Abstract
The content of flavonoids especially baicalin and baicalein determined the medical quality of Scutellaria baicalensis which is a Chinese traditional medicinal plant. Here, we investigated the mechanism responsible for the content and composition of flavonoids in S. baicalensis under water deficit condition. The transcription levels of several genes which are involved in flavonoid biosynthesis were stimulated by water deficit. Under water deficit condition, fifteen up-regulated proteins, three down-regulated proteins and other six proteins were detected by proteomic analysis. The identified proteins include three gibberellin (GA)- or indoleacetic acid (IAA)-related proteins. Decreased endogenous GAs level and increased IAA level were observed in leaves of S. baicalensis which was treated with water deficit. Exogenous application of GA or α-naphthalene acelic acid (NAA) to plants grown under water deficit conditions led to the increase of endogenous GAs and the decrease of IAA and flavonoids, respectively. When the synthesis pathway of GA or IAA in plants was inhibited by application with the inhibitors, flavonoid levels were recovered. These results indicate that water deficit affected flavonoid accumulation might through regulating hormone metabolism in S. baicalensis Georgi.
Introduction
Flavonoids are important plant secondary metabolites which have important effect on plant physiology [1]. Plant flavonoids exhibit several medicinal properties, such as antioxidant activity and anti-inflammatory activity [2], and these flavonoids largely determine the quality of medicinal plants. For example, flavonoids are regarded as one of the most important determinants of quality in red grapes and wine [3]. Various biotic and abiotic stress conditions also affected the accumulation of flavonoids in plant vegetative tissues and organs [4].
The roots of Scutellaria baicalensis Georgi are used to treat various diseases in Chinese traditional medicine. The active compounds of S. baicalensis include baicalin, baicalein, wogonoside, wogonin, neobaicalein, visidulin I, and oroxylin A, and these compounds exhibit anti-inflammatory, anti-tumor, and anti-HIV activities [5]. These flavonoids, especially baicalin and baicalein, are regarded as the most important determinants of the quality of S. baicalensis [6]. Baicalin is synthesized via the phenylpropanoid pathway by the activities of several enzymes, including phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase, 4-coumarate:CoA ligase, chalcone synthase (CHS), and chalcone isomerase [7]. β-glucuronidase (GUS) catalyze baicalin to baicalein which then can be catalyzes to 6,7-dehydrobaicalein by peroxidase (POD), detoxifying H2O2 to water [8], [9]. Baicalein can be catalyzed back to baicalin by UDP-glucuronate: baicalein 7-O-glucuronosyltransferase (UBGAT) [10].
Water deficit affects flavonoid biosynthesis in plants. Vincent et al. [11] found the lower lignin levels in leaves of plants treated with water deficit, compared with those well-watered plants. It has also been found that drought treatment strongly upregulated the anthocyanin biosynthesis in ripening fruit [12]. Regulated deficit irrigation can be used to improve berry and wine quality, because water deficit early in the season can lead to more anthocyanins and phenolics [13], [14]. Water deficit can also enhance the flavonoid production in cell suspension culture of Glycyrrhiza inflata Batal [15]. Guidi et al. [16] reported that antioxidant phenylpropanoid concentrations increased in response to water stress in shade leaves. In a previous study, we found that light conditions could affect the expression of GUS and UBGAT, and the baicalein∶baicalin ratio by influencing the flavonoid metabolism and the conver between individual flavonoids [17].
Flavonoid biosynthesis is also affected by plant hormones which regulating the plant growth and development. Anthocyanin accumulation could be enhanced by abscisic acid (ABA) treatment and suppressed by application of synthetic auxins or 1-naphthaleneacetic acid (NAA) [18]–[20]. Flavonoid compounds have been shown to modulate the transport of phytohormone auxin [21], [22]. Besseau et al. [23] reported that flavonoid accumulation affects auxin transport and plant growth in Arabidopsis.
Our previous work revealed that low levels of rainfall are closely associated with baicalein content in 19 production areas [24], indicating that water status is an important factor affecting the flavonoid mechanisms that in turn determine the quality of S. baicalensis. Here, we investigated the mechanism responsible for the content and composition of flavonoids in S. baicalensis grown under water deficit conditions.
Results
Water deficit affected the flavonoid accumulation
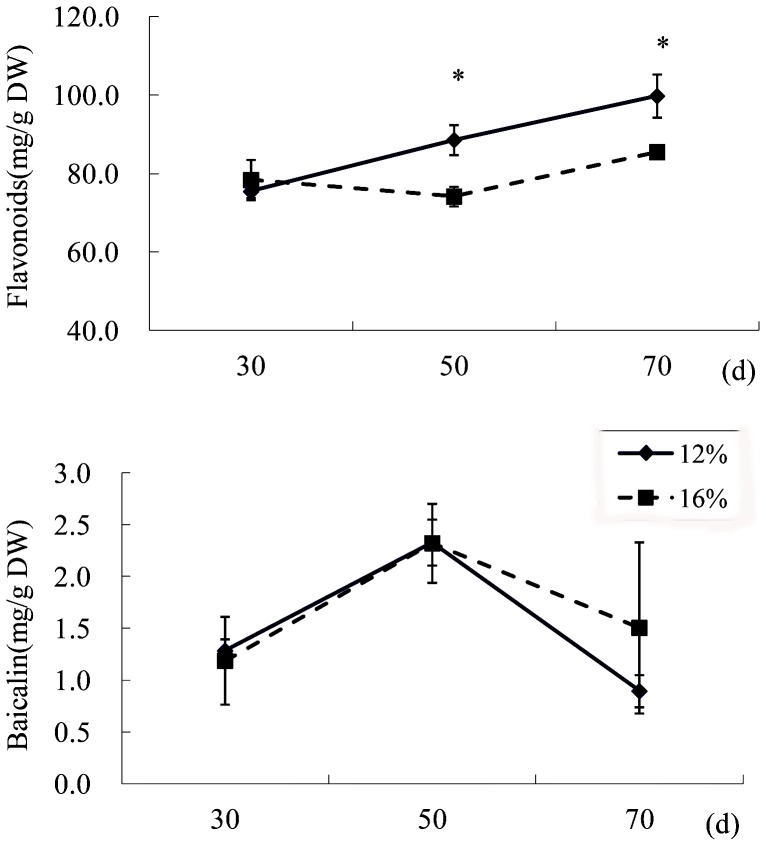
S. baicalensis plants grow in the north of China where 15–20% of soil water content (SWC) is usually suitable for crop growth, whereas 12–15% SWC and 8–12% SWC is considered as mild and moderate drought stress, respectively (http://www.natesc.gov.cn). In this study, three-month-old plants which have grown under well-water condition were then kept SWC as 12% SWC (water deficit) or 16% SWC (control) condition. Water deficit significantly increased the total flavonoid contents both in roots [25] and in leaves at 50 d and 70 d (Figure 1), whereas the content of baicalin did not change much in leaves (Figure 1). HPLC analysis revealed that the major active compounds were baicalin and baicalein, and that these compounds mainly accumulated in roots (Data not shown).
Figure 1. Effects of water deficit on flavonoid accumulation in S. baicalensis.
Total flavonoids and baicalin in the leaves of S. baicalensis grown under 16% SWC as a control (broken line) and 12% SWC as a water deficit treatment (solid line). Vertical bars indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
Water deficit increased the expression of several flavonoid biosynthesis genes
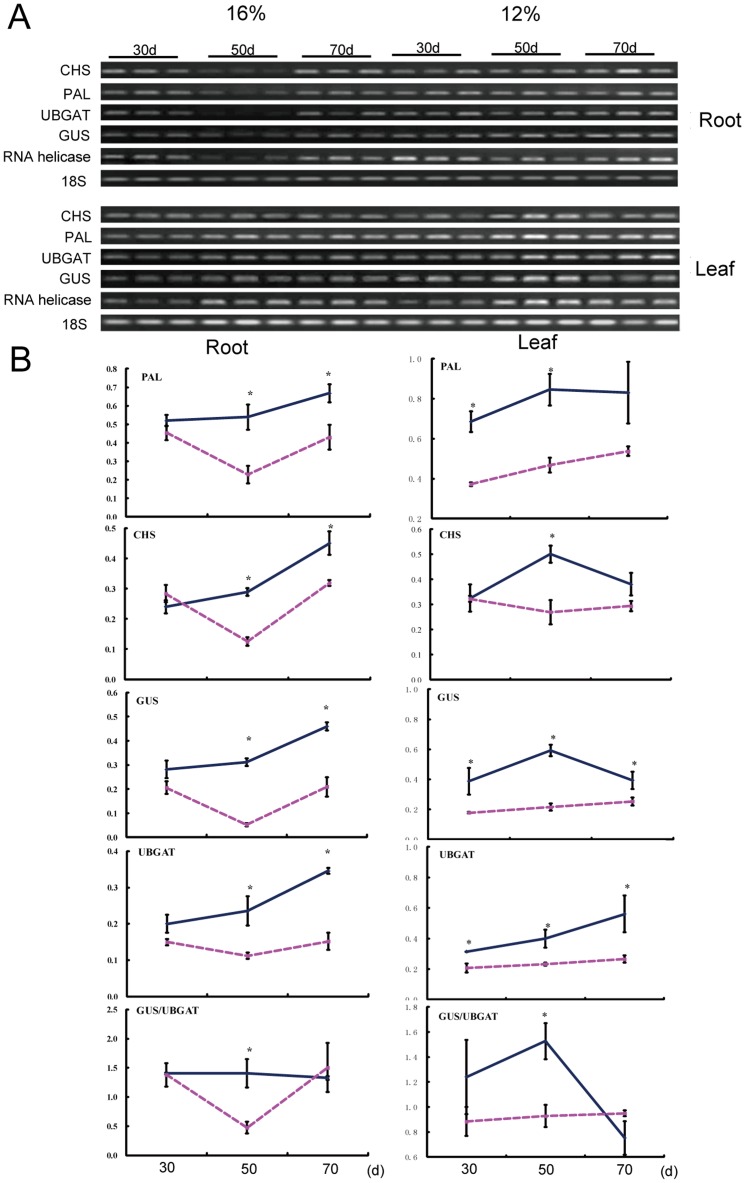
We further to investigate whether water deficit affected the expression of the genes involved in flavonoid biosynthesis. Baicalin and baicalein are synthesized via the phenylpropanoid pathway by the activities of several enzymes, including PAL, cinnamate 4-hydroxylase, 4-coumarate:CoA ligase, CHS and chalcone isomerase. By blasting in GenBank with the known gene sequence of Arabidopsis, we found the S. baicalensis EST sequences encoding for PAL (EF501766), CHS (AB008748), UBGAT (EF512580) and GUS (AB040072). The specific primers for these genes were designed and semiquantitative RT-PCR was performed (The primers were shown in Table S1). The results showed that water deficit increased the expression of PAL, CHS, GUS and UBGAT both in leaves and roots (Figure 2), and the expression pattern were similar in leaves and roots. Transcript level of GUS was increased to a greater extent than those of UBGAT. In further investigations, we were more interested in what happened in S. baicalensis root, because root is used in Chinese medicine and contains the highest concentrations of flavonoids than other organs.
Figure 2. Effects of water deficit on the expression of flavonoid biosynthesis genes in S. baicalensis.
A, RT-PCR analysis of the transcript levels of interested genes; B, Quantification of the RT-PCR results. The broken line is for the 16% SWC control and the solid line is for the 12% SWC water stress treatment. Vertical bars indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
Proteome changes in S. baicalensis roots under water deficit
To further elucidate the mechanisms that stimulated the accumulation of flavoinds in the roots of S. baicalensis under water deficit, the proteome change was investigated using two-dimensional gel electrophoresis (2-DE) method. For high reproducibility and low background on gels, a silver staining approach was used to detect protein spots on 2-DE gels, and the 2-D protein patterns was shown in Figure S1. These 2-DE gels averaged around 1300 spots/gel and more than 600 spots overlapped on these gels. The protein profile was highly reproducible among three replicate samples.
The protein spots showing a significant change in volume were selected; and a total of 29 spots showed altered expression patterns following water deficit. These 29 spots were excised and identified by mass spectrometry (MS) analysis. Only 24 spots could be successfully identified as unique hits in searches of the NCBInr database generated by the BGI and S. baicalensis cDNA library using the MASCOT program. Protein identifications were considered significantly when at least three peaks matched the protein. The MS spectrum and the matched peptide fragments of three identified proteins were shown in Figure S2. Among the 24 proteins, 15 proteins such as a putative R2R3-Myb transcription factor, a putative electron transporter, an adenosylhomocyteinase, and a chloroplast heat shock protein 70B were up-regulated, and three proteins were down-regulated by water deficit. The expression patterns of other six proteins varied with sampling time (Table 1; Table S2). The proteins identified from the three sets of 2-DE gels were grouped according to their functions as documented in the EBI (http://www.ebi.ac.uk/InterPro) and NCBI databases. Most of the proteins with differential expression belonged to a group of metabolism-related proteins according to the KO pathway (Figure S3).
Table 1. Differentially expressed proteins which have S. baicalensis cDNA sequence.
| Spot no. | NCBI accession No. | Protein name | Average -fold changea | Score | Mb | Cc (%) | NCBI accession Nod. | ||
| 30 d | 50 d | 70 d | |||||||
| Up-regulated protein Spots | |||||||||
| 001 | AAO42850 | 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase superfamily protein | 1.088 | 3.830 | nse | 68 | 5 | 23 | JX068528 |
| 004 | EEF28373 | R2r3-myb transcription factor, putative | ns | 1.789 | 1.879 | 71 | 7 | 26 | JX068530 |
| 005 | EEF33225 | Electron transporter, putative | 2.954 | 5.156 | ns | 77 | 8 | 13 | JX068531 |
| 009 | XP_002313027 | BEL1-related homeotic protein, putative | ns | 1.192 | 1.731 | 78 | 9 | 21 | JX068534 |
| 014 | NP_193130 | Adenosylhomocysteinase/copper ion binding | ns | 1.969 | ns | 88 | 10 | 23 | JX068535 |
| 015 | ACJ24804 | Chloroplast heat shock protein 70B | ns | 1.258 | ns | 75 | 7 | 73 | JX068536 |
| 020 | AAU84988 | Anthranilate synthase alpha 1 | ns | 1.361 | ns | 78 | 9 | 20 | JX068542 |
| 021 | AAT40104 | ADH-like UDP-glucose dehydrogenase | ns | 1.306 | ns | 73 | 9 | 20 | JX068532 |
| 023 | BAC98579 | ATP-dependent RNA-helicase | 1.424 | 1.326 | 1.891 | 79 | 15 | 19 | ADD65372 |
| 024 | ACO62538 | Mitochondrial carrier family | ns | 2.058 | ns | 73 | 11 | 33 | JX068538 |
| Down-regulated protein Spots | |||||||||
| 022 | EEF43612 | d-3-phosphoglycerate dehydrogenase | ns | 0.759 | ns | 72 | 11 | 17 | JX068537 |
| 026 | AAF79147 | Alpha-tubulin | ns | 0.441 | ns | 100 | 8 | 35 | JX068539 |
| Others | |||||||||
| 008 | XP_002318651 | pantothenate kinase, putative | 0.456 | 1.642 | ns | 74 | 12 | 12 | JX068533 |
| 027 | NP_001142255 | Glycosyltransferase, CAZy family GT8 | ns | 0.294 | 3.658 | 72 | 7 | 31 | JX068540 |
| 028 | EEF39512 | Chitin-inducible gibberellin-responsive protein, putative | 4.840 | 0.000 | ns | 44 | 4 | 10 | JX068541 |
Spot abundance is expressed as the ratio of intensities of up-regulated or down-regulated proteins between stress and control. -Fold changes had p values<0.05. 30 d, 50 d, and 70 d represent water stress treatment for 30,50,70 d, respectively.
Number of mass values matched.
Sequence coverage.
identified proteins in protein database of Scutellaria baicalensis in our group.
ns indicates no significant change of spot abundance between stress and control.
Our proteome analysis results clearly showed that water deficit affected the expression level of several proteins related to GA or auxin metabolism. The expression of a GA-responsive protein (EEF39512) increased after 30 d and decreased after 50 d. Water deficit increased the expression level of an R2R3-Myb transcription factor (EEF28373), and an anthranilate synthase (AAU84988), which is related to indoleacetic acid (IAA) synthesis (Table 1).
Water deficit affected flavonoid accumulation by regulating GAs and IAA metabolism
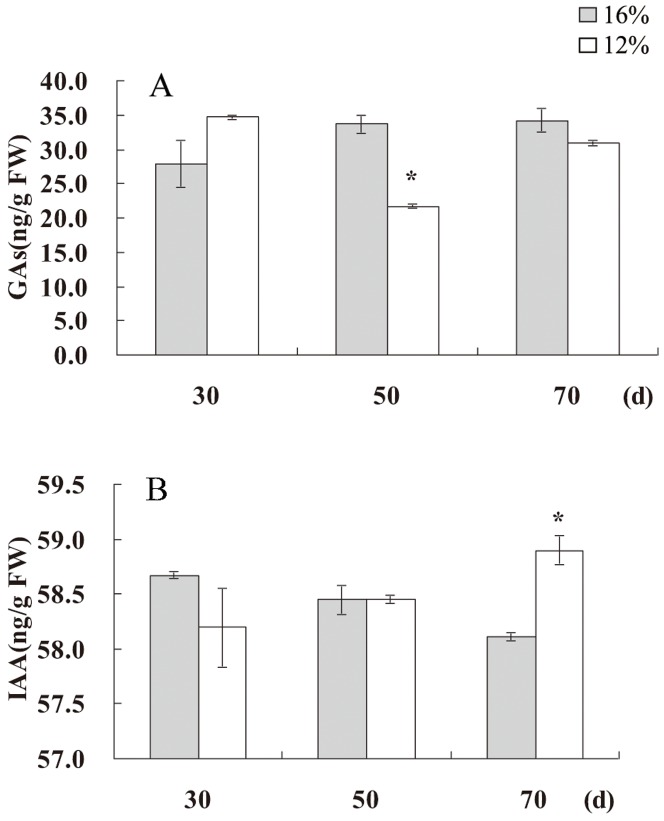
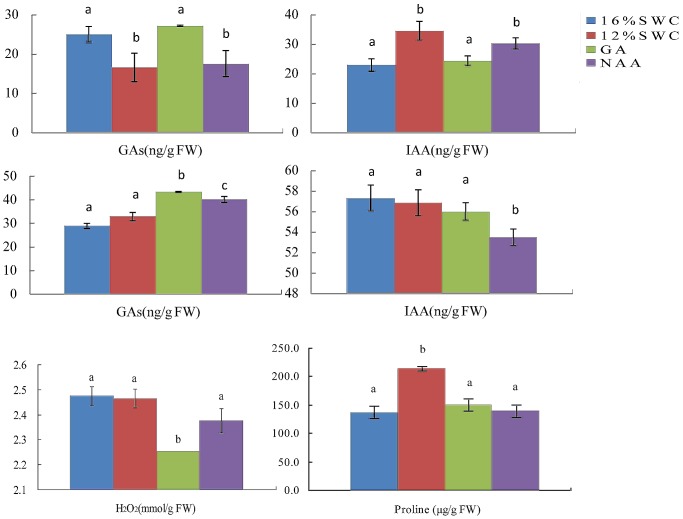
The influence of water deficit on GA- and IAA-related proteins and flavonoid accumulation indicated a possible linkage among water deficit, flavonoid accumulation, and hormone metabolism. To investigate this possibility, we measured endogenous GAs and IAA levels in S. baicalensis. Under water deficit conditions, decreased endogenous GAs levels and increased IAA levels were observed in leaves of S. baicalensis (Figure 3) and in roots [25], indicating that water deficit affected both GA and IAA metabolism. When exogenous GA3 was sprayed on S. baicalensis plants grown under water deficit conditions, endogenous GAs levels were increased both in roots and leaves, whereas endogenous IAA levels were decreased in roots (Figure 4). Exogenous GA3 also caused a decrease of H2O2 level (Figure 4). When plants were treated with exogenous auxin NAA, the increased GAs levels and decreased IAA levels in leaves were observed, however GA and IAA levels in roots were not affected. These results indicate that GAs and IAA could affect each other (Figure 4).
Figure 3. Effects of water deficit on endogenous GAs and IAA.
The GA and IAA content of leaves of S. baicalensis grown under 16% SWC as a control and 12% SWC as a water deficit treatment. Vertical lines indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
Figure 4.
Effects of plant growth regulators on levels of endogenous GAs and IAA in roots (A, B) and leaves (C, D), and on the content of H2O2 (E) and proline (F) in roots of S. baicalensis. Vertical lines indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
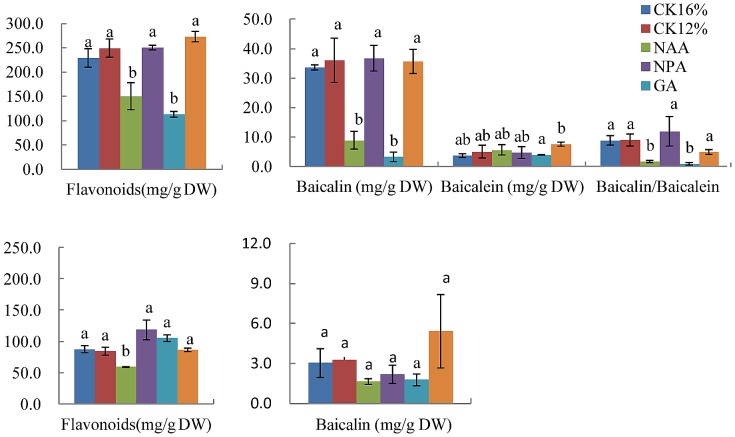
To further analyze whether GAs or IAA influence flavonoid metabolism in S. baicalensis plants, we examined the flavonoid contents of S. baicalensis plants sprayed with exogenous GA3, NAA, and their synthesis inhibitors, paclobutrazol and 1-N-naphthylphthalamic acid (NPA), respectively. The content of total flavonoids and baicalin and the ratio of baicalin and baicalein decreased in roots under water stress after application of GA3, and these decreases were reversed by application of paclobutrazol (Figure 5). Application of NAA and its synthesis inhibitor 1-N-naphthylphthalamic acid led to results similar to GA3 treatment. However application of GA3 and its synthesis inhibitor paclobutrazol did not affect total flavonoid content or the baicalin content in leaves under water stress, and NAA treatment decreased flavonoid content (Figure 5). Exogenous IAA treatment led to the similar results as NAA treatment (Figure S4)..When the plants at 16% SWC were sprayed with GA3 or NAA, the contents of total flavonoids and baicalin were decreased and baicalein were increased in roots, and these decreases were reversed by application of paclobutrazol and NPA (Figure S5). However, baicalein content was increased by treatment with NAA or GA3.
Figure 5. Effects of plant growth regulators on flavonoid levels in S. baicalensis.
Total flavonoids, baicalin, baicalein, and the ratio of baicalin to baicalein in roots (A, B) and leaves (C, D) of S. baicalensis. Vertical bars indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
Discussion
The composition of active compounds in Chinese medicinal plants determines the quality of these herbal medicines [26]. For S. baicalensis, the flavonoid content and the ratio of baicalin to baicalein are the determinants. The metabolism of flavonoids follows a complex pathway, and some environmental factors such as temperature, water status, light condition, and nitrogen affect the accumulation of flavonoids [27]–[30]. In a previous study, we showed that low rainfall levels are closely associated with the content of flavonoids, especially of baicalein, in S. baicalensis plants from 19 production areas [24], indicating that water status is an important factor affecting flavonoid accumulation in S. baicalensis.
Water deficit can initiate a complex of responses in plants at the cellular, physiological, and developmental levels [31]. Water deficit also can enhance flavonoid production in ripening fruit [3] and berries [13]–[14], and in cell suspension cultures of Glycyrrhiza inflata Batal [15] and Ligustrum vulgare [16]. Our previous study showed that total flavonoid accumulation was accelerated after 50 d of water deficit treatment [25]. Expression levels of PAL and CHS, two key genes in the biosynthesis of flavonoids, were also elevated by water deficit. These results are consistent with those of Yamaguchi et al. [32], who found that water stress significantly increased the total isoflavonoid content in portions of soybean primary root. However, Vincent et al. [11] found that lignin levels were lower in leaves of plants subjected to water deficit than in those of well-watered plants. We did not find any change in baicalin accumulation with water deficit in leaves of S. baicalensis.
The mechanism of water deficit tolerance in S. baicalensis and its relation to changes of flavonoids was further examined. The 2-DE analysis combined with MS is a powerful approach to identify the proteins involved in plant drought tolerance. The proteins involved in ROS metabolism, isoflavonoid biosynthesis, control of apoptosis-like cell death, and control of protein degradation in soybean root were affected by water stress [32]. In Arabidopsis, 30% of the transcriptome responds stress and 1008 mRNAs were specifically upregulated by water deficit [33]. In our experiment, water deficit affected four functional categories in S. baicalensis including hormone metabolism, carbohydrate metabolism, proteins responsive to stress, and transcript factors.
In our experiment, a total of 29 spots showed altered expression patterns following water deficit treatments, and 24 spots were identified. In this study, we run the MS analysis for the protein identification. MS data may have false discovery compared to MS/MS data, so we identified the protein with at least three match peaks. Among the 24 proteins, 15 proteins were upregulated and three proteins were down-regulated by water deficit. The other six proteins exhibited diverse expression patterns depending on sampling time. In the 24 identified proteins, the expressed level of ATP-dependent RNA-helicase (BAC98579) increased at all times under water deficit conditions compared to the control (Figure S6). The results of semiquantitative RT-PCR analysis showed that transcript pattern was similar to protein expression (Figure S6), confirming the reliability of the 2-DE results. Wehmeyer et al. [34] suggested that heat shock proteins are involved in desiccation tolerance, and Hajheidari et al. [35] reported that two sHSPs were up-regulated under drought stress in sugar beets grown in the field. In this experiment, a chloroplast heat shock protein 70B (ACJ24804) was somewhat up-regulated. In other reports on proteomics analysis of drought stress, many more significantly changed proteins were identified. For example, 58 proteins were identified in Elymus elongatum [36] and 49 changed proteins were found in peanut [37]. Fewer significantly changed proteins were identified in our experiment compared with these reports, might due to the fact that the S. baicalensis plants adapted to the water deficit treatment (12% SWC) in our experiment, resulting in fewer proteins being affected. In other previous works, the water deficit condition similar to that used in this study led to the increase of detoxifying proteins such as superoxide dismutase and dehydroascorbate reductase in Elymus elongatum [36], and proteins relating to biosynthesis of jasmonic acid and ABA signaling [37].
In our proteome analysis, the expression levels of several proteins related to GA or auxin metabolism were affected. The expression of a GA-responsive protein (EEF39512) increased after 30 d, but then decreased after 50 d. Water deficit increased the expression level of an R2R3-Myb transcription factor (EEF28373) and an anthranilate synthase (AAU84988), which is related to IAA synthesis (Table 1). The MYB transcription factors play important roles in the regulation of many secondary metabolites at the transcriptional level, and Arabidopsis R2R3-Myb transcription factors have been reported to play roles in flavonoid biosynthesis in lettuce [38]. Devaiah et al. [39] reported on the role of MYB62 in the regulation of phosphate starvation responses via changes in GA metabolism and signaling. We also detected an increased expression level of a putative R2R3-Myb transcription factor in S. baicalensis roots that has high identity with AtMYB113, which is involved in the regulation of anthocyanin biosynthesis, indicating that the R2R3-Myb transcription factor is also involved in flavonoid biosynthesis in S. baicalensis.
The effect of water deficit on GA- and IAA-related proteins and flavonoid accumulation prompted us to consider the possible linkage among water deficit, flavonoid accumulation and hormone metabolism. Plant hormones affect the accumulation of secondary metabolites. Decrease of the CsPAL expression level and catechin content in response to ABA and GA3 treatment has been observed [40]. GA3 may inhibit the phenylpropanoid pathway at the level of PAL in Myrica rubra [41], pea [42] and carrot [43]. Exogenous ABA application can also increase the expression level of anthocyanin synthesis-related genes and the concentration of anthocyanins in grape berries [20]. Meanwhile, plant flavonoids inhibit auxin transport primarily at the shoot apex and root tip, and flavonoid changes are subsequent to auxin accumulation in some auxin-accumulating tissues [44]. In S. baicalensis, the total flavonoid content increased in leaves and did not change significantly in roots [25]. Water deficit decreased the level of endogenous GAs and increased IAA levels in leaves and roots of S. baicalensis. Exogenous GA3 increased endogenous GAs levels in both roots and leaves of S. baicalensis grown under water deficit conditions, and decreased endogenous IAA levels in roots. Exogenous NAA increased GA levels and decreased IAA levels in leaves, but their levels in roots were not affected. These results indicate that GAs and IAA may affect each other. Ribnicky et al. [45] reported that NAA treatment for 4 weeks led to a 50% decrease in the concentration of total IAA in carrot. The reason that exogenous NAA affect endogenous IAA might be due to the decrease of the biosynthesis of endogenous IAA. The levels of total flavonoids and baicalin and the ratio of baicalin to baicalein in roots were decreased under water deficit condition after application of GAs, and these decreases were recovered after application of paclobutrazol. Application of NAA and its synthesis inhibitor NPA produced similar results as GAs and paclobutrazol treatment. However, application of GAs and its synthesis inhibitor paclobutrazol did not affect the levels of total flavonoids and baicalin in leaves under water stress, and IAA treatment decreased flavonoid content. These results clearly show that plant hormones, particularly GAs and IAA, affected flavonoid metabolism, and that water deficit affected flavonoid accumulation by regulating hormone metabolism in S. baicalensis.
Materials and Methods
Plant material and experimental conditions
The seeds of S. baicalensis were obtained from Institute of Chinese Materia Medica, Academy of Chinese Medical Sciences, Beijing, China), sterilized in 0.5% NaOCl for 5 min, then washed 3 times with sterile water, and placed in petri dishes to germinate. The seedlings five days after germination were transferred to individual pots (10 seedlings per pot) containing 500 g dried soil in climate chamber at 25°C with 16 h-light photoperiod under well-water condition. Three months later, pots were weighted at 9 am each day, and the soil water content at saturation was determined experimentally adding a known volume of water to the pots. Plants were irrigated with calculated volume of distilled water to maintain the soil water content at 16% or 12%. The roots and leaves were sampled three times at 30, 50, 70 d of water stress.
GA3, NAA and their inhibitor P and NPA (100 mg/L) were spayed every five days for one month on leaves of 3-month-old plants grown at 12% SWC. The roots and leaves were sampled three times. The sample were rinsed three times in distilled water, and then stored at −80°C for further experiments.
Two-Dimensional Gel Electrophoresis (2-DE)
The S. baicalensis root samples from plants exposed to water-stress for 30, 50, and 70 d and control roots were homogenized to powder in liquid nitrogen. Proteins were extracted with phenol according to Wang et al. [46]. Total protein content was estimated according to the method described by Bradford [47].
The proteins (0.2 mg/gel) were mixed with rehydration buffer containing 8 M urea, 2% (w/v) CHAPS, 20 mM DTT, 0.5% (v/v) IPG buffer (pH 4–7), and 0.002% bromophenol blue and rehydrated overnight with IPG strips (18 cm with a linear gradient of pH 4–7). Isoelectric focusing was carried out for 50 kVh using IPGphor (Amersham Biosciences) at 20°C. Subsequently, the IPG strips were treated with reduction and alkylation in SDS-PAGE running buffer at equilibrium. These strips were then loaded and run on 12% acrylamide Laemmli gels (26×20 cm) using the Ettan DALT II system (Amersham Biosciences) with a programmable power control for 0.5 h at 2.5 W per gel, and then at 15 W per gel until the dye front reached the gel bottom. The separated proteins were visualized using silver staining. The 2-DE gels were made in triplicate from six independent protein extractions.
Image analysis
Images of the stained gels were acquired with an Image Scanner (Amersham Biosciences) in a transmission mode. The gel images were subsequently analyzed by ImageMaster 2D Platinum (Amersham Biosciences). To produce comparable data for quantitative analysis, several key parameters in the image analysis were fixed as the constants, such as smooth at 2, saliency at 300, and Min Area at 100. Based on the analyzed results from the software, the gel images were further analyzed manually, with particular attention paid to checking the differential spots on the gels.
Tryptic digestion
The gel spots were excised, successively destained, and dehydrated with 50% acetonitrile (ACN) in 25 mM ammonium bicarbonate. The proteins were then reduced with 10 mM DTT in 25 mM ammonium bicarbonate at 56°C for 1 h and alkylated by 55 mM iodoacetamide in 25 mM ammonium bicarbonate in the dark at room temperature for 45 min. Finally, the gel pieces were thoroughly washed with 25 mM ammonium bicarbonate in water/ACN (50/50) solution and completely dried in a SpeedVac Concentrator. Proteins were incubated in 2.5 mL of modified trypsin solution (10 ng/mL in 25 mM ammonium bicarbonate) for 30 min on ice, and 10 mL 25 mM ammonium bicarbonate were added for digestion overnight at 37°C. The digestion reaction was stopped using 1 mL 10% TFA.
Peptide identification and classification
The digested peptide (10–50 fmoles) was loaded onto a target well of an AnchorChip plate. After drying, 0.1 mL CHCA (4 mg/mL in 70% CH3CN, 0.1% TFA) matrix solution was added to the target well followed by air drying. Subsequently, the sample target was washed twice using 0.1% TFA for desalting. The AnchorChip plate with protein sample was injected into the Bruker AutoFlex MALDI-TOF MS. The mass spectrometer was operated under 19 kV accelerating voltage in reflection mode with an m/z range of 600–4000. The monoisotopic peptide masses obtained from MALDI-TOF MS were analyzed by m/z software. Mass spectra were internally calibrated with peptides arising from trypsin autoproteolysis at m/z = 842.509 and m/z = 2211.105 to reach a typical mass measurement accuracy of 100 ppm.
Monoisotopic peptide masses obtained from MALDI-TOF MS were used to search the NCBInr database generated by the BGI using the MASCOT program (Matrix Science, London, UK), and also search in our S. baicalensis cDNA library with 6941 full-length cDNAs, some of which have been submitted to GenBank. The proteins identified from the three sets of 2-DE gels were categorized according to their functions as documented in the EBI (http://www.ebi.ac.uk/InterPro) and NCBI databases. The output from InterProScan and NCBI was analyzed to obtain GO and KO categories of each sequence.
ROS and Proline measurement
The H2O2 concentration was measured by monitoring the absorbance of titaniumperoxide complex at 415 nm, following the method of Patterson et al. [48]. Proline was extracted and its concentration determined following the method of Bates et al. [49]. Roots were homogenized with 3% sulfosalicylic acid and centrifuged. The supernatant was treated with acetic acid and acid-ninhydrin, boiled for 1 h, and extracted with toluene. Then, its OD at 520 nm was read. Proline content was expressed as µg/g FW.
HPLC analysis of flavonoid
To determine flavonoid content, 100 mg powdered material was extracted for 1 h in 1 mL ethyl alcohol. The solution was filtered through a membrane filter (0.2 µm), and flavonoid concentrations were determined using an HPLC system with a 1.0 mL/min flow rate. HPLC was performed on a diamonsil C18 column (4.6 mm×250 mm, 5 µm). The detection wavelength was set at 280 nm and the column temperature was maintained at 30°C. The mobile phase consisted of acetonitrile-deionized water-methanoic acid (A; 21∶78∶1, v/v) and acetonitrile-deionized water-methanoic acid (B; 80∶20∶1, v/v). The initial condition was A–B (100∶0, v/v) for 15 min, and this was linearly changed to A–B (87∶13, v/v) at 25 min, to A–B (52∶48, v/v) at 40 min, and to A–B (0∶100, v/v) at 60 min. HPLC grade acetonitrile (E. Merck, Darmstadt, Germany) was used for the HPLC analysis. Peaks were identified using retention time standards from the National Institute for the Control of Pharmaceutical and Biological Products (China). The standard solutions contained 0.208 mg/mL baicalin and 0.602 mg/mL baicalein. The injection volume of the sample solution was 20 µl, and the experiment was repeated six times. The amount of baicalin and baicalein was calculated using the method of Li et al. [50].
Total flavonoid assay
Total flavonoid content was quantitatively analyzed in samples using the aluminum chlorimetric assay [51], calculated using a standard solution of baicalin. The test was repeated six times.
ELISA assay for IAA, and GAs
Extraction, purification, and determination of IAA and GAs in leaves by indirect ELISA techniques were performed using the methods of He [52], Yang et al. [53], and Teng et al. [54]. Samples were homogenized in liquid nitrogen and extracted overnight at 4°C in cold 80% (v/v) methanol containing 1 mM butylated hydroxytoluene. The extracts were collected after centrifugation at 10,000 g at 4°C for 20 min, then passed through a C18 Sep-Pak cartridge (Waters) and dried in N2. The residues were dissolved in PBS (0.01 M, pH 7.4) in order to determine the levels of IAA and GAs. Microtitration plates were coated with IAA- or GAs-ovalbumin conjugates in NaHCO3 buffer (50 mM, pH 9.6) and left overnight at 37°C. Ovalbumin solution (10 mg/mL) was added to each well to block nonspecific binding. After incubation for 30 min at 37°C, standard IAA and GA samples and specific monoclonal antibodies (provided by Dr. Baomin Wang, Chinese Agricultural University) were added and incubated for an additional 45 min at 37°C. Horseradish POD-labeled goat anti-rabbit immunoglobulin was then added to each well and incubated for 1 h at 37°C. Finally, the buffered enzyme substrate (o-phenylenediamine) was added, and the enzyme reaction was carried out in the dark at 37°C for 15 min and then terminated with 3 M H2SO4. The absorbance was recorded at 490 nm. Calculations of the enzyme-immunoassay data were performed as described by Weiler et al. [55]. The percentage recovery of each hormone was calculated by adding known amounts of standard hormone to a split extract. Percentage recoveries were all above 90%, and all sample extract dilution curves paralleled the standard curves, indicating the absence of nonspecific inhibitors in the extracts.
RNA extraction and semiquantitative RT-PCR analysis
For analysis of gene expression by semiquantitative RT-PCR, total RNA was isolated from water-stress roots and control roots using Trizol reagent (Invitrogen). Semiquantitative RT-PCR was carried out for PAL (EF501766), CHS (AB008748), UBGAT(EF512580), GUS(AB040072), RNA helicase (GU561857) and 18S(FJ527609) using the One-Step RT-PCR kit (TakaRa) with primers (Table S1). The 18S gene was chosen as a loading control. The one-step RT-PCR was done as follows: 94°C for 3 min, 31 cycles of 94°C for 30 s, annealing temperature for 40 s, and 72°C for 40 s, and 72°C for 10 min.
Supporting Information
Primers used in this paper.
(DOCX)
Differentially expressed proteins identified by MALDI-TOF MS.
(DOCX)
Differentially expressed proteins in S. baicalensis roots exposed to water deficit. Separated proteins from 16% SWC treated roots at 30 d (A), 50 d (C), and 70 d (E) are compared with protein profiles resulting from 12% SWC treated roots at 30 d (B), 50 d (D), and 70 d (F). Marked proteins (T) are named in accordance with Table 1 and Table S2 and were identified by MALDI-TOF MS. The numbers at the top of the gel T denote the pH gradient in the first dimension, while the molecular masses of the 2-D standards are displayed on the right.
(TIF)
The MS spectrum and the matched peptide fragments of protein spot number 4(A), 20 (B) and 28 (C).
(TIF)
Functional classification and distribution of identified proteins. Unknown proteins include those whose functions have not been described.
(TIF)
Effects of plant growth regulators on flavonoid levels in S. baicalensis after spay IAA and NAA. Baicalin (A), baicalein (B) and total flavonoids (C) in roots of S. baicalensis. Vertical bars indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
(TIF)
Effects of plant growth regulators on flavonoid levels in S. baicalensis under SWC16%. Total flavonoids, baicalin, and baicalein in roots of S. baicalensis. Vertical bars indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
(TIF)
Effects of water deficit on the expression of RNA helicase in S. baicalensis . Protein expression (A) and transcript abundance (B) of RNA helicase in roots of S. baicalensis grown under 16% SWC as a control (shaded bars) and 12% SWC as a water deficit treatment (white bars). Vertical lines indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
(TIF)
Acknowledgments
We thank the editor and two anonymous reviewers for the constructive suggestion for the manuscript improvement.
Funding Statement
This work was supported by the Scientific and technological research of Chinese medicine Program (06-07ZP51), Beijing nova program (2008B82) and the Self-selection program of Scientific and technological research of Chinese medicine Program (06-07ZP51) (ZZ2008062). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pourcel L, Routaboul JM, Cheynier V (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12: 29–36. [DOI] [PubMed] [Google Scholar]
- 2. Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochem 55: 481–504. [DOI] [PubMed] [Google Scholar]
- 3. Castellarin SD, Pfeifeer A, Sivilotti P, Degan M, Peterlumger E, et al. (2007) Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ 30: 1381–1399. [DOI] [PubMed] [Google Scholar]
- 4. Braidot E, Zancani M, Petrussa E, Peresson C, Bertolini A, et al. (2008) Transport and accumulation of flavonoids in grapevine (Vitis vinifera L.). Plant Signal Behav 3: 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blach-Olszewska Z, Jatczak B, Rak A, Lorenc M, Gulanowski B, et al. (2008) Production of cytokines and stimulation of resistance to viral infection in human leukocytes by Scutellaria baicalensis flavones. J Interf Cytok Res 28: 571–81. [DOI] [PubMed] [Google Scholar]
- 6. Yuan Y, Liu YJ, Luo YJ, Huang LQ, Chen SQ, et al. (2011) High temperature effects on flavones accumulation and antioxidant system in Scutellaria baicalensis Georgi cells. Afr J Biotech 10: 5182–5192. [Google Scholar]
- 7. Xu H, Park NI, Li X, Kim YK, Lee SY, et al. (2010) Molecular cloning and characterization of phenylalanine ammonia-lyase, cinnamate 4-hydroxylase and genes involved in flavone biosynthesis in Scutellaria baicalensis . Bioresource Technol 101: 9715–22. [DOI] [PubMed] [Google Scholar]
- 8. Morimoto TN, Matsuda T, Tanaka H, Taura F, Furuya N, et al. (1998) Novel hydrogen peroxide metabolism in suspension cells of Scutellaria baicalensis Georgi. J Biol Chem 273: 12606–12611. [DOI] [PubMed] [Google Scholar]
- 9. Sasaki K, Taura F, Shoyama Y, Morimoto S (2000) Molecular characterization of a novel β-Glucuronidase from Scutellaria baicalensis Georgi. J Biol Chem 275: 27466–27472. [DOI] [PubMed] [Google Scholar]
- 10. Nagashima S, Hirotani M, Yoshikawa T (2000) Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. cell suspension cultures. Phytochem 53: 533–538. [DOI] [PubMed] [Google Scholar]
- 11. Vincent D, Lapierre C, Pollet B, Cornic G, Negroni L, et al. (2005) Water deficits affect caffeate O-methyltransferase, lignification, and related enzymes in maize leaves. A proteomic investigation. Plant Physiol 137: 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castellarin SD, Pfeifeer A, Sivilotti P, Degan M, Peterlumger E, et al. (2007) Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ 30: 1381–1399. [DOI] [PubMed] [Google Scholar]
- 13. Matthews MA, Anderson MM (1988) Fruit ripening in Vitis vinifera L. responses to seasonal water deficits. Am J Enol Viticult 39: 313–320. [Google Scholar]
- 14. Roby G, Harbertson JF, Adams DA, Matthews MA (2004) Berry size and vine water deficits as factors in winegrape composition: anthocyanins and tannins. Aust J Grape Wine 10: 100–107. [Google Scholar]
- 15. Yang Y, He F, Yu L, Chen X, Lei J, et al. (2007) Influence of drought on oxidative stress and flavonoid production in cell suspension culture of Glycyrrhiza inflata Batal. Z Naturforsch C 62: 410–416. [DOI] [PubMed] [Google Scholar]
- 16. Guidi L, Degl'Innocenti E, Remorini D, Massai R, Tattini M (2008) Interactions of water stress and solar irradiance on the physiology and biochemistry of Ligustrum vulgare . Tree Physiol 28: 873–883. [DOI] [PubMed] [Google Scholar]
- 17. Chen SQ, Yuan Y, Luo YJ, Huang LQ, Li XM (2010) Effects of light on flavonoids accumulation and related gene expression in suspension cultures of Scutellaria baicalensis . China J Chin Mat Med 35: 49–52. [PubMed] [Google Scholar]
- 18. Davies C, Boss PK, Robinson SP (1997) Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol 115: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ban T, Ishimaru M, Kobayashi S, Shiozaki S, Goto-Yamamoto N, et al. (2003) Abscisic acid and 2,4-dichlorohenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. J Hort Sci Biotech 78: 586–589. [Google Scholar]
- 20. Jeong ST, Goto-Yamamotob N, Kobayashi S, Esaka M (2004) Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci 167: 247–252. [Google Scholar]
- 21. Brown D, Rashotte A, Murphy A, Normanly J, Tague B, et al. (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12: 556–563. [DOI] [PubMed] [Google Scholar]
- 23. Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, et al. (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19: 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan Y, Hao JD, Yang B, Li H, Li Z, et al. (2010) Climate change affected the best producing area of Chinese herbal medicine Scutellaria baicalensis Georgi. J Tradition Med (Russia) 3s: 241–248. [Google Scholar]
- 25. Qin SS, Chen SQ, Huang LQ (2010) Effect of water stress on relationship of endogenous phytohormone and active compound content in roots of Scutellaria baicalensis Georgi. Chin J Exp Tradition Med Form 16: 99–101. [Google Scholar]
- 26. Murch SJ, Rupasinghe HP, Goodenowe D, Saxena PK (2004) A metabolomic analysis of medicinal diversity in Huang-qin (Scutellaria baicalensis Georgi) genotypes: discovery of novel compounds. Plant Cell Rep 23: 419–425. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez I, Alegre L, Munne-Bosch S (2004) Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol 24: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 28. Albert NW, Lewis DH, Zhang H, Irving LJ, Jameson PE, et al. (2009) Light-induced vegetative anthocyanin pigmentation in Petunia. J Exp Bot 60: 2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olsen KM, Slimestad R, Lea US, Brede C, Lovdal T, et al. (2009) Temperature and nitrogen effects on regulators and products of the flavonoid pathway: experimental and kinetic model studies. Plant Cell Environ 32: 286–299. [DOI] [PubMed] [Google Scholar]
- 30. Steyn WJ, Wand SJ, Jacobs G, Rosecrance RC, Roberts SC (2009) Evidence for a photoprotective function of low-temperature-induced anthocyanin accumulation in apple and pear peel. Physiol Plant 136: 461–472. [DOI] [PubMed] [Google Scholar]
- 31. Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamaguchi M, Valliyodan B, Zhang J, Lenoble ME, Yu O, et al. (2010) Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant Cell Environ 33: 223–243. [DOI] [PubMed] [Google Scholar]
- 33. Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, et al. (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130: 2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E (1996) Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol 112: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hajheidari M, Abdollanhian-Noghabi M, Askari H, Hedari M, Sadeghian SY, et al. (2005) Proteome analysis of sugar beet leaves under drought stress. Proteomics 5: 950–960. [DOI] [PubMed] [Google Scholar]
- 36. Gazanchian A, Hajheidari M, Khoshkholgh SN, Hosseini SG (2007) Proteome response of Elymus elongatum to severe water stress and recovery. J Exp Bot 58: 291–300. [DOI] [PubMed] [Google Scholar]
- 37. Kottapalli KR, Rakwal R, Shibato J, Burow G, Tissue D, et al. (2009) Physiology and proteomics of the water-deficit stress response in three contrastin peanut genotypes. Plant Cell Environ 32: 380–407. [DOI] [PubMed] [Google Scholar]
- 38. Park JS, Kim JB, Cho KJ, Cheon CI, Sung MK, et al. (2008) Arabidopsis R2R3-Myb transcription factor AtMYB60 functions as a transcriptional repressor of anthocyanin biosynthesis in lettuce (Lactuca sativa). Plant Cell Rep 27: 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2: 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh K, Kumar S, Rani A, Gulati A, Singh AP (2009) Phenylalanine ammonia-lyase (PAL) and cinnamate-4-hydroxylase (C4H) and catechins (flavan-3-ols) accumulation in tea. Funct Integr Genomic 9: 125–134. [DOI] [PubMed] [Google Scholar]
- 41. Li X, Li S, Lin J (2003) Effect of GA3 spraying on lignin and auxin contents and the correlated enzyme activities in bayberry (Myrica rubra Bieb.) during flower bud induction. Plant Sci 164: 549–556. [Google Scholar]
- 42. Russel DW, Galston AW (1969) Blockange by gibberellic acid on phytochrome effects on growth, auxin responses, and flavonoid biosynthesis in etiolated pea internodes. Plant Physiol 44: 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hinderer W, Peterson M, Seitz HU (1983) Inhibition of flavonoid biosynthesis by gibberellic acid in cell suspension cultures of Daucus carota . Planta 160: 544–549. [DOI] [PubMed] [Google Scholar]
- 44. Peera W, Bandyopadhyaya A, Blakesleea J, Makama S, Chenb R, et al. (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ribnicky DM, Ilic N, Cohen JD, Cooke TJ (1996) The Effects of Exogenous Auxins on Endogenous Indole-3-Acetic Acid Metabolism (The Implications for Carrot Somatic Embryogenesis). Plant Physiol 112: 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang W, Vignani R, Scali M, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27: 2782–2786. [DOI] [PubMed] [Google Scholar]
- 47. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 48. Patterson BD, Macrae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 134: 487–492. [DOI] [PubMed] [Google Scholar]
- 49. Bates LS (1973) Rapid determination of free proline for water stress studies. Plant Soil 39: 205–207. [Google Scholar]
- 50. Li SM, Luo YJ, Yuan Y, Huang LQ (2009) HPLC determination of baicalin and baicalein in the callus of Scutellariae baicalensis Georgi. Chin J ExpTradition Med Form 8: 1–3. [Google Scholar]
- 51. Marinova D, Ribarova F, Atanassova M (2005) Total phenolics and total flavonoids in bulgarian fruits and vegetables. J Univf Chem Tech Met 40: 255–260. [Google Scholar]
- 52.He ZP (1993) A laboratory Guide to Chemical Control Technology on Field Crop. Ed. by He ZP, Beijing Agricultural University Press, Beijing, China, pp. 60–68.
- 53. Yang YM, Xu CN, Wang BM, Jia JZ (2001) Effects of Plant Growth Regulators on Secondary Wall Thickening of Cotton Fibres. Plant Growth Regul 35: 233–237. [Google Scholar]
- 54. Teng NJ, Wang J, Chen T, Wu XQ, Wang YH, et al. (2006) Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana . New Phytol 172: 92–103. [DOI] [PubMed] [Google Scholar]
- 55. Weiler EW, Jourdan PS, Conrad W (1981) Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 153: 561–571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used in this paper.
(DOCX)
Differentially expressed proteins identified by MALDI-TOF MS.
(DOCX)
Differentially expressed proteins in S. baicalensis roots exposed to water deficit. Separated proteins from 16% SWC treated roots at 30 d (A), 50 d (C), and 70 d (E) are compared with protein profiles resulting from 12% SWC treated roots at 30 d (B), 50 d (D), and 70 d (F). Marked proteins (T) are named in accordance with Table 1 and Table S2 and were identified by MALDI-TOF MS. The numbers at the top of the gel T denote the pH gradient in the first dimension, while the molecular masses of the 2-D standards are displayed on the right.
(TIF)
The MS spectrum and the matched peptide fragments of protein spot number 4(A), 20 (B) and 28 (C).
(TIF)
Functional classification and distribution of identified proteins. Unknown proteins include those whose functions have not been described.
(TIF)
Effects of plant growth regulators on flavonoid levels in S. baicalensis after spay IAA and NAA. Baicalin (A), baicalein (B) and total flavonoids (C) in roots of S. baicalensis. Vertical bars indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
(TIF)
Effects of plant growth regulators on flavonoid levels in S. baicalensis under SWC16%. Total flavonoids, baicalin, and baicalein in roots of S. baicalensis. Vertical bars indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
(TIF)
Effects of water deficit on the expression of RNA helicase in S. baicalensis . Protein expression (A) and transcript abundance (B) of RNA helicase in roots of S. baicalensis grown under 16% SWC as a control (shaded bars) and 12% SWC as a water deficit treatment (white bars). Vertical lines indicate the standard deviation of three biological replicates. Asterisks indicate a significant difference at the P<0.05 level.
(TIF)