Abstract
Human monoclonal antibodies have been generated from heterohybridomas obtained by fusing mouse myeloma cells with peripheral lymphocytes from patients with active Graves disease. This report characterizes four antibodies as presumptive thyrotropin receptor antibodies because they specifically inhibit thyrotropin binding and competitively inhibit thyrotropin-induced cAMP levels in human thyroid cells. Two of these antibodies, 208F7 and 206H3, are representative of autoimmune stimulators in Graves disease sera because they stimulate thyroid function in all assays, including the mouse bioassay; their ability to inhibit thyrotropin-induced cAMP increases in thyroid cells competitively is complemented by more than additive agonism at low (10 pM) thyrotropin concentrations. These stimulating antibodies interact more potently with human thyroid ganglioside preparations than with bovine thyroid or brain gangliosides; in contrast, they are poor inhibitors of 125I-labeled thyrotropin binding to liposomes containing the glycoprotein component of the human thyrotropin receptor. Antibodies 129H8 and 122G3 appear to be representative of inhibiting or "blocking" antibodies in Graves disease sera. Thus they have no intrinsic stimulatory action in assays of thyroid function but rather inhibit thyrotropin activity in the assays tested. These two antibodies do not react with human thyroid gangliosides but are strong inhibitors of thyrotropin binding to liposomes containing the high-affinity glycoprotein component from human, bovine, and rat thyroid membranes. The data unequivocally establish the pluritopic nature of the immunoglobulins in Graves disease and relate individual components or determinants of the thyrotropin receptor structure with specific autoimmune immunoglobulins.
Full text
PDF
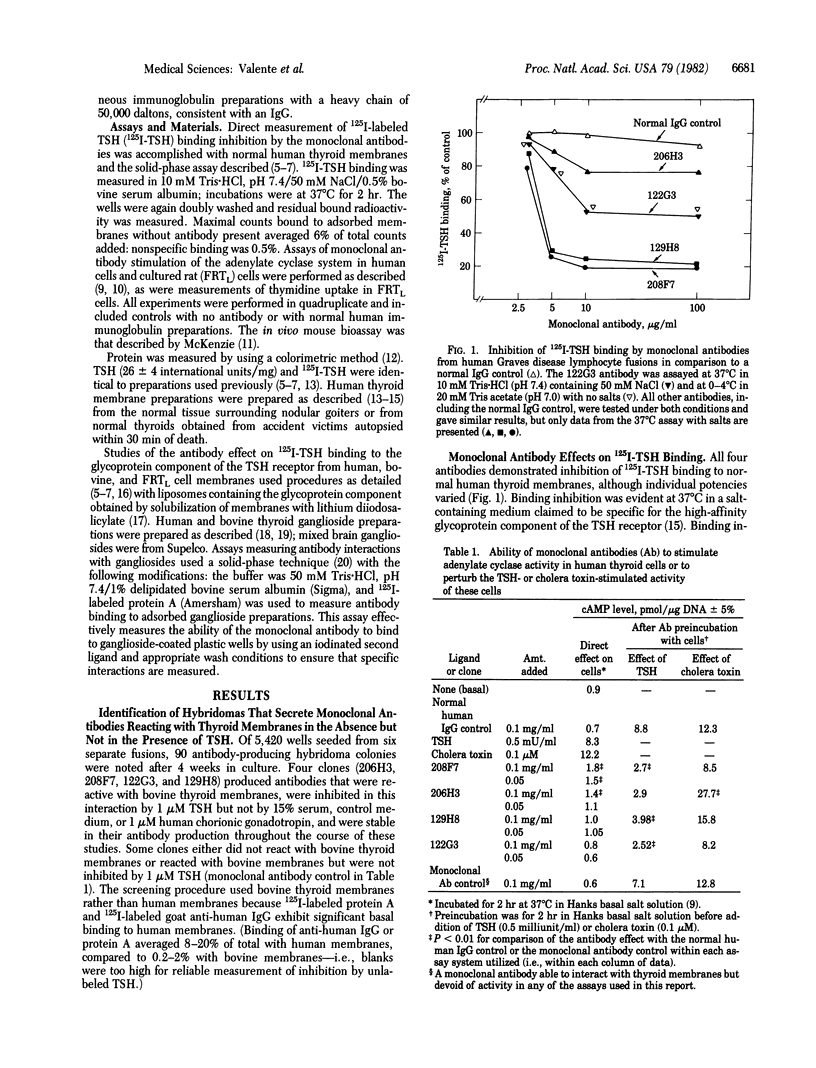
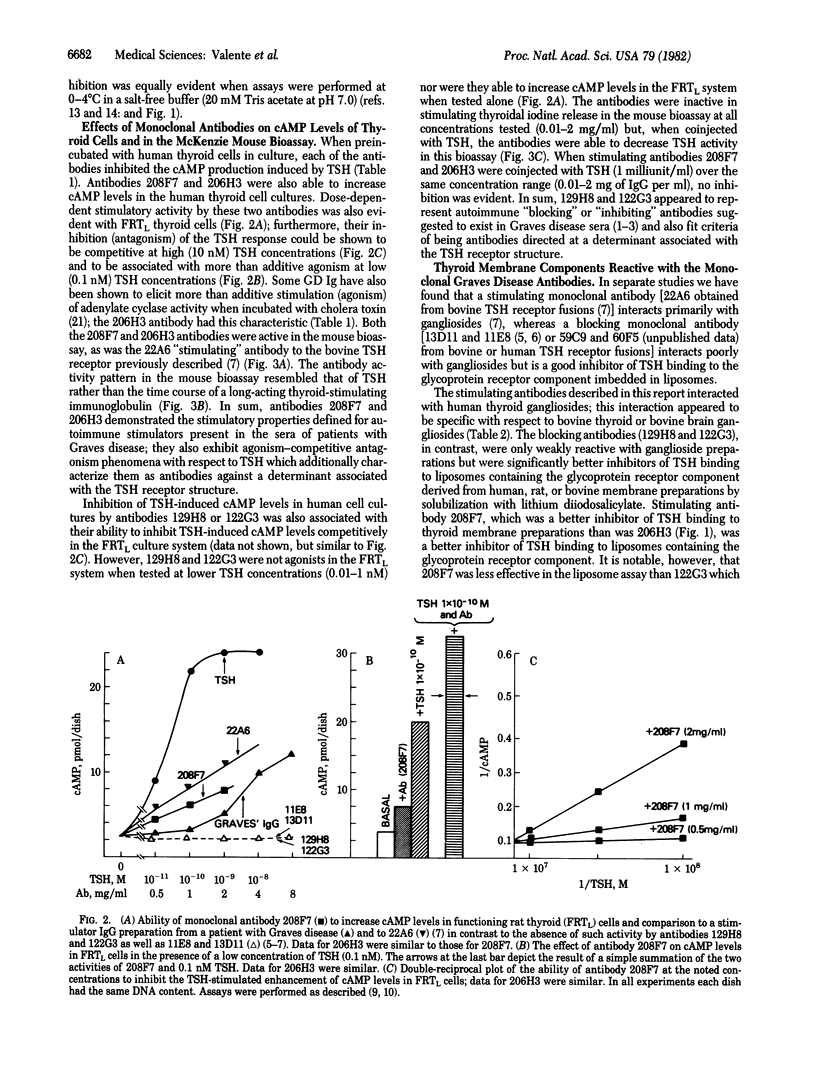
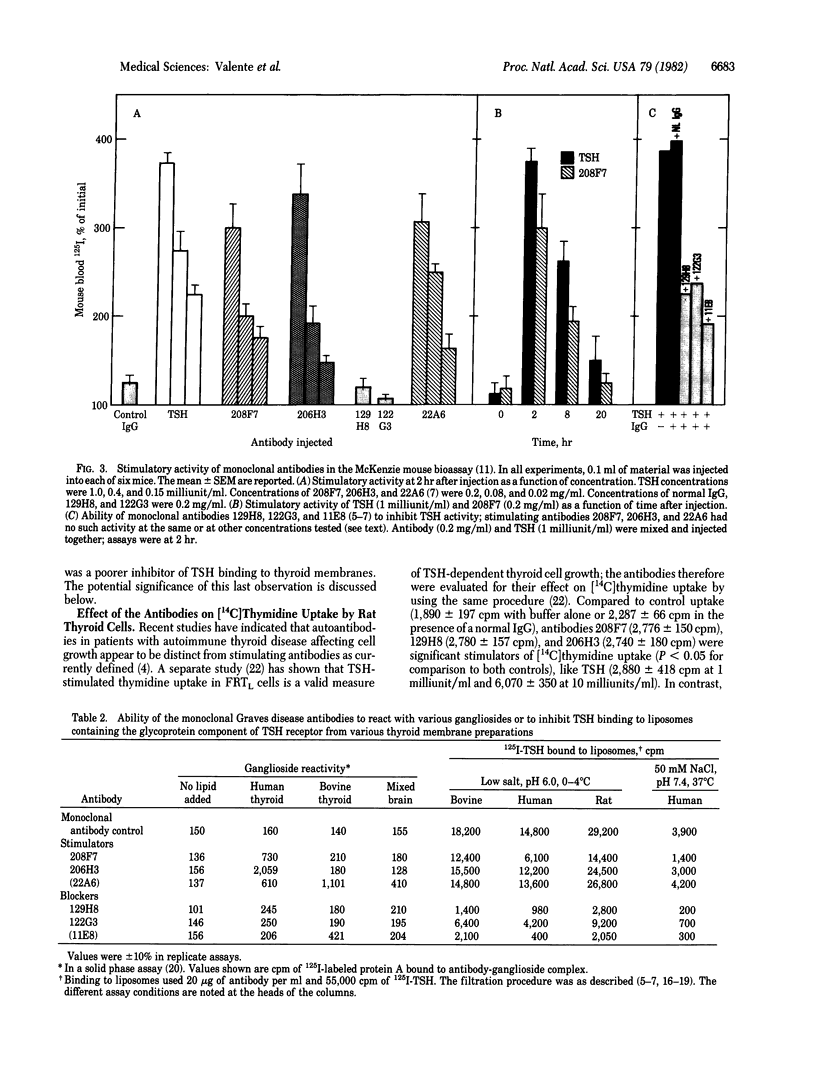

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloj S. M., Lee G., Grollman E. F., Beguinot F., Consiglio E., Kohn L. D. Role of phospholipids in the structure and function of the thyrotropin receptor. J Biol Chem. 1979 Sep 25;254(18):9040–9049. [PubMed] [Google Scholar]
- Amir S. M., Carraway T. F., Jr, Kohn L. D., Winand R. J. The binding of thyrotropin to isolated bovine thyroid plasma membranes. J Biol Chem. 1973 Jun 10;248(11):4092–4100. [PubMed] [Google Scholar]
- Brockhaus M., Magnani J. L., Blaszczyk M., Steplewski Z., Koprowski H., Karlsson K. A., Larson G., Ginsburg V. Monoclonal antibodies directed against the human Leb blood group antigen. J Biol Chem. 1981 Dec 25;256(24):13223–13225. [PubMed] [Google Scholar]
- Drexhage H. A., Bottazzo G. F., Bitensky L., Chayen J., Doniach D. Thyroid growth-blocking antibodies in primary myxoedema. Nature. 1981 Feb 12;289(5798):594–596. doi: 10.1038/289594a0. [DOI] [PubMed] [Google Scholar]
- Grollman E. F., Lee G., Ramos S., Lazo P. S., Kaback H. R., Friedman R. M., Kohn L. D. Relationships of the structure and function of the interferon receptor to hormone receptors and establishment of the antiviral state. Cancer Res. 1978 Nov;38(11 Pt 2):4172–4185. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKENZIE J. M. The bioassay of thyrotropin in serum. Endocrinology. 1958 Sep;63(3):372–382. doi: 10.1210/endo-63-3-372. [DOI] [PubMed] [Google Scholar]
- McKenzie J. M., Zakarija M. A reconsideration of a thyroid-stimulating immunoglobulin as the cause of hyperthyroidism in Graves' disease. J Clin Endocrinol Metab. 1976 Apr;42(4):778–781. doi: 10.1210/jcem-42-4-778. [DOI] [PubMed] [Google Scholar]
- Mullin B. R., Pacuszka T., Lee G., Kohn L. D., Brady R. O., Fishman P. H. Thyroid gangliosides with high affinity for thyrotropin: potential role in thyroid regulation. Science. 1978 Jan 6;199(4324):77–79. doi: 10.1126/science.199.4324.77. [DOI] [PubMed] [Google Scholar]
- Pekonen F., Weintraub B. D. Salt-induced exposure of high affinity thyrotropin receptors on human and porcine thyroid membranes. J Biol Chem. 1980 Sep 10;255(17):8121–8127. [PubMed] [Google Scholar]
- Tate R. L., Holmes J. M., Kohn L. D., Winand R. J. Characteristics of a solubilized thyrotropin receptor from bovine thyroid plasma membranes. J Biol Chem. 1975 Aug 25;250(16):6527–6533. [PubMed] [Google Scholar]
- Tate R. L., Schwartz H. I., Holmes J. M., Kohn L. D. Thyrotropin receptors in thyroid plasma membranes. Characteristics of thyrotropin binding and solubilization of thyrotropin receptor activity by tryptic digestion. J Biol Chem. 1975 Aug 25;250(16):6509–6515. [PubMed] [Google Scholar]
- Toccafondi R. S., Aterini S., Medici M. A., Rotella C. M., Tanini A., Zonefrati R. Thyroid-stimulating antibody (TSab) detected in sera of Graves' patients using human thyroid cell cultures. Clin Exp Immunol. 1980 Jun;40(3):532–539. [PMC free article] [PubMed] [Google Scholar]
- Trisler G. D., Schneider M. D., Nirenberg M. A topographic gradient of molecules in retina can be used to identify neuron position. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2145–2149. doi: 10.1073/pnas.78.4.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitti P., Valente W. A., Ambesi-Impiombato F. S., Fenzi G. F., Pinchera A., Kohn L. D. Graves' IgG stimulation of continuously cultured rat thyroid cells: a sensitive and potentially useful clinical assay. J Endocrinol Invest. 1982 May-Jun;5(3):179–182. doi: 10.1007/BF03349476. [DOI] [PubMed] [Google Scholar]
- Yavin E., Yavin Z., Schneider M. D., Kohn L. D. Monoclonal antibodies to the thyrotropin receptor: implications for receptor structure and the action of autoantibodies in Graves disease. Proc Natl Acad Sci U S A. 1981 May;78(5):3180–3184. doi: 10.1073/pnas.78.5.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M. Influences of cholera toxin on thyroid stimulation by thyrotropin and thyroid-stimulating antibody. Endocrinology. 1980 Dec;107(6):2051–2054. doi: 10.1210/endo-107-6-2051. [DOI] [PubMed] [Google Scholar]