.4 ± 2.2 nmol per mg protein (n = 5; P < 0.05) in normoxic and hypoxic conditions, respectively. In the AK1-KO heart, the hypoxia-induced surge in AMP was lost. Accordingly, AMP levels were 5.7 and 5.5 nmol per mg protein (n = 5) in normoxic and hypoxic conditions, respectively. This was associated with a blunted response in AK phosphotransfer in the AK1-KO heart under hypoxia compared with the wild type (Fig. 4D). Thus, AMP levels and AMP-related AK phosphotransfer respond with high fidelity to hypoxia, a property lost in hearts lacking the AK1 gene. By effectively responding to stress conditions, AK phosphotransfer may accurately transmit information reflecting the cellular metabolic state.
Figure 4.
AK phosphotransfer under metabolic stress. (A) 31P NMR spectra of guinea pig heart extracts under normoxic and hypoxic conditions. Incorporation of 18O into β-phosphoryls of ADP, which reflects the rate of AK-catalyzed phosphotransfer, induces the appearance of three 18O-labeled species (18O1, 18O2, and 18O3). Incorporation of 18O was significantly higher under hypoxia. (B) Average values for AK-catalyzed phosphotransfer flux in normoxia (n = 5; ▩) and hypoxia (n = 5; ■) in guinea pig heart determined by 18O/31P NMR spectroscopy. (C) AMP levels in wild-type (WT, n = 5; □) and AK1-KO (n = 5; ▨) mouse heart under normoxia and hypoxia determined by HPLC. (D) Blunted AK phosphotransfer response in the AK1-KO (n = 5; ░⃞) compared with the wild-type (n = 5; ■) mouse heart in hypoxia.
AK Facilitates Communication of Mitochondrial Signals to KATP Channels.
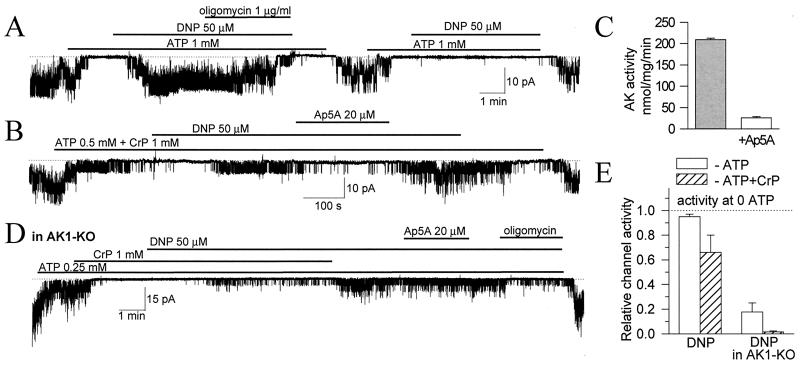
Mitochondria, which are responsible for production of the majority of cellular ATP, rapidly respond to metabolic stress by releasing signaling molecules that ultimately can determine the fate of a cell (26–28). Mitochondria contribute to metabolic oscillations that drive fluctuations of membrane current and set membrane excitability through regulation of KATP channels (2). At the cellular level, metabolic stress can be readily induced by the mitochondrial uncoupler, dinitrophenol (DNP). In the presence of DNP, mitochondrial F0F1-ATP synthase reverses its activity from ATP synthesis to ATP hydrolysis, leading to generation of ADP and vigorous KATP channel opening (Fig. 5A). The DNP-induced KATP channel opening was abolished by oligomycin, an inhibitor of F0F1-ATPase activity, indicating tight coupling of mitochondria with channel activity (Fig. 5A).
Figure 5.
AK communicates mitochondrial signals to KATP channels. (A) The mitochondrial uncoupler, DNP, induced vigorous KATP channel activation which was irreversibly inhibited by oligomycin, a mitochondrial FoF1-ATPase antagonist (n = 5). (B) In the presence of CrP, DNP produced lower activation of KATP channels as part of mitochondria-generated ADP was scavenged by the CrP/CK system. Ap5A, which at 20 μM inhibited AK activity in cardiac sarcolemma (n = 3; C), suppressed AK-mediated channel activation (n = 5). (D) In AK1-KO cardiomyocytes, KATP channel activation by DNP was blunted and insensitive to Ap5A, but inhibited by oligomycin (n = 3). (E) Average (mean ± SE) DNP-induced KATP channel activity in wild-type (left) and AK1-KO (right) cardiomyocytes in 0.5 mM ATP (□) or 0.5 mM ATP plus 1 mM CrP (▨). Bars were constructed from three to five recordings for each data point.
Through feedback regulation of nucleotide-sensitive processes, AK has been recognized as an integral part of dynamic cellular metabolic networks (29–33). Involvement of AK phosphotransfer in linking mitochondrial events with KATP channels was probed using diadenosine pentaphosphate (Ap5A), an inhibitor of AK (34, 35). Ap5A was applied under conditions when mitochondria-generated ADP, not involved in AK trafficking, was scavenged by the CK system (Fig. 5B). Ap5A inhibited AK activity in cardiac membranes (Fig. 5C) and antagonized DNP-induced KATP channel activation (Fig. 5B). Thus, pharmacological inhibition of AK phosphotransfer impeded delivery of mitochondrial signals to the KATP channel site.
Furthermore, in AK1-KO cardiomyocytes the KATP channel response to DNP-induced mitochondrial ATPase activation was blunted, indicating uncoupling of mitochondrial signals from KATP channel regulation following deletion of the AK1 gene (Fig. 5D). In AK1-deficient cells, the modest DNP effect was sensitive to oligomycin but insensitive to Ap5A (Fig. 5D), suggesting that in AK1-KO cells mitochondrial signals can be generated but their delivery is compromised. Thus, disarrangement of the AK-catalyzed phosphorelay impedes transmission of mitochondrial signals to KATP channels and disrupts coordination of channel behavior in response to metabolic stress. This provides direct evidence that simple diffusion, in the absence of catalyzed phosphotransfer, is insufficient to secure effective metabolic signaling.
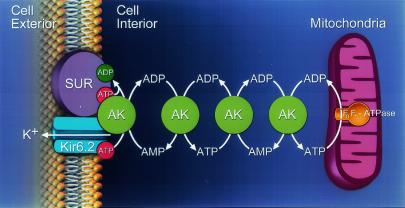
In summary, this study identifies catalyzed phosphorelay as essential for efficient coupling of cellular energetics with membrane excitability. This mechanism represents a novel and possibly general aspect in the control of ATP-sensitive cellular components. Specifically, KATP channel-associated AK coupled with cellular phosphotransfer facilitated nucleotide exchange and transduction of mitochondrial signals into membrane electrical events (Fig. 6). The significance of this finding is underscored by defective metabolic sensing, associated with electrical instability and functional degeneration, in diseased states with compromised cellular phosphotransfer (2, 9, 10, 14, 36).
Figure 6.
AK phosphotransfer communicates mitochondria-generated signals to KATP channels. AK molecules form a phosphorelay network connecting mitochondria with the cell membrane. Under hypoxic stress, mitochondrial FoF1-ATPase consumes cellular ATP generating ADP, which is delivered to the KATP channel through the chain of sequential AK-catalyzed phosphotransfer reactions. The inwardly rectifying potassium channel (Kir6.2) and the SUR are the pore-forming and regulatory subunits of the KATP channel complex.
Acknowledgments
We are grateful to Drs. J. Bryan, Y. Kurachi, and S. Seino for KATP channel clones. This work was supported by National Institutes of Health (GM-19567, HL-07111, HL-64822), American Heart Association, Miami Heart Research Institute, Bruce and Ruth Rappaport Program in Vascular Biology and Gene Delivery, Marriott Foundation, American Physicians Fellowship for Medicine in Israel, Guidant Foundation, Netherlands organization for Scientific Research-GMW Program (901-01-095), and Nederlandse Kankerbestrijding/KWF (KUN 98-1808). A.T. is an Established Investigator of the American Heart Association.
Abbreviations
- KATP
ATP-sensitive K+
- SUR
sulfonylurea receptor
- AK
adenylate kinase
- KO
knockout
- DNP
dinitrophenol
- Ap5a
diadenosine pentaphosphate
- Cr
creatine
- CrP
Cr phosphate
- CK
Cr kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Weiss J N, Lamp S T. Science. 1987;238:67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- 2.O'Rourke B, Ramza B M, Marban E. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 3.Noma A. Nature (London) 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft F M, Harrison D E, Ashcroft S J. Nature (London) 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki N, Gonoi T, Clement J P, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 6.Nichols C G, Shyng S, Nestorowicz A, Glaser B, Clement J, Gonzalez G, Aguilar-Bryan L, Permutt M, Bryan J. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 7.Bienengraeber M, Alekseev A E, Abraham M R, Carrasco A J, Moreau C, Vivaudou M, Dzeja P P, Terzic A. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- 8.Bessman S P, Geiger P J. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- 9.Steeghs K, Benders A, Oerlemans F, de Haan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, et al. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 10.Dzeja P P, Terzic A. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 11.Saupe K W, Spindler M, Hopkins J C, Shen W, Ingwall J S. J Biol Chem. 2000;275:19742–19746. doi: 10.1074/jbc.M001932200. [DOI] [PubMed] [Google Scholar]
- 12.Dzeja P P, Zeleznikar R J, Goldberg N D. Mol Cell Biochem. 1998;184:169–182. [PubMed] [Google Scholar]
- 13.Dzeja P P, Vitkevicius K T, Redfield M M, Burnett J C, Terzic A. Circ Res. 1999;84:1137–1143. doi: 10.1161/01.res.84.10.1137. [DOI] [PubMed] [Google Scholar]
- 14.Janssen E, Dzeja P P, Oerlemans F, Simonetti A, Heerschap A, de Haan A, Rush P S, Terjung R R, Wieringa B, Terzic A. EMBO J. 2000;19:6371–6381. doi: 10.1093/emboj/19.23.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pucar D, Janssen E, Dzeja P P, Juranic N, Macura S, Wieringa B, Terzic A. J Biol Chem. 2000;275:41424–41429. doi: 10.1074/jbc.M007903200. [DOI] [PubMed] [Google Scholar]
- 16.Bandlow W, Strobel G, Zoglowek C, Oechsner U, Magdolen V. Eur J Biochem. 1998;178:451–457. doi: 10.1111/j.1432-1033.1988.tb14469.x. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez J A, Csonka L N. J Bacteriol. 1995;177:390–400. doi: 10.1128/jb.177.2.390-400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson L K, Schroeder W, Robertson R P, Goldberg N D, Walseth T F. J Biol Chem. 1996;271:16544–16552. doi: 10.1074/jbc.271.28.16544. [DOI] [PubMed] [Google Scholar]
- 19.Elvir-Mairena J R, Jovanovic A, Gomez L A, Alekseev A E, Terzic A. J Biol Chem. 1996;271:31903–31908. doi: 10.1074/jbc.271.50.31903. [DOI] [PubMed] [Google Scholar]
- 20.Alekseev A E, Brady P A, Terzic A. J Gen Physiol. 1998;111:381–394. doi: 10.1085/jgp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alekseev A E, Kennedy M, Navarro B, Terzic A. J Membr Biol. 1997;156:161–168. doi: 10.1007/s002329900279. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki N, Gonoi T, Clement J P, Wang C Z, Aguilar-Bryan L, Seino S, Bryan J. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz E, Alekseev A E, Krapivinsky G B, Carrasco A J, Clapham D E, Terzic A. Mol Cell Biol. 1998;18:1652–1659. doi: 10.1128/mcb.18.3.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz E, Terzic A. J Mol Cell Cardiol. 1999;31:425–434. doi: 10.1006/jmcc.1998.0876. [DOI] [PubMed] [Google Scholar]
- 25.Saks V A, Tiivel T, Kay L, Novel-Chate V, Daneshrad Z, Rossi A, Fontaine E, Keriel C, Leverve X, Ventura-Clapier R, et al. Mol Cell Biochem. 1998;160–161:195–208. doi: 10.1007/BF00240050. [DOI] [PubMed] [Google Scholar]
- 26.Saraste M. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 27.Wallace D C. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 28.Kroemer G, Reed J C. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 29.Mair T, Muller S C. J Biol Chem. 1996;271:627–630. doi: 10.1074/jbc.271.2.627. [DOI] [PubMed] [Google Scholar]
- 30.Schellenberger W, Eschrich K, Hofmann E. Acta Biol Med Ger. 1978;37:1425–1441. [PubMed] [Google Scholar]
- 31.Kremen A. J Theor Biol. 1982;96:425–441. doi: 10.1016/0022-5193(82)90119-9. [DOI] [PubMed] [Google Scholar]
- 32.Appleby J L, Parkinson J S, Bourret R B. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 33.Jeong H, Tombor B, Albert R, Oltval Z, Barabasi A-L. Nature (London) 2000;407:651–654. doi: 10.1038/35036627. [DOI] [PubMed] [Google Scholar]
- 34.Lienhard G E, Secemski I I. J Biol Chem. 1973;248:1121–1123. [PubMed] [Google Scholar]
- 35.Jovanovic A, Jovanovic S, Mays D C, Lipsky J J, Terzic A. FEBS Lett. 1998;423:314–318. doi: 10.1016/s0014-5793(98)00114-8. [DOI] [PubMed] [Google Scholar]
- 36.Dzeja P P, Pucar D, Redfield M M, Burnett J C, Terzic A. Mol Cell Biochem. 1999;201:33–40. doi: 10.1023/a:1007016703229. [DOI] [PubMed] [Google Scholar]