Abstract
Purpose
Outline the biomechanics of reaching both in healthy individuals and in individuals with acquired brain injury (ABI), and to discuss the clinical implications for using valid biomechanical models to assess reaching.
Methods
A review of current literature, including a MEDLINE search using keywords of reaching, acquired brain injury, stroke, biomechanics and motor control.
Results
Current assessments of the upper extremity in acquired brain injury (ABI) are focused on single joint characteristics of range of motion, strength, and spasticity. However, reaching is a functional multijoint task requiring interjoint coordination in addition to feedback and feedforward control to optimally position the hand at a desired location so that it may interact with the environment. From the literature, biomechanical measures of reaching such as movement time, movement distance and interjoint coordination have been shown to discriminate changes to hand path quality following brain injury. These measures also have been shown to correlate with measures of sensorimotor function (e.g., Fugl-Meyer) in the upper extremity.
Conclusions
Further development of reliable and valid multijoint biomechanical evaluations is required, particularly for natural and goal-oriented reaching movements. The biomechanical assessment of reaching in ABI can provide an understanding of the specific deficits in physiological structures or motor planning underlying altered reaching ability, assist in the evaluation of new therapies, and characterize the recovery process following ABI.
Keywords: Upper Extremity, Assessment, Motor Control
1. Introduction
The ability to reach is critical for virtually all activities of daily living such as grooming, toileting, feeding, transfers, and dressing.1 Reaching has been defined as the voluntary positioning of the hand at or near a desired location so that it may interact with the environment.2 It requires coordination of multiple joints and involves both the musculoskeletal and neural systems. Although grasping3 and postural control4 actions often accompany a reach, these elements are controlled by distinct motor programs.5 For the purpose of this review, we will restrict our discussion to the reaching process alone.
Patients with stroke and traumatic brain injury (TBI) suffer high rates of impairment to the upper extremity with approximately 85% incurring acute impairment and 40% incurring chronic impairment.6 In over half of individuals with a stroke, the affected upper extremity remains severely impaired despite intensive and prolonged rehabilitation.7 Furthermore, in both stroke and TBI patients, the upper extremity recovers less than the lower.8 Stroke patients rate return of upper extremity function as a high priority9 and failure to substantially recover upper limb function can lead to depression and withdrawal.10
Reaching ability following an ABI is generally assessed on an ordinal scale as one component of a standardized upper extremity function scale, e.g., Frenchay Arm Test.11 These global scales are useful for measuring gross changes in functional performance but lack sensitivity to small yet important changes. Furthermore, ordinal scales provide little information as to the underlying causes of the motor dysfunction. A full understanding of human movement requires the integration of kinematic (movement) and kinetic (force) analyses to identify the internal forces (e.g., from muscles, ligaments) and external forces (e.g., from contact with an object such as a door or from a load such as a fork) acting upon the body.12–14 Electromyography, kinematic, and kinetic measures during movement are sensitive to the effects of neurological and orthopaedic conditions and their treatments.15,16
Movement analysis of reaching can identify changes in interjoint coordination or the quality of the hand path (e.g., directness, smoothness) following brain injury. In fact, kinematic measures of movement time, movement distance and interjoint coordination during a reaching task are strongly correlated to functional measures of upper extremity function (e.g., Fugl-Meyer upper extremity score) in individuals with stroke.13 Kinematic and kinetic measures can be used not only to assess performance but also to elucidate the motor strategies used during a goal-oriented reach. In addition, movement analysis of reaching may be useful for the evaluation of existing17 and developing upper extremity therapies in acquired brain injury (ABI) such as the force use paradigm where the less affected arm is restrained to encourage use of the more affected extremity,18 the use of neuromuscular blocks (e.g., botulinum toxin) to upper extremity muscle to relieve spasticity,19 and robotic assisted reaching exercises.20
Despite the fact that reaching is one of the major functions of the upper extremity and has poor recovery in ABI,6 the biomechanics of this multijoint task are largely ignored in undergraduate rehabilitation curriculums relative to the emphasis placed upon the multijoint function of the lower extremity. Therefore, the purposes of this paper are (1) to outline the biomechanics of reaching in healthy individuals, (2) to review the uses of current clinical assessments of reaching function in ABI, (3) to describe the findings of biomechanical investigations in ABI, and (4) to discuss the clinical implications and future considerations for the use of valid biomechanical models for the assessment of reaching. We performed a systematic literature search and based on a MEDLINE search using the keywords “reaching”, “acquired brain injury”, “stroke”, “biomechanics” and “motor control”. Additional references were gleaned from the articles identified.
2. Biomechanics of Normal Reaching
Reaching to a target within arm’s length involves the shoulder, elbow, and wrist. Reaching to targets beyond arm’s length involves movements at all these joints, as well as trunk and hip motion.21 These joints work together as a coordinated mechanical system in healthy individuals to accurately place the hand in a desired position. Understanding the biomechanical and neuromotor control processes underlying reaching in the healthy population can help clinicians to identify where deficits may occur in persons with ABI.
Segments of the upper limb may move about seven possible degrees of freedom (DOF) (i.e., joint rotations), in the shoulder (3 DOF), elbow (1 DOF), forearm (1 DOF) and wrist (2 DOF), in addition to elevation/depression and protraction/retraction of the shoulder-scapular complex. This natural excess of joints affords the central nervous system (CNS) the ability to employ an infinite number of paths and when reaching to a specific target. Despite the many available degrees of freedom, joint motion during reaching is similar for a given start position, end position, and hand orientation across the healthy population.21,22 Functionally, the redundancy of joints provides the ability to adaptively and optimally control movements to account for internal and external environmental factors23 such as compensating for an injury or altering the hand trajectory to avoid collision with an object.
Neuromuscular control of reaching is computationally complex and requires the synchronization of muscle activation at all the moving joints as well as all the muscles involved in postural stabilization. The acceleration of each joint during a reaching movement depends upon both the net joint torque (i.e. rotational force) and inertia (resistance of an object to any change in motion); rotational inertias experienced by upper extremity joints are coupled by the movements and configurations of the upper arm, forearm, and hand.24 The net joint torque is due not only to muscle activity but also to the effects of gravity, joint viscoelasticity, and externally applied forces (e.g. the reaction force of a door on the hand as it is being opened). Gravitational effects depend upon the weight and general orientation of the arm segments. Viscoelasticity is the inherent mechanical property of passive tissues (i.e. muscles and tendons) to stabilize joint position.
The central nervous system (CNS) planning of reaching movements may be considered as a hierarchical control in which spatial information is converted to motor patterns at the shoulder and elbow to move the hand through space. A series of transformations convert sensory signals into hand trajectories, then into corresponding joint trajectories, required muscular torques, and finally into the actual patterns of muscle activity.25 While there is general agreement regarding this motor control process, there are debates over the specific coordinate system used in planning reaching movements and whether or not the muscle torques are explicitly represented by the CNS.26 For example, some have suggested that at the highest level, planning could occur in terms of a joint angle co-ordinate system (e.g., control of shoulder, elbow and wrist angles)27 or in terms of the final endpoint co-ordinates (i.e., target).28
The CNS uses both feedforward and feedback strategies to control reaching movements.5 The first phase of reaching is feedforward (preplanned) controlled, sensory information is used to anticipate disturbances to limb dynamics and plan appropriate muscle activation based on experience. Feedforward control is characterized by a profile of continuous movement that contains one acceleration and one deceleration phase. The second phase of reaching is feedback controlled and corrects for discrepancies between where and how one wants to place the arm versus the current position and speed of the arm. In feedback control, signals from peripheral receptors provide information back to the nervous system about the events occurring in the muscles, joints and other tissues. Feedback control is characterized by a profile of discontinuous movement that contains multiple accelerations and decelerations of progressively shorter duration as the error between the hand and the target approaches zero.29 Control of voluntary movements improves with practice as we learn to anticipate and correct for disturbances resulting from internal and external forces acting on the body.30 People of all ages exhibit this ability to adapt their reaching strategies to changing environmental and physiological factors.31
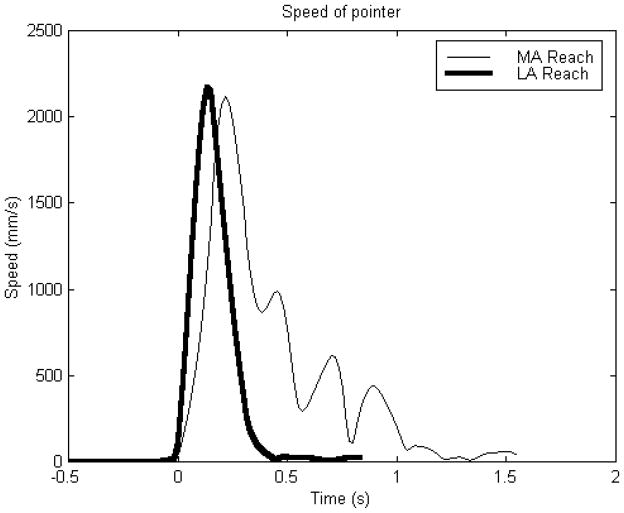
Normal multijoint reaching is characterized by a smooth bell shaped velocity profile with a peak velocity approximately halfway between the start and endpoints (see line LA in Figure 1). The peak velocity corresponds to the changeover from the acceleration and deceleration phases and its location within the velocity profile is an indicator of strategy. As requirements for accuracy increase, the bell shaped velocity profile becomes skewed and the peak velocity occurs earlier in movement. Conversely, as requirements for speed increase, the peak velocity occurs later in movement. The relationship between movement speed and accuracy during reaching movements is known as Fitts’ law32 where an increase in accuracy (decreasing the target width) is related to a reduction in reaching speed.
Figure 1.
Fingertip speeds for the more affected (MA) and less affected (LA) arms while during a reaching task. The subject (SR01: age = 57, injury = hemorrhagic stroke affecting the right side, time since stroke = 6 years, Fugl-Meyer upper extremity function = 19/66) was seated with a belt across the chest to restrain trunk movement. An auditory tone cued the subject to reach from a start position on the mid thigh to a target at shoulder height as “fast as possible”. The speed profile of the MA arm is less symmetric and more segmented than the LA arm.
Hand paths during reaching movements are straight or slightly curved.28 Producing such a path requires a subject to coordinate rotations of both shoulder and elbow joints, typically characterized by a roughly constant ratio of joint angular velocities.33,34 A measure of the straightness of the hand path (known as the hand directness) is the ratio of the actual path length to that of the direct path.35 Straight hand paths require simultaneous rotation of the elbow and shoulder so that inter-joint coordination in healthy individuals is demonstrated by a near constant ratio of the elbow and shoulder angular velocities throughout the reaching movement.33,34 Deviations from straight line paths is caused by reduced coordination of the shoulder and elbow joint movements. Analyses of interjoint coordination may be helpful in understanding the nature of movement deficits in individuals with CNS lesions.36,37
3. Upper Extremity Assessments in ABI
Reaching may be affected by a number of impairments following an ABI, including spasticity, a decreased range of motion (ROM), coordination difficulties and weakness resulting from peripheral muscle atrophy or decreased central motor recruitment.38
Weakness from either peripheral (e.g., muscle atrophy) or central sources (e.g., reduced motor unit recruitment) may be the major impairment underlying the functional disability of the more affected upper limb in ABI injury.38–40 Classifying muscle strength by manual testing41 has limited sensitivity to strength deficits in ABI.42 Muscle strength is better measured using hand held dynamometry, which is sensitive to low and high levels of force43,44 and is a reliable45 and valid46 measure of hemiparetic muscle strength.
Spasticity is one of the principal factors affecting rehabilitation47 following an upper motor neuron lesion (UMN). Spasticity has been defined as a velocity dependent increase in the tonic stretch reflex.48 The sensation of resistance while moving the joint passively through its range of motion, and the degree of resistance is one manifestation of spasticity that is commonly assessed using the Ashworth scale.49,50 The 5-point modified Ashworth scale has a high inter-rater reliability for an extension movement of the elbow joint50 but tends to cluster scores in the middle range rendering the scale insensitive to subtle changes that may result from treatment.51,52 There is growing recognition that tone is only one dimension of spasticity as spasticity can also manifest in contractures, reduced active joint range, muscle spasms, pain, heterotopic ossification, and clonus.53,54
Isolated joint measurements of weakness under isometric loading and spasticity in passive motion may not be functionally significant. Reaching tasks are inherently multijointed and require the integration of musculoskeletal and neural components. The relationship between single joint assessments and reaching is largely undetermined. The effect that these impairments may have on reaching can be better understood in the context of an integrated model of the neuromuscular system. Such models have been well used in simulating reaching movements in the healthy population.55
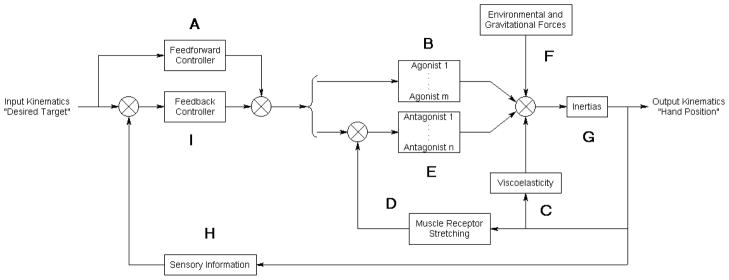
Consider the process of initiating and completing a reaching movement using a spastic hemiparetic arm in a simple model that we propose (Figure 2). Once a target is specified, the feedforward controller (A) generates patterns of muscle activation that are used to drive agonist muscles (B) (e.g., triceps brachii). However, decreased motor recruitment and disuse atrophy may limit the forces that agonist muscles can generate. As the hand moves towards the target, elbow extension and shoulder flexion may be restrained by increases in tone. Increased tone may be due to structural changes in muscles that result in elevated viscoelasticity (C). Increased tone may also be due to hyperreflexia (increased sensitivity to muscle receptor stretching) (D), which causes antagonist muscles (e.g., biceps brachii) (E) to contract.52 The resultant forces from muscle contractions, the environment, gravity (F), and viscoelasticity in addition to the inertias of the arm (G) determine the accelerations of the upper arm and forearm. If the original motor command does not adequately compensate for arm dynamics, the hand may not reach the desired target. New positions of the hand and joints will be detected by the sensory system (H) and iteratively corrected by the feedback controller (I).
Figure 2.
Simple model of the neuromuscular system applied to a spastic hemiparetic arm during forward reaching. Junctions that sum or compare signals are represented by “⊗”. Agonist and antagonistic muscles are indexed to m and n respectively. Reaching impairment could arise at various components of the model, eg. inability to activate muscles due to decreased motor recruitment (A & I), inability to generate muscle force due to disuse atrophy (B), changes in muscle structure that result in increased viscoelasticity (C), and increased gain (sensitivity) to stretching of muscle receptors (hyperreflexia) (D).
Task-based instruments for the upper extremity, e.g. Action Research Arm,56 Frenchay Arm Test,11 Peg Tests,57 Rivermead Motor Assessment,58 and Motor Assessment Scale59 score function on a “can” or “cannot” basis. In addition to the lack of sensitivity to partially completed movements, these assessments fail to provide valuable information about the strategies and mechanisms underlying abnormal reaching. These instruments are only concerned with the ability to achieve a preset goal, treating all the physiological structures and possible impairments as a black box. A more comprehensive assessment is the upper extremity component of the Fugl-Meyer Scale,60 which is composed of ordinal scales for sensation, proprioception, joint pain, and range of motion (shoulder, elbow, wrist and fingers), reflex activity, and joint coordination. The Fugl-Meyer Scale has a high inter-rater reliability.61,62 However, its individual test components neither assess purposeful reaching tasks nor quantify the functional impairments due to spasticity or weakness.
4. Biomechanics of Reaching in ABI
Although biomechanical analyses have been used to identify reaching characteristics in healthy individuals, basic principles such as Fitts’ Law have yet to be evaluated in individuals with brain injuries during functional reaching tasks.
Krebs et al.63 used kinematic analyses of hand paths to track the recovery pattern and identify adaptive strategies during unconstrained reaching tasks following brain injury. They found an improvement in twenty stroke survivors of acute stroke in both accuracy and smoothness in reaching movements and drawing tasks and the re-acquisition of bell-shaped velocity profiles over an 11 week period following their strokes. In addition, improvements of hand velocity in reaching during the acute period following stroke have also been reported.64,65
Kinematic analyses of hand paths have produced evidence that persons with chronic stroke may select a strategy that optimizes their environment and neuromuscular system.66–68 Trombly68 found that the kinematic profile of the non-paretic arm during reach is fast and continuous whereas the profile of the paretic arm is slow and discontinuous. She suggested that the CNS adapts a feedback control in the paretic arm to correct deviations from the desired trajectory. We have found in our lab (unpublished findings) that the reaching kinematics of the spastic hemiparetic arm is more segmented and less symmetrical than the healthier arm (see line MA in Figure 1). Roby-Brami67 tested reaching to cone shaped targets in the horizontal plane and found that individuals with chronic stroke selected strategies that compensated for their specific impairments; those with predominantly proximal impairments slid their hands along a supportive table while patients with predominantly distal impairments made downward stabs. Cirstea and Levin66 found that patients with moderate and severe impairments in the paretic arm would involve trunk movements to targets that were within arm’s length and that the recruitment of an extra degree of freedom may be related to the severity of the impairment.
Goal-directed actions seem to produce significantly smoother and faster reaching movements of the non-paretic arm in persons with chronic stroke than “no object” conditions.69 Moreover, practically preferred and meaningful targets such as food items have resulted in even faster and smoother movements.70 This suggests that movement efficiency may be enhanced in therapy and assessment by using functionally significant target objects during goal oriented reaching.
Elbow and shoulder coordination has been investigated in individuals with stroke using a reaching task in the horizontal plane with the arm supported by a table. During these tasks, kinematic measures of interjoint co-ordination (ratio of the shoulder-elbow velocity) were more strongly correlated with impairment as measured by the Fugl-Meyer test than with spasticity scores.13 In addition, it has been suggested that individuals with stroke lack the required compensation for inertial torques (i.e., torques dependent upon movements from other joints) during fast reaching movements and consequently result in larger deviations between the initial direction of reaching movement and the actual target direction.71,72 These directional errors are associated with excessive rotation of the elbow with respect to the shoulder. Beer et al.71,72 suggested that the inability of patients to specify joint co-ordination may be partly due to decreased ability to predict limb dynamics in feedforward control.
Elbow and shoulder coordination has also been investigated using reaching tasks in which the hand path is physically restricted to straight paths of specified direction (e.g., forward/backward, lateral/medial). These constrained tasks can provide complementary information to unconstrained natural movements. If the elbow and shoulder joints act in a coordinated fashion during a constrained task, the hand will accelerate towards the target. However, in individuals with stroke, altered coupling of the elbow and shoulder joints results in forces acting in directions other than the intended direction.14,15,73 These forces, known as constraint forces, can differentiate between the pathological extensor and flexor synergies found in ABI (as defined by the Fugl-Meyer scale).73 The magnitude of these constraint forces increases when reaching movements are exerted against greater gravitational loads (e.g., reaching in increasingly upward directions).14,73
A constrained reaching task was also used to investigate the multijoint effects of tone by measuring the resistive force when the upper limb is passively extended into a reach position and moved at a slow enough rate to avoid excitation of stretch reflexes and inertial effects.14,73 The force required to hold the arm in an extended position was consistently higher in the paretic arm when compared to the non-paretic arm.14,73,74 Joint torque analysis indicated that this increase was due to muscle or joint based contractures at both the shoulder and elbow.14,73–75 Although a constrained reaching paradigm can characterize passive and active mechanical properties of the upper limb, predefined paths may not represent naturally occurring unconstrained reaching movements.76
Results from constrained reaching assessments have shown that spastic reflexes are not elicited at speeds found in normal reaching.73 Furthermore, stretch responses when the muscle is activated are comparable between the paretic and non-paretic arms.14,73 Together, these studies suggest that the inability of persons with spastic hemiparesis to extend their arm in a reaching motion is primarily related to agonist muscle weakness and not restraint from spastic antagonist muscles.14,73
5. Clinical Utility of Biomechanical Assessments and Models
The use of biomechanical analysis techniques in assessing patients with ABI is still in its infancy, and much remains to be done to develop these techniques. The development of multi-joint biomechanical evaluations of natural and goal-oriented reaching movements is required. The majority of biomechanical evaluations performed to date involve reaching movements performed at one speed in the horizontal plane with the arm supported. We need to evaluate the effects of different reaching conditions in ABI such as speed, effect of gravity (e.g., reaching upwards or downwards), accuracy, direction and sitting posture.
Kinematic and kinetic descriptions of reaching in the affected arm have indicated that hand paths become less smooth and rotations at the elbow and shoulder become less coordinated as targets progressively move farther contralateral, upwards, and forwards.13,14,73 The effect of this increasing taxation on the motor system could be used to develop a biomechanically valid hierarchical performance assessment of reaching in which targets are placed in increasingly more difficult locations. Such assessments would likely be more valid if functionally relevant objects (e.g., cups) were used as targets.60,70 In addition, there needs to be further study of the reliability and validity of biomechanical parameters of reaching ability. These investigations have been initiated and the results are promising; measures of force magnitude and directional error during a reaching task in chronic stroke exhibited moderate to high test-retest reliability and measures of directional force error in the more affected arm were a valid and sensitive measure of impairment when correlated with Fugl-Meyer upper extremity assessments.15
Most current upper extremity assessments focus on measures (e.g., ROM, spasticity, strength) across a single joint and there is little information as to how these clinical findings relate to multijoint functional reaching ability. Since the complex devices required to evaluate multijoint reaching are not likely to soon make the transition from research to clinical settings, it is important to establish the relationships and develop valid models between single joint and multijoint assessments. Such models have proven useful in relating muscle strength to multijoint lower extremity gait performance.77,78
The biomechanical studies of reaching have demonstrated the importance of variables not traditionally evaluated in rehabilitation, such as hand path and movement speed that could be integrated into traditional clinical assessments. In fact, observational kinematic assessments of upper limb movements have shown that therapists using visual analogue scales are capable of making accurate judgments of movement speed (r>=0.87), jerkiness (r>=0.78), and hand path indirectness (r>=0.68) when compared to kinematic assessments using computerized video systems.78
Finally, biomechanical studies of a reaching task have identified specific deficits in upper extremity function that could direct future clinical studies. For example, reaching performance is not likely related to antagonist muscle spasticity but to agonist muscle weakness and the inability to transform desired hand trajectories into coordinated elbow and shoulder joint movements.13,14,73 Therapeutically, this suggests that improved reaching function may result from strength training and the restoration of normal sensorimotor relationships between joints13,14,73 through practice.20 Thus, future clinical studies need to test these hypotheses that are based on sound biomechanics.
In conclusion, biomechanical assessments of a reaching task can play a complementary role to current clinical assessments to provide an understanding of the mechanisms underlying altered reaching ability following ABI.
Acknowledgments
We would like to thank members of the GF Strong Rehabilitation and the UBC Neuromotor Control laboratories for their comments on this manuscript.
Grant Support: Rick Hansen Neurotrauma Initiative Operating Grant, the Canadian Institute of Health Research Operating Grant (MOP-57862), and Studentship and the BC Health Research Foundation.
References
- 1.Granger CV, Hamilton BB, Sherwin FS. Uniform Data System for Medical Rehabilitation. New York: Project Office, Buffalo General Hospital; 1986. Guide for the use of uniform data set for medical rehabilitation. [Google Scholar]
- 2.Carr J, Shepherd R. Reaching and Manipulation. In: Carr J, Shepherd R, editors. Neurological Rehabilitation, Optimizing Motor Performance. Oxford: Reed Educational and Professional Publishing Ltd; 1999. pp. 126–153. [Google Scholar]
- 3.Edwards MG, Humphreys GW. Pointing and grasping in unilateral visual neglect: effect of on-line visual feedback in grasping. Neuropsychologia. 1999;37:959–973. doi: 10.1016/s0028-3932(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 4.Eng JJ, Winter DA, Mackinnon CD, Patla AE. Interaction of the Reactive Moments and Centre of Mass Displacement for Postural Control During Voluntary Arm Movements. Neuroscience Research Communications. 1992;11:73–80. [Google Scholar]
- 5.Jeannerod M. The neural and behavioural organization of goal-directed movements. Oxford: Clarendon Press; 1990. [Google Scholar]
- 6.Parker VM, Wade DT, Langton Hewer R. Loss of arm function after stroke: measurement, frequency, and recovery. International Rehabilitation Medicine. 1986;8:69–73. doi: 10.3109/03790798609166178. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Compensation in Recovery of Upper Extremity Function After Stroke: The Copenhagen Stroke Study. Archives of Physical Medicine. 1994;75:852–857. doi: 10.1016/0003-9993(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 8.Eng JJ, Rowe SJ, McLaren L. Mobility Status during Inpatient Rehabilitation: A comparison of Patients with Stroke and Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation. doi: 10.1053/apmr.2002.31203. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. Brief Research Report – International Journal of Rehabilitation Research. 1988;11:181–183. [Google Scholar]
- 10.Balliet R, Levy B, Blood KM. Upper extremity sensory feedback therapy in chronic cerebrovascular accident patients with impaired expressive aphasia and auditory comprehension. Archives of Physical Medicine and Rehabilitation. 1986;67:304–310. [PubMed] [Google Scholar]
- 11.deSouza LH, Langton Hewer R, Miller S. Assessment of recovery of arm control in hemiplegic stroke patients. Arm Function Test. International Rehabilitation Medicine. 1980;2:3–9. doi: 10.3109/09638288009163947. [DOI] [PubMed] [Google Scholar]
- 12.Eng JJ, Winter DA, Patla AE. Intralimb dynamics simplify reactive control strategies during walking. Journal of Biomechanics. 1997;30:581–588. doi: 10.1016/s0021-9290(97)84507-1. [DOI] [PubMed] [Google Scholar]
- 13.Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119:281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- 14.Reinkensmeyer DJ, Kahn LE, Averbuch M, McKenna-Cole A, Schmit BD, Rymer WZ. Understanding and treating arm movement impairment after chronic brain injury: Progress with the ARM Guide. Journal of Rehabilitation Research and Development. 2000;37:653–662. [PubMed] [Google Scholar]
- 15.Lum PS, Burgar CG, Kenney DE, Van der Loos HF. Quantification of force abnormalities during passive and active-assisted upper-limb reaching movements in post-stroke hemiparesis. IEEE Transactions and Biomedical Engineering. 1999;46:652–662. doi: 10.1109/10.764942. [DOI] [PubMed] [Google Scholar]
- 16.Winter DA, Olney SJ. Adaptability of motor patterns in pathological gait. In: Winter JM, Woo SL, editors. Multiple Muscle Systems. New York: Springer-Verlag; 1990. pp. 680–693. [Google Scholar]
- 17.van der Lee JH, Snels IA, Beckerman H, Lankhorst GJ, Wagenaar RC, Bouter LM. Exercise therapy for arm function in stroke patients: a systematic review of randomized control trials. Clinical Rehabilitation. 2001;15:20–31. doi: 10.1191/026921501677557755. [DOI] [PubMed] [Google Scholar]
- 18.Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Archives of Physical Medicine and Rehabilitation. 1993;74:347–354. [PubMed] [Google Scholar]
- 19.Gracies JM, Simpson DM. Botulinum Toxin Therapy. Neurologist. 2000;6:98–115. [Google Scholar]
- 20.Volpe BT, Krebs HI, Hogan N, Edelstein L, Diels MA, Aisen M. A novel approach to stroke rehabilitation, Robot-aided sensorimotor stimulation. Neurology. 2000;54:1938–1944. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- 21.Dean CM, Shepherd RB. Task-related training improves performance of seated reaching tasks after stroke. A randomized controlled trial. Stroke. 1997;28:722–728. doi: 10.1161/01.str.28.4.722. [DOI] [PubMed] [Google Scholar]
- 22.Kaminski TR, Bock C, Gentile AM. The coordination between trunk and arm motion during pointing movements. Experimental Brain Research. 1995;106:457–466. doi: 10.1007/BF00231068. [DOI] [PubMed] [Google Scholar]
- 23.Latash M, Anson J. What are “normal movements” in atypical populations? Behavioural and Brain Sciences. 1996;19:55–106. [Google Scholar]
- 24.Gribble PL, Ostry DJ. Compensation for Interaction Torques During Single- and Multijoint Limb Movements. Journal of Neurophysiology. 1999;82:2310–2326. doi: 10.1152/jn.1999.82.5.2310. [DOI] [PubMed] [Google Scholar]
- 25.Scott SH. Role of motor cortex in coordinating multijoint movements: Is it time for a new paradigm? Canadian Journal of Physiology and Pharmacology. 2000;78:923–933. [review] [PubMed] [Google Scholar]
- 26.Feldman AG, Levin MF. The origin and use of positional frames of reference in motor control. Behavioural and Brain Sciences. 1995;18:723–744. [Google Scholar]
- 27.Hollerbach JM. Planning of arm movements. In: Osherson DN, Kosslyn SM, Hollerbach JM, editors. Visual cognition and action: an invitation to cognitive science. Vol. 2. Cambridge: MIT Press; 1990. pp. 183–211. [Google Scholar]
- 28.Morasso P. Spatial control of arm movements. Experimental Brain Research. 1981;42:223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- 29.Brooks VB. Controlled variables. In: Brooks VB, editor. The neural basis of motor control. New York: Oxford University Press; 1986. pp. 129–149. [Google Scholar]
- 30.Shademehr R, Moussavi ZMK. Spatial Generalization from Learning Dynamics of Reaching Movements. Journal of Neuroscience. 2000;20:7807–7815. doi: 10.1523/JNEUROSCI.20-20-07807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan JH, Stelmach GE, Thomas JR, Thomas KT. Developmental Features of Rapid Aiming Arm Movements Across the Lifespan. Journal of Motor Behavior. 2000;32:121–140. doi: 10.1080/00222890009601365. [DOI] [PubMed] [Google Scholar]
- 32.Fitts P. The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology. 1954;47:381–391. [PubMed] [Google Scholar]
- 33.Flanagan RJ, Ostry DJ, Feldman AG. Control of trajectory modifications in target-directed reaching. Journal of Motor Behavior. 1993;25:175–192. doi: 10.1080/00222895.1993.9942045. [DOI] [PubMed] [Google Scholar]
- 34.Lacquaniti F. Automatic control of limb movement and posture [review] Current Opinions in Neurobiology. 1992;2:807–814. doi: 10.1016/0959-4388(92)90138-b. [DOI] [PubMed] [Google Scholar]
- 35.Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. Journal of Neurophysiology. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- 36.Flanders M, Tillery SIH, Soechting JF. Early stages in a sensorimotor transformation. Behavioral Brain Sciences. 1992;15:309–362. [Google Scholar]
- 37.Kelso JAS, Tuller B. Toward a theory of apractic syndromes. Brain Language. 1981;12:224–227. doi: 10.1016/0093-934x(81)90016-x. [DOI] [PubMed] [Google Scholar]
- 38.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112:749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- 39.Bourbonnais D, Vanden Noven S, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain. 1989;112:85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- 40.Gowland C, deBruin H, Basmajian JV, Plews N, Burcea I. Agonist and antagonist activity during voluntary upper-limb movement in patients with stroke. Physical Therapy. 1992;72:624–633. doi: 10.1093/ptj/72.9.624. [DOI] [PubMed] [Google Scholar]
- 41.Medical Research Council. Aids to the Examination of the Peripheral Nervous System. London: Her Majesty’s Stationary Office; 1976. [Google Scholar]
- 42.van der Ploeg RJO, Oosterhuis HJGH, Reuvekamp J. Measuring muscle strength. Journal of Neurology. 1984;231:200–203. doi: 10.1007/BF00313939. [DOI] [PubMed] [Google Scholar]
- 43.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Physical Therapy. 1996;67:931–933. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 44.Bohannon RW. Muscle strength changes in hemiparetic stroke patients during inpatient rehabilitation. Journal of Neurological Rehabilitation. 1988;2:163–166. [Google Scholar]
- 45.Bohannon RW, Andrews AW. Interrater reliability of hand-held dynamometry. Physical Therapy. 1987;67:931–933. doi: 10.1093/ptj/67.6.931. [DOI] [PubMed] [Google Scholar]
- 46.Bohannon RW. Determinants of transfer capacity in patients with hemiplegia. Physiotherapy Canada. 1988;40:236–239. [Google Scholar]
- 47.Levin MF, Hui-Chan C. Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? Journal of Neurology. 1993;240:63–71. doi: 10.1007/BF00858718. [DOI] [PubMed] [Google Scholar]
- 48.Lance JW. Symposium Synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: Disordered Motor Control. Chicago: Year Book Publishers; 1980. pp. 485–494. [Google Scholar]
- 49.Ashworth B. Preliminary trial of carisprodol in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 50.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 51.Harburn KL, Hill KM, Vadervoort AA, Helewa A, Goldsmith C, Chareles H, Kertesz A, Teasell RW. Spasticity and Measurement in Stroke: A Pilot Study. Canadian Journal of Public Health Supplement. 1992;2:S41–45. [PubMed] [Google Scholar]
- 52.Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Archives of Physical Medicine and Rehabilitation. 1989;70:144–155. [Review] [PubMed] [Google Scholar]
- 53.Djergaian RS. Management of musculoskeletal complications. In: Horn LJ, Zasler ND, editors. Medical rehabilitation of traumatic brain injury. Philadelphia: Hanley & Belfus; 1996. pp. 459–477. [Google Scholar]
- 54.Doraisamy P. Spasticity - Implications in Rehabilitation. Annals Academy of Medicine. 2000;21:414–419. [PubMed] [Google Scholar]
- 55.Winters JM, Stark L. Muscle Models: What is Gained and What is Lost by Varying Model Complexity. Biological Cybernetics. 1987;55:403–420. doi: 10.1007/BF00318375. [DOI] [PubMed] [Google Scholar]
- 56.Crow JL, Lincoln NNB, Nouri FM, De Weerdt W. The effectiveness of EMG biofeedback in the treatment of arm function after stroke. International Disability Studies. 1989;11:155–160. doi: 10.3109/03790798909166667. [DOI] [PubMed] [Google Scholar]
- 57.Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. Journal of Neurology, Neurosurgery, and Psychiatry. 1989;52:1267–1272. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collin C, Wade DT. Assessing motor impairment after stroke: a pilot reliability study. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53:576–579. doi: 10.1136/jnnp.53.7.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carr JH, Shepherd RB, Nordholm L, Lynne D. Investigation of a new motor assessment scale for stroke patients. Physical Therapy. 1985;65:175–180. doi: 10.1093/ptj/65.2.175. [DOI] [PubMed] [Google Scholar]
- 60.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. Poststroke hemiplegic patient: evaluation of physical performance. Scandanavian Journal of Rehabilitation Medicine. 1975;7:13–31. [PubMed] [Google Scholar]
- 61.Berglund K, Fugl-Meyer AR. Upper extremity function in hemiplegia. A cross-validation study of two assessment methods. Scandanavian Journal of Rehabilitation Medicine. 1986;18:155–157. [PubMed] [Google Scholar]
- 62.Duncan PW, Propst M, Nelson SG. Reliability of Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Physical Therapy. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 63.Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proceedings of the National Academy of Sciences. 1999;96:4645–4649. doi: 10.1073/pnas.96.8.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trombly CA. Observations of improvement in reaching in five subjects with left hemiparesis. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56:40–45. doi: 10.1136/jnnp.56.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wing AM, Lough W, Turton A, Fraser C, Jenner JR. Recovery of elbow function in voluntary positioning of the hand following hemiplegia due to stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53:126–134. doi: 10.1136/jnnp.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 67.Roby-Brami A, Fuchs S, Mokhtari M, Bussel B. Reaching and Grasping Strategies in Hemiparetic Patients. Motor Control. 1997;1:72–91. [Google Scholar]
- 68.Trombly CA. Deficits of reaching in subjects with left hemiparesis: a pilot study. American Journal of Occupational Therapy. 1992;46:887–897. doi: 10.5014/ajot.46.10.887. [DOI] [PubMed] [Google Scholar]
- 69.Trombly CA, Wu CY. Effect of rehabilitation tasks on organization of movement after stroke. American Journal of Occupational Therapy. 1999;53:333–344. doi: 10.5014/ajot.53.4.333. [DOI] [PubMed] [Google Scholar]
- 70.Wu C, Wong M, Lin K, Chen J. Effects of task goal and personal preference on seated reaching kinematics after stroke. Stroke. 2001;32:70–76. doi: 10.1161/01.str.32.1.70. [DOI] [PubMed] [Google Scholar]
- 71.Beer R, Dewald J, Rymer Z. Disturbances of voluntary movement in stroke: problems of planning or execution? Progress in Brain Research. 1999;123:455–460. doi: 10.1016/s0079-6123(08)62881-2. [DOI] [PubMed] [Google Scholar]
- 72.Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Experimental Brain Research. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- 73.Reinkensmeyer DJ, Schmit BD, Rymer WZ. Assessment of Active and Passive Restraint During. Guided Reaching After Chronic Brain Injury. Annals of Biomedical Engineering. 27:805–81. doi: 10.1114/1.233. [DOI] [PubMed] [Google Scholar]
- 74.Given JD, Dewald JPA, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;59:271–279. doi: 10.1136/jnnp.59.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldspink G, Williams PE. Muscle fiber and connective tissue changes associated with use and disuse. In: Ada L, Canning C, editors. Foundations for Practice: Topics in Neurological Pysiotherapy. London: Heinemann; 1990. pp. 197–218. [Google Scholar]
- 76.Desmurget M, Jordan M, Prablanc C, Jeannerod M. Constrained and unconstrained movements involve different control strategies. Journal of Neurophysiology. 1977;77:1644–1650. doi: 10.1152/jn.1997.77.3.1644. [DOI] [PubMed] [Google Scholar]
- 77.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. A Mechanical Model to Study the Relationship Between Gait Speed and Muscular Strength. IEEE Transactions on Rehabilitation Engineering. 1996;4:386–394. doi: 10.1109/86.547940. [DOI] [PubMed] [Google Scholar]
- 78.Olney SJ, Griffen MP, McBride ID. Temporal, Kinematic, and Kinetic Variables Related to Gait Speed in Subjects with Hemiplegia: A Regression Approach. Physical Therapy. 1994;74:872–885. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- 79.Bernhardt J, Bate PJ, Matyas TA. Accuracy of Observational Kinematic Assessment of Upper-Limb Movements. Physical Therapy. 1998;78:259–270. doi: 10.1093/ptj/78.3.259. [DOI] [PubMed] [Google Scholar]