Abstract
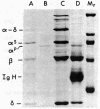
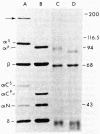
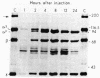
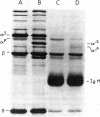
The alpha-chain of murine fourth component of complement (C4) secreted by cells in vitro and in vivo has a Mr that is larger by approximately equal to 4,000 than that of the alpha-chain of the principal form of C4 in plasma. By using in vivo labeling of C4 with [35S]methionine, C4 was shown to be first synthesized with the higher Mr ("secreted") alpha-chain, which was then quickly processed (t1/2 approximately equal to 1 hr) extracellularly to the mature ("plasma") C4 possessing the lower Mr alpha-chain. Both forms of C4 were functional as assayed by the ability of their alpha-chains to be cleaved by the protease C1, to bind methylamine, and to undergo denaturation-dependent autolysis. When secreted C4 and plasma C4 were activated to C4b, the Mr difference of 4,000 was maintained in the alpha'-chains. The Mr difference was localized to the carboxyl-terminal autolytic fragment of the alpha-chain and was unaffected by the removal of carbohydrate. C4 from resident peritoneal macrophage cultures could be converted to the plasma form by incubation with heparin/plasma. This conversion could be blocked by EDTA or 1,10-phenanthroline. These data suggest that an enzyme, presumably a neutral proteinase present in mouse plasma, cleaves the carboxyl terminus of newly synthesized C4 alpha-chains, thereby creating the major form of C4 in plasma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., McGinnis K., Brown L., Peterein J., Shreffler D. A murine C4 molecule with reduced hemolytic efficiency. J Exp Med. 1980 Feb 1;151(2):492–497. doi: 10.1084/jem.151.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. The many forms and functions of cellular proteinases. Fed Proc. 1980 Jan;39(1):9–14. [PubMed] [Google Scholar]
- Carroll M. C., Capra J. D. Studies on murine Ss protein: demonstration that S region encodes structural gene for fourth component of complement. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4641–4645. doi: 10.1073/pnas.76.9.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. G., Sim R. B. Intramolecular general acid catalysis in the binding reactions of alpha 2-macroglobulin and complement components C3 and C4. Biosci Rep. 1981 Jun;1(6):461–468. doi: 10.1007/BF01121579. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Nussenzweig V., Gigli I. Structural and functional differences between the H-2 controlled Ss and Slp proteins. J Exp Med. 1978 Nov 1;148(5):1186–1197. doi: 10.1084/jem.148.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G., Odink K., Chapuis R. M. Synthesis of the mouse complement component C4 (Ss-protein) by peritoneal macrophages: kinetics of secretion and glycosylation of the subunits. Eur J Immunol. 1980 Feb;10(2):75–82. doi: 10.1002/eji.1830100202. [DOI] [PubMed] [Google Scholar]
- Gorski J. P., Howard J. B. Effect of methylamine on the structure and function of the fourth component of human complement, C4. J Biol Chem. 1980 Nov 10;255(21):10025–10028. [PubMed] [Google Scholar]
- Graves C. B., Munns T. W., Carlisle T. L., Grant G. A., Strauss A. W. Induction of prothrombin synthesis by prothrombin fragments. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4772–4776. doi: 10.1073/pnas.78.8.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B. Methylamine reaction and denaturation-dependent fragmentation of complement component 3. Comparison with alpha2-macroglobulin. J Biol Chem. 1980 Aug 10;255(15):7082–7084. [PubMed] [Google Scholar]
- Howard J. B. Reactive site in human alpha 2-macroglobulin: circumstantial evidence for a thiolester. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2235–2239. doi: 10.1073/pnas.78.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B., Vermeulen M., Swenson R. P. The temperature-sensitive bond in human alpha 2-macroglobulin is the alkylamine-reactive site. J Biol Chem. 1980 May 10;255(9):3820–3823. [PubMed] [Google Scholar]
- Hugli T. E., Gerard C., Kawahara M., Scheetz M. E., 2nd, Barton R., Briggs S., Koppel G., Russell S. Isolation of three separate anaphylatoxins from complement-activated human serum. Mol Cell Biochem. 1981 Dec 4;41:59–66. doi: 10.1007/BF00225297. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Kells D. I. Conformational and functional changes in the fourth component of human complement produced by nucleophilic modification and by proteolysis with C1s-. Biochemistry. 1982 Mar 16;21(6):1109–1117. doi: 10.1021/bi00535a001. [DOI] [PubMed] [Google Scholar]
- Janatova J., Lorenz P. E., Schechter A. N., Prahl J. W., Tack B. F. Third component of human complement: appearance of a sulfhydryl group following chemical or enzymatic inactivation. Biochemistry. 1980 Sep 16;19(19):4471–4478. [PubMed] [Google Scholar]
- Janatova J., Tack B. F. Fourth component of human complement: studies of an amine-sensitive site comprised of a thiol component. Biochemistry. 1981 Apr 28;20(9):2394–2402. doi: 10.1021/bi00512a005. [DOI] [PubMed] [Google Scholar]
- Karp D. R., Atkinson J. P., Shreffler D. C. Genetic variation in glycosylation of the fourth component of murine complement. Association with hemolytic activity. J Biol Chem. 1982 Jul 10;257(13):7330–7335. [PubMed] [Google Scholar]
- Karp D. R., Capra J. D., Atkinson J. P., Shreffler D. C. Structural and functional characterization of an incompletely processed form of murine C4 and Slp. J Immunol. 1982 May;128(5):2336–2341. [PubMed] [Google Scholar]
- Karp D. R., Parker K. L., Shreffler D. C., Slaughter C., Capra J. D. Amino acid sequence homologies and glycosylation differences between the fourth component of murine complement and sex-limited protein. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6347–6349. doi: 10.1073/pnas.79.20.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Holcombe F. H., Levine R. P. Interaction between the labile binding sites of the fourth (C4) and fifth (C5) human complement proteins and erythrocyte cell membranes. J Immunol. 1980 Aug;125(2):634–639. [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Leung M. K., Fessler L. I., Greenberg D. B., Fessler J. H. Separate amino and carboxyl procollagen peptidases in chick embryo tendon. J Biol Chem. 1979 Jan 10;254(1):224–232. [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Moon K. E., Gorski J. P., Hugli T. E. Complete primary structure of human C4a anaphylatoxin. J Biol Chem. 1981 Aug 25;256(16):8685–8692. [PubMed] [Google Scholar]
- Newell S. L., Shreffler D. C., Atkinson J. P. Biosynthesis of C4 by mouse peritoneal macrophages. I. Characterization of an in vitro culture system and comparison of C4 synthesis by "low" vs "high" C4 strains. J Immunol. 1982 Aug;129(2):653–659. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. L., Atkinson J. P., Roos M. H., Shreffler D. C. Genetic and structural characterization of H-2-controlled allelic forms of murine C4. Immunogenetics. 1980 Jul;11(1):55–63. doi: 10.1007/BF01567769. [DOI] [PubMed] [Google Scholar]
- Parker K. L., Roos M. H., Shreffler D. C. Structural characterization of the murine fourth component of complement and sex-limited protein and their precursors: evidence for two loci in the S region of the H-2 complex. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5853–5857. doi: 10.1073/pnas.76.11.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton K. W., Deloria L. B., Mannering G. J. Effects of polyribonoinosinic acid polyribocytidylic acid and a mouse interferon preparation on cytochrome P-450-dependent monooxygenase systems in cultures of primary mouse hepatocytes. Mol Pharmacol. 1978 Jul;14(4):672–681. [PubMed] [Google Scholar]
- Roos M. H., Atkinson J. P., Shreffler D. C. Molecular characterization of the Ss and Slp (C4) proteins of the mouse H-2 complex: subunit composition, chain size polymorphism, and an intracellular (PRO-Ss) precursor. J Immunol. 1978 Sep;121(3):1106–1115. [PubMed] [Google Scholar]
- Saunders D., Edidin M. Sites of localization and synthesis of Ss protein in mice. J Immunol. 1974 Jun;112(6):2210–2218. [PubMed] [Google Scholar]
- Sim R. B., Sim E. Autolytic fragmentation of complement components C3 and C4 under denaturing conditions, a property shared with alpha 2-macroglobulin. Biochem J. 1981 Jan 1;193(1):129–141. doi: 10.1042/bj1930129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skok J., Solomon E., Reid K. B., Thompson R. A. Distinct genes for fibroblast and serum C1q. Nature. 1981 Aug 6;292(5823):549–551. doi: 10.1038/292549a0. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zabern I., Bloom E. L., Chu V., Gigli I. The fourth component of human complement treated with amines or chaotropes or frozen-thawed (C4b-like C4): interaction with C4 binding protein and cleavage by C3b/C4b inactivator. J Immunol. 1982 Mar;128(3):1433–1438. [PubMed] [Google Scholar]
- von Zabern I., Gigli I. Conformational changes in complement component C4 induced by activation, treatment with amines, chaotropes, or freezing-thawing, detectable by radioiodination using lactoperoxidase. J Immunol. 1982 Mar;128(3):1439–1442. [PubMed] [Google Scholar]