Abstract
Male orangutans (Pongo spp.) display an unusual characteristic for mammals in that some adult males advance quickly to full secondary sexual development while others can remain in an adolescent-like form for a decade or more past the age of sexual maturity. Remarkably little is understood about how and why differences in developmental timing occur. While fully-developed males are known to produce higher androgen levels than arrested males, the longer-term role of steroid hormones in male life history variation has not been examined. We examined variation in testosterone and cortisol production among 18 fully-developed (“flanged”) male orangutans in U.S. captive facilities. Our study revealed that while testosterone levels did not vary significantly according to current age, housing condition, and species origin, males that had undergone precocious development had higher testosterone levels than males that had experienced developmental arrest. While androgen variation had previously been viewed as a state-dependent characteristic of male developmental status, our study reveals that differences in the physiology of early and late developing males are detectable long past the developmental transition and may instead be trait-level characteristics associated with a male’s life history strategy. Further studies are needed to determine how early in life differences in testosterone levels emerge and what consequences this variation may have for male behavioral strategies.
Introduction
While it is common for alternative reproductive strategies to exist within a species, orangutans are unusual among mammals in that fertile, adult males occur in two distinct morphological types. Flanged males (Figure 1a) exhibit massive body size accompanied by prominent fatty cheek flanges, and a throat sac facilitating the long call vocalization of this morph [1]–[3]. Some males, although able to reproduce, remain in the adolescent-like unflanged form (Figure 1b) for 10 or more years following puberty [4], [5]. While flanged males appear to gain preferential access to fecundable females [6], it remains unclear why some males develop earlier than others, what factors trigger secondary sexual development, and what the relative fitness advantages of each morph are. Early observations from captivity suggested that development was suppressed whenever a dominant flanged male could be seen or heard [7]–[9], yet results from male pairings are inconsistent. Additionally, a survey of captive orangutans found higher levels of glucocorticoids in males undergoing secondary sexual development than those in developmental arrest, suggesting that stress from competitors was not a mechanism for delayed maturation [10]. A failure to understand the mechanisms for the male developmental transition has hindered understanding the evolution of this peculiar life history pattern.
Figure 1. Sexual bimaturism in orangutans.
Flanged (A) and unflanged (B) male orangutans from Gunung Palung National Park, Indonesia, illustrating dramatic differences in morphology. Photos by Tim Laman.
As a general rule, testosterone and related androgenic hormones are responsible for the development of male genitalia, as well as a range of sexually-dimorphic characteristics, such as musculature and ornamental coloration. Testosterone additionally supports behaviors pursuant to male reproduction, including sexual motivation, breeding displays, and male-male competition [11]–[13]. Thus, it is not surprising that studies have reported higher testosterone levels among flanged versus unflanged males [8], [14]. The precise role of testosterone during development is still questionable, since one study in captivity found the levels of actively-developing males to be significantly higher than either arrested or fully-flanged males [14], while another found the levels of developing males to be intermediate between arrested and flanged males [8]. In neither case were young males followed to determine whether variation in testosterone levels is merely informative about current developmental status (“state-dependent”) or whether this variation might influence or predict future developmental trajectories (“trait-dependent”). Similarly, these studies do not indicate whether testosterone levels continue to change with age after development is complete. In fact, no published research explores variation in testosterone levels among orangutan males beyond the level of current developmental stage. Such research would importantly inform our understanding of alternative life history variation in orangutans by revealing whether life history strategies diverge only in age of development or encompass longer-term processes. Additionally, insights into physiological variation among flanged males may help explain the highly variable behavior patterns observed among flanged males in the wild [15], [16].
Stress can be a potent inhibitor of development, with glucocorticoids acting directly to inhibit growth hormone, thyroid hormone, and other growth factors [17], [18]. Elevated glucocorticoids can also be a mechanism for the reproductive suppression of subordinate animals [19], [20], though this can be ruled out as a mechanism for suppression in many cases [21], [22]. While it has been suggested that development in orangutan males may be suppressed directly or indirectly by the presence of dominant flanged males, a prior endocrine study found growth hormone levels of seven arrested males to be suppressed [23] without elevation of glucocorticoids [10]. Nevertheless, because glucocorticoids have interactions both with development and with the reproductive axis, they may be expected to be correlates of male life history strategy. Additionally, because some captive housing conditions may lead to chronic stress, it is important to determine whether variation in androgen production may be a correlate of variation in the stress response.
While longitudinal studies of physiology during development are needed to fully address the relationship of hormones to male life history in orangutans, the exceptionally slow developmental process in orangutans makes realizing this goal a time-consuming challenge. As we continue to gather such data, we present here an examination of variation in testosterone and glucocorticoids among fully-flanged male orangutans in relation to several important life history variables: current age, age of flange development, the presence of other flanged males in the same facility, and specific origin (Pongo pygmaeus, P. abelii, or hybrid). We predict that, if divergence of male life history strategies persists beyond the age of flange development, males that developed at a relatively young age will have higher testosterone than males that developed relatively late. We further predict that this difference should not be explained by variation in stress, as measured by glucocorticoid levels.
Methods
We recruited subjects by contacting accredited U.S. captive facilities for collaboration. Thirteen zoos collected urine samples from 18 flanged male orangutans (Table 1). All subjects were captive-born and thus of known age. For 15 of the subjects, facility records provided information on developmental age, defined as the first year in which noticeable changes in secondary sexual characteristics (chiefly, body and flange size) were recorded. It should be noted that male development, once initiated, proceeds relatively rapidly and is typically completed within 1–2 years. Five of the males initiated flange development early, between the ages of 9 and 11, while 10 of the males initiated development late, between the ages of 14 and 18. Though these categories unevenly divide the males in the study, they were most appropriate because of a discrete gap in the distribution; no males of known developmental age in our sample initiated development at ages 12 or 13. For an additional 3 males of uncertain developmental age, contextual information based on developmental status at the time of transfer between facilities allowed us to determine whether they had begun development prior to age 14 (N = 1) or after age 14 (N = 2). Therefore, our final sample included 18 males that were designated as to whether their age of development was early (prior to age 14, N = 6) or late (after age 14, N = 12). All males except one were housed in social groups with at least one adult female; while mutual intolerance prevents two flanged males from living as cagemates just as it prevents direct association in the wild, some facilities maintained more than one group, meaning that males would have had some auditory, visual, and/or olfactory contact with another flanged male. Thus, we considered in our analysis whether each male was housed in the same facility as one or more flanged males. Unfortunately, record keeping was not generally adequate to determine housing conditions at the time of male secondary sexual development. In the process of collecting samples from flanged males, two collaborating zoos also submitted 25 samples from 3 males that were undergoing development at the time of sampling.
Table 1. Study subjects and characteristics.
| Stud ID | Zoo | Species | Age at Sampling | Age at Flanging | # Samples | Mean ± S.D. Morning T(ng/mg-Cr) | Current Housed with Flanged |
| 2431 Brunei | Brookfield | Hybrid | 15 | 9 | 3 | 276±219 | Yes |
| 2132 Kiko | National | Hybrid | 19 | 10 | 3 | 660 | Yes |
| 1818 Urban | Sacramento | P. abelii | 25 | 11 | 10 | 146±93 | No |
| 1801 Chewbacca | Sedgwick County | P. abelii | 26 | 11 | 8 | 237±198 | No |
| 2021 Rok | Little Rock | Hybrid | 22 | 11 | 7 | 123±59 | No |
| 1886 Pongo | Brookfield | Hybrid | 24 | <14 | 6 | 197±71 | Yes |
| 2255 Heran | Woodland Park | Hybrid | 18 | 14 | 10 | 52±10 | Yes |
| 1932 Mias II | Denver | P. abelii | 23 | 15 | 6 | 111±31 | Yes |
| 2009 Doc | Houston | P. pygmaeus | 24 | 15 | 10 | 113±34 | Yes |
| 2139 Teak | Louisville | Hybrid | 20 | 15 | 10 | 146±58 | Yes |
| 2137 Segundo | Louisville | P. abelii | 20 | 15 | 10 | 220±89 | Yes |
| 2201 Mawas | Topeka | P. pygmaeus | 18 | 15 | 4 | 63±5 | No |
| 1614 Rudi | Houston | Hybrid | 31 | 16 | 10 | 77±9 | Yes |
| 1403 Rango | Lowry Park | P. pygmaeus | 33 | 16 | 6 | 38±19 | No |
| 1616 Azy | Great Ape Trust | Hybrid | 29 | 17 | 18 | 175±45 | No |
| 2034 Butch | Miami Metro | P. abelii | 21 | 18 | 6 | 251±148 | Yes |
| 1671 Ben | Brookfield | P. pygmaeus | 28 | >14 | 4 | 172±70 | Yes |
| 1504 Robin | Denver | Hybrid | 30 | >14 | 5 | 85±29 | Yes |
| Actively Developing Males | |||||||
| 2718 Panji | Sedgwick County | P. abelii | 11 | 8 | 317±197 | Yes | |
| 2626 Sulango | Atlanta | P. pygmaeus | 14 | 7 | 59±30 | Yes | |
| 2259 Jantan | Atlanta | P. abelii | 18 | 10 | 50±15 | Yes | |
Urine samples were collected from each male on multiple days within a 1–4 week time period (Table 1). Samples were collected either by direct urination into a cup or by pipetting from a clean, dry cage floor. Samples were placed into polypropylene tubes, frozen immediately, and subsequently shipped to the laboratory on ice for analysis within 1–3 months. In other apes, urinary testosterone and cortisol are reported to have circadian patterns, decreasing from morning to afternoon [24]–[26]. We, therefore, centered our analysis on 120 samples collected prior to noon before orangutans went on display (of which >80% were collected between 7 and 9 am). Among samples collected prior to noon, there was no significant decline in either hormone among samples with a specific time recorded (log-transformed testosterone, Pearson’s r = 0.070, N = 110, p = 0.466; log-transformed cortisol, r = −0.178, N = 110, p = 0.063), which is consistent with our observations for wild orangutans (Knott and Emery Thompson, unpublished data). For six subjects sampled both in the morning and afternoon (on different days), average hormonal determinations from the two time periods were highly correlated (log-transformed testosterone, Pearson’s r = 0.932, N = 6, p = 0.007; cortisol, r = 0.904, N = 6, p = 0.013). Prior to assays, samples were deconjugated with beta-glucuronidase (Helix pomatia, Calbiochem, <2% aryl sulfatase activity) to recover the major urinary metabolite of testosterone [27]. Though a recent paper has questioned whether Escherichia coli derived enzyme might be superior to H. pomatia for this procedure [28], we found that the two enzyme derivations produced comparable testosterone concentrations from orangutan urine (log-transformed, Pearson’s r = 0.709, N = 35, p<0.001; E. coli VII-A, Sigma Aldrich). After drying down and reconstituting in phosphate-buffered saline, samples were assayed for immunoreactive testosterone and cortisol metabolites using enzyme-immunoassay reagents and protocols provided by the Clinical Endocrinology Laboratory at UC Davis [29].
The testosterone antibody employed (R156/7, U.C. Davis) has demonstrated a high cross-reactivity with only one exclusive metabolite of testosterone, 5alpha-dihydrotestosterone (DHT, 57%), and low cross-reactivity with the adrenal androgen androstenedione (0.3%, C. Munro personal communication). Cross-reactivities with other steroids were minimal (≤0.04). Assay accuracy was assessed by the recovery of testosterone in an orangutan urine sample added to the standard curve doses. Recoveries averaged 121±14 (SD)% (N = 7). This suggests that our assay systematically overestimates the concentrations of testosterone in orangutan urine. However, variation in recovery did not covary with the dosage of standard and could not be attributable to differences in sample matrix as both standard and sample were brought up in the same buffer. We also tested the parallelism of binding curves generated from serial dilutions of testosterone standard and an extracted orangutan urine sample. The resulting regressions were not significantly different (standard y = −0.380x +1.512, sample: y = −0.337x +1.322; t = 0.932, df = 10, p = 0.373). Interassay CVs were 12.4% for low samples and 15.4% for high sample (N = 12), and intrassay CVs for duplicates (N = 161) averaged 7.7%. Sensitivity of the assay was approximately 16 pg/ml.
The cortisol antibody (R4866, U.C. Davis) used in our assay cross-reacts to a limited degree with cortisone (5%), while all other cross-reactivities with natural steroids are <1%. Recoveries of cortisol in orangutan urine averaged 90.5±8.7% (N = 7). Parallel curves were obtained from diluted standard and urine samples (standard y = −0.115x +0.979, sample: y = −0.138x +0.978; t = −0.392, df = 9, p = 0.704). Interassay CVs were 10.9% for low samples and 13.6% for high samples (N = 15), and intraassay CVs averaged 7.1% (N = 161). Sensitivity of the assay was approximately 16 pg/ml. Steroid concentrations were indexed to creatinine using the Jaffe reaction [30] and log-transformed prior to analysis.
Statistical analyses were performed using PASW 18.0. Generalized Linear Models included two discrete factors (age of development early/late and presence/absence of flanged male in the same facility) and one continuous covariate (age), with each male’s mean immunoreactive testosterone or cortisol as the response variable. Non-significant variables were sequentially eliminated until the model contained only predictors with p-values <0.05. Parameter and probability estimates for excluded predictors, as presented in the tables, are calculated by adding each back, one at a time, to the final model. We tested for possible significant interaction terms, but found none. We also verified that the models produced normally-distributed and uncorrelated residuals and that significant predictors exhibited homogeneity of variance. Species origin does not qualitatively affect our results if included in such models, but we did not include it because there was an empty category (all Bornean were late maturers). Effects of species were examined in separate one-way ANOVAs.
Non-invasive sample collection was conducted with approvals by the Animal Care and Use Committees at Harvard University (protocol 95-04) and the University of New Mexico (protocol 11-100726-MCC). Samples were collected without direct contact with the animals and with minimal disturbance to their daily routine.
Results
While neither age nor presence of other flanged males was a significant predictor of urinary testosterone levels, age of flange development was (Table 2). Males that completed their development before age 14 had higher testosterone levels as adults than did males that completed development after that age (Figure 2). One male with unusually high testosterone levels and a single morning sample did not bias the results, as the model was qualitatively the same and the effect of developmental timing was statistically significant with his exclusion (Estimate = 0.240, df = 1, p = 0.039).
Table 2. Results of Generalized Linear Models for Urinary Testosterone in 18 Flanged Male Orangutans.
| Variable | Estimate | Std. Error | Df | p-value |
| Intercept | 2.033 | 0.069 | 1 | <0.001 |
| Early/Late Development | 0.325 | 0.120 | 1 | 0.007 |
| Excluded Variables | ||||
| Age | −0.015 | 0.011 | 1 | 0.201 |
| Other Flanged | 0.137 | 0.122 | 1 | 0.261 |
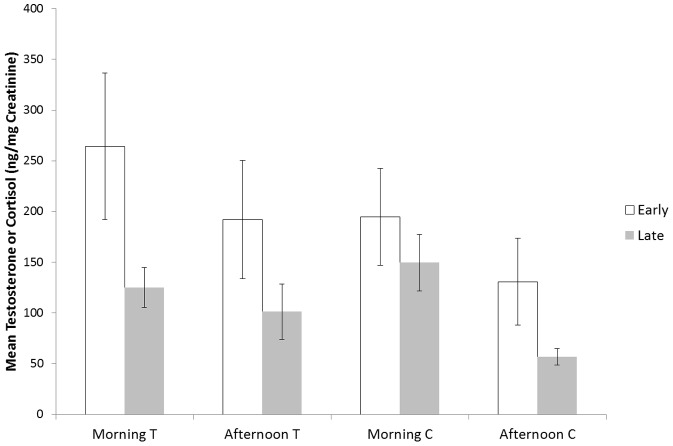
Figure 2. Boxplot of urinary testosterone and cortisol in captive male orangutan subjects according to categorical age of maturation: early (flanging before age 14) or late (after age 14).
Morning samples were obtained from 18 subjects and comprised the dataset for statistical analysis; afternoon samples were available for only 6 males but corroborate the differences observed in the morning. Plots indicate median (horizontal line), 25th and 75th percentile (box), minimum and maximum excluding outliers (whiskers), and outliers (circles).
Urinary cortisol levels of males were not correlated with their testosterone levels (Pearson’s r = 0.233, N = 21, p = 0.310). Cortisol levels did not vary significantly between early- and late-maturing males, between species, between males housed in proximity with other flanged males and those not, nor with male age (Table 3).
Table 3. Results of Generalized Linear Models for Urinary Cortisol in 18 Flanged Male Orangutans.
| Variable | Estimate | Std. Error | Df | p-value |
| Intercept | 2.143 | 0.059 | 1 | <0.001 |
| Excluded Variables | ||||
| Age | −0.017 | 0.011 | 1 | 0.135 |
| Early/Late Development | 0.131 | 0.121 | 1 | 0.281 |
| Other Flanged | 0.002 | 0.121 | 1 | 0.990 |
Because all four Bornean orangutans in our sample matured late, we tested whether there was a species effect on either testosterone or cortisol and found no evidence of such an effect in either the full dataset (ANOVA, testosterone: F = 1.833, df = 2,15, p = 0.194; cortisol: F = 0.378, df = 2,15, p = 0.691) or in the subset of subjects that matured late (ANOVA, testosterone: F = 1.975, df = 2,9, p = 0.195; cortisol: F = 0.205, df = 2,9, p = 0.818).
Discussion
In this first analysis of variation in testosterone among flanged male orangutans, we found that males that had completed their secondary sexual development at a young age maintained higher testosterone levels than those with delayed maturation. This is the first evidence that testosterone levels in male orangutans are linked not only with developmental status, but with developmental timing. This extends our understanding of a very rare form of developmental variation by demonstrating persistent differences in the steroid physiology of males with divergent life history strategies. The fact that developmentally delayed males maintain low levels of androgen production many years after transitioning to a flanged status suggests that they may continue to pursue differences in behavioral strategies when compared to males with early development, a possibility which has not yet been raised in the literature on this species. Indeed, if it is the case that all males eventually complete secondary sexual development, categorization of behavior according to current status may have blurred the lines between males pursuing dramatically different life history strategies.
One possible alternative explanation for the link between testosterone production and developmental age is that some males may have experienced persistent stress in their environments, simultaneously delaying development and suppressing testosterone. Indeed, the prevailing hypothesis for arrested development in orangutans is that male secondary sexual development is delayed and subsequently triggered in response to external stimuli, such as visual or auditory cues to the presence of other developed males [7], [9]. No such triggers have been consistently linked with developmental timing, and a prior study reported that arrested males actually had lower stress hormone production than flanging males [10]. Our results found no difference in stress hormone levels between early and late developing males. Because flanged males compete intensely with one another but are relatively tolerant of unflanged males, it has been suggested that delayed development could actually serve as a mechanism for stress avoidance in the face of intense competition [10]. If this is the case, then we would expect stress hormone levels to equalize after flanging, consistent with our findings. Nevertheless, the stress- avoidance hypothesis suggests that there should be stress responses to male competitors, and we did not find that the presence of other flanged males in the same facility produced an effect on either testosterone or cortisol levels of the subjects.
Neither current age nor species origin was a significant predictor of testosterone or cortisol in our sample. Age declines in testosterone are detectable in some human populations [31], as well as some non-human primates (e.g., baboons [32], but not muriquis [33]). Our data showed a trend in this direction, but given a large amount of individual variability, age trends are difficult to discern in small samples. The solitary nature of orangutan males makes it difficult to acquire a sufficiently large sample from either the wild or captivity to gain sufficient power for such a test. Additionally, the eldest males in our sample were in their mid-30s, whereas orangutans can live a decade or two longer [34], [35]. There is a debate in the literature as to whether the Bornean and Sumatran species of orangutans maintain evolved differences in life history trajectories [36]. While some authors have argued for slower life histories for Sumatran orangutans in the wild based on small samples of interbirth intervals [35], demographic statistics for the two species converge in captivity when ecological conditions are stable [34]. Thus, while it has been suggested that developmental arrest might be more common or more prolonged among Sumatran orangutans, where the relative proportion of unflanged males is higher [37], this was not apparent in our captive sample: all four Bornean males were late-maturers, and species origin did not modify the relationship between maturation timing and hormonal parameters. However, the majority of individuals in our sample were hybrids.
Our results cannot currently distinguish between two alternative interpretations, that young male orangutans that produce high levels of testosterone initiate secondary sexual development earlier than those with low levels, or that males that developed early attain higher testosterone levels only after flanging. Distinguishing these alternatives will require monitoring young male orangutans throughout the long juvenile and adolescent periods. Acquiring such a sample will require many years of continuing research, and is an objective we are actively pursuing. However, in acquiring the sample for the present study, we incidentally received samples from 3 males that were beginning flange development. Among them, one was developing at a young age (8 yrs) and had testosterone levels more than five times higher than the two males that were developing at late ages (14 and 18 yrs). Other studies have identified predictive effects of testosterone on future status. Testosterone levels of chacma baboons over a 1-yr study period were better predictors of future than current rank and mating activity [38]. In the closest primate analog to male bimaturism in orangutans, male mandrills commonly experience delayed reproductive development. Among adolescent male mandrills, there is already considerable individual variation in testosterone levels, which correlates with individual differences in secondary sexual development, testicular volume, body size, and dominance and with early attainment of adult weight [39], [40]. Evidence of similar effects in orangutans would modify hypotheses about developmental timing in this species to focus less on the role of external factors and more on the role of intrinsic individual variation.
It is not currently known what consequences the observed variation in testosterone would have for orangutans on different life history trajectories. Elevated testosterone is generally indicative of behavioral and physiological allocations to mating effort, including competition with other males [12], [13]. Encounters among flanged male orangutans are invariably aggressive and entail significant risk of injury or death [1], [16], [41]. Therefore, it is possible that testosterone variation influences the willingness of males to provoke an encounter with another male. Flanged males differ amongst one another in the use of sexually coercive aggression with females. Ranging patterns of flanged males also vary, with some establishing home ranges and others pursuing transient strategies [4], [42]. Physiological correlates of these behaviors are not yet known, yet either might reflect persistence of high or low risk strategies past the age of development. Finally, though empirical evidence from mammals is scarce, testosterone, either directly or through its diversion of energy to mating effort, is theoretically predicted to compromise immune function and other investments in somatic maintenance [43], [44]. Just as alternative developmental trajectories in orangutans have been theorized to optimize the costs of benefits of large body size and secondary sexual adornments [1], [14], [45], [46], males are predicted to balance the costs and benefits of elevated testosterone in pursuit of a life history strategy.
In conclusion, we report that male orangutans that undergo arrested secondary sexual development maintain lower levels of androgen production, even many years following the developmental transition, than do males that develop at a young age. These differences cannot be explained by differences in housing conditions, physiological stress, or species origin, and are independent of the ages of the subject. We conclude that testosterone production is not only elevated in orangutans as a state-dependent consequence of becoming a flanged male, but is also elevated as a persistent trait of males that adopt an early maturation strategy. Thus, a full understanding of sexual bimaturism as a life history strategy in orangutans entails a longer-term perspective than that which has previously been applied and requires investigation of how developmental timing correlates with differences in behavior and physical condition after, and perhaps before, the developmental transition.
Acknowledgments
We thank the veterinary and animal care staffs of the facilities that provided samples (Table 1).
Funding Statement
Funding was provided by the National Science Foundation (grant 0936199). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Galdikas BMF (1985) Subadult male orangutan sociality and reproductive behavior at Tanjung Puting. American Journal of Primatology 8: 87–99. [DOI] [PubMed] [Google Scholar]
- 2. MacKinnon JR (1974) Behavior and ecology of wild orangutans (Pongo pygmaeus). Animal Behaviour 22: 3–74. [Google Scholar]
- 3.Rijksen H (1978) A field study on Sumatran orang-utans (Pongo pygmaeus abelli, Lesson 1827): ecology, behaviour and conservation: Veenman & Zonen, Wageningen, Netherlands. [Google Scholar]
- 4. te Boekhorst IJA, Schürmann CL, Sugardjito J (1990) Residential status and seasonal movements of wild orang-utans in the Gunung Leuser Reserve (Sumatra, Indonesia). Animal Behaviour 39: 1098–1109. [Google Scholar]
- 5. Utami SS, Goossens B, Bruford MW, de RuiterJR, van Hooff JARAM (2002) Male bimaturism and reproductive success in Sumatran orangutans. Behavioral Ecology 13: 643–652. [Google Scholar]
- 6. Knott CD, Emery Thompson M, Stumpf RM, McIntyre MH (2010) Female reproductive strategies in orangutans, evidence for female choice and counterstrategies to infanticide in a species with frequent sexual coercion. Proceedings of the Royal Society of London Series B: Biological Sciences 277: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maple T (1980) Orangutan Behavior. New York: Van Nostrand Reinhold. [Google Scholar]
- 8.Kingsley S (1982) Causes of non-breeding and the development of secondary sexual characteristics in the male orang-utan: a hormonal study. In: de Boer LEM, editor. The Orang-Utan, Its Biology and Conservation. The Hague: W. Junk. 215–229. [Google Scholar]
- 9.Graham CE, Nadler RD (1990) Socioendocrine interactions in great ape reproduction. In: Ziegler TE, Bercovitch FB, editors. Socioendocrinology of Primate Reproduction: Wiley-Liss, Inc, New York. 33–58. [Google Scholar]
- 10. Maggioncalda A, Czekala NM, Sapolsky RM (2002) Male orangutan subadulthood: a new twist on the relationships between chronic stress and developmental arrest. American Journal of Physical Anthropology 118: 25–32. [DOI] [PubMed] [Google Scholar]
- 11.Nelson RJ, 3rd (2005) An Introduction to Behavioral Endocrinology, Third Edition: Sinauer Associates, Inc, Sunderland, MA. 816 pp p. [Google Scholar]
- 12. Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF (1990) The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist 136: 829–846. [Google Scholar]
- 13.Dixson AF (1998) Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Human Beings: Oxford University Press, Oxford. [Google Scholar]
- 14. Maggioncalda AN, Sapolsky RM, Czekala NM (1999) Reproductive hormone profiles in captive male orangutans: implications for understanding developmental arrest. American Journal of Physical Anthropology 109: 19–32. [DOI] [PubMed] [Google Scholar]
- 15.Knott CD (2009) Orangutans: sexual coercion without sexual violence. In: Muller MN, Wrangham RW, editors. Sexual Coercion in Primates and Humans: An Evolutionary Perspective on Male Aggression Against Females. Cambridge, MA: Harvard University Press. 81–111. [Google Scholar]
- 16. Mitani JC (1985) Mating behaviour of male orangutans in the Kutai Game Reserve, Indonesia. Animal Behaviour 33: 392–402. [Google Scholar]
- 17. Chrousos GP (1998) Stressors, stress, and neuroendocrine integration of the adaptive response: the 1997 Hans Selye Memorial Lecture. Annals of the NY Academy of Sciences 851: 311–335. [DOI] [PubMed] [Google Scholar]
- 18.Sapolsky RM (1992) Neuroendocrinology of the stress response. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral Endocrinology: MIT Press, Cambridge. 284–324. [Google Scholar]
- 19. Hacklander K, Mostl E, Arnold W (2003) Reproductive suppression in female Alpine marmots, Marmota marmota . Animal Behaviour 65: 1133–1140. [Google Scholar]
- 20. Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, et al. (2002) Trends of reproductive hormones in male rates during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biology of Reproduction 67: 1750–1755. [DOI] [PubMed] [Google Scholar]
- 21. Ziegler TE, Scheffler G, Snowdon CT (1995) The relationship of cortisol levels to reproductive functioning in female cotton-top tamarins, Saguinus oedipus . Hormones and Behavior 29: 407–424. [DOI] [PubMed] [Google Scholar]
- 22. Creel SF (2005) Dominance, aggression, and glucocorticoid levels in social carnivores. Journal of Mammalogy 86: 255–264. [Google Scholar]
- 23. Maggioncalda AN, Czekala NM, Sapolsky RM (2000) Growth hormone and thyroid stimulating hormone concentrations in captive male orangutans: Implications for understanding developmental arrest. American Journal of Primatology 50: 67–76. [DOI] [PubMed] [Google Scholar]
- 24. Muller MN, Lipson SF (2003) Diurnal patterns of urinary steroid excretion in wild chimpanzees. American Journal of Primatology 60: 161–166. [DOI] [PubMed] [Google Scholar]
- 25. Czekala NM, Lance VA, Sutherland-Smith M (1994) Diurnal urinary corticoid excretion in the human and gorilla. American Journal of Primatology 34: 29–34. [DOI] [PubMed] [Google Scholar]
- 26. Whitten PL, Brockman DK, Stavisky RC (1998) Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Yearbook of Physical Anthropology 41: 1–23. [DOI] [PubMed] [Google Scholar]
- 27. Robbins MM, Czekala NM (1997) A preliminary investigation of urinary testosterone and cortisol levels in wild male mountain gorillas. American Journal of Primatology 43: 51–64. [DOI] [PubMed] [Google Scholar]
- 28. Hauser B, Schulz D, Boesch C, Deschner T (2008) Measuring urinary testosterone levels of great apes – problems with enzymatic hydrolysis using Helix pomatia juice. General and Comparative Endocrinology 158: 77–86. [DOI] [PubMed] [Google Scholar]
- 29. Munro CJ, Lasley BL (1988) Non-radiometric methods for immunoassay of steroid hormones. Prog Clin Biol Res 285: 289–329. [PubMed] [Google Scholar]
- 30. Taussky HH (1954) A microcolorimetric determination of creatinine in urine by the Jaffe reaction. Journal of Biological Chemistry 208: 853–861. [PubMed] [Google Scholar]
- 31. Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, et al. (2002) Population variation in age-related decline in male salivary testosterone. Human Reproduction 17: 3251–3253. [DOI] [PubMed] [Google Scholar]
- 32. Altmann J, Gesquiere L, Galbany J, Onyango PO, Alberts SC (2010) Life history context of reproductive aging in a wild primate model. Annals of the NY Academy of Sciences 1204: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strier KB, Ziegler TE, Wittwer DJ (1999) Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides). Hormones and Behavior 35: 125–134. [DOI] [PubMed] [Google Scholar]
- 34. Anderson HB, Emery Thompson M, Knott CD, Perkins L (2008) Fertility and mortality patterns of captive Bornean and Sumatran orangutans: is there a species difference in life history?. Journal of Human Evolution 54: 34–42. [DOI] [PubMed] [Google Scholar]
- 35. Wich SA, Utami-Atmoko SS, Mitra Setia T, Rijksen HD, Schurmann C, et al. (2004) Life history of wild Sumatran orangutans (Pongo abelii). Journal of Human Evolution 47: 385–398. [DOI] [PubMed] [Google Scholar]
- 36.Knott CD, Emery Thompson M, Wich SA (2009) The ecology of reproduction in wild orangutans. In: Wich SA, Utami SS, Mitra Setia T, van Schaik CP, editors. Orangutans: Ecology, Evolution, Behaviour, and Conservation. London: Oxford University Press. 171–188. [Google Scholar]
- 37. Delgado R, van Schaik CP (2000) The behavioural ecology and conservation of the orangutan (Pongo pygmaeus): a tale of two islands. Evolutionary Anthropology 9: 201–218. [Google Scholar]
- 38. Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL (2006) Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behavioral Ecology and Sociobiology 59: 469–479. [Google Scholar]
- 39. Setchell JM, Dixson AF (2002) Developmental variables and dominance rank in adolescent male mandrills (Mandrillus sphinx). American Journal of Primatology 56: 9–25. [DOI] [PubMed] [Google Scholar]
- 40. Wickings EJ, Dixson AF (1992) Testicular function, secondary sexual development, and social status in male mandrills (Mandrillus sphinx). Physiology & Behavior 52: 909–916. [DOI] [PubMed] [Google Scholar]
- 41.Knott CD (1999) Orangutan behavior and ecology. In: Dolhinow P, Fuentes A, editors. The Nonhuman Primates. Mountain View, CA: Mayfield Publishing. 50–57. [Google Scholar]
- 42. Singleton I, van Schaik CP (2001) Orangutan home range size and its determinants in a Sumatran swamp forest. International Journal of Primatology 22: 877–911. [Google Scholar]
- 43. Muehlenbein MP, Bribiescas RG (2005) Testosterone-mediated immune functions and male life histories. American Journal of Human Biology 17: 527–558. [DOI] [PubMed] [Google Scholar]
- 44. Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. The American Naturalist 139: 603–622. [Google Scholar]
- 45.Utami Atmoko SS (2000) Bimaturism in orang-utan males: reproductive and ecology strategies: Utrecht University, The Netherlands. [Google Scholar]
- 46. Schürmann CL, van Hooff JA (1986) Reproductive strategies of the orang-utan: new data and a reconsideration of existing sociosexual models. International Journal of Primatology 7: 265–287. [Google Scholar]