Abstract
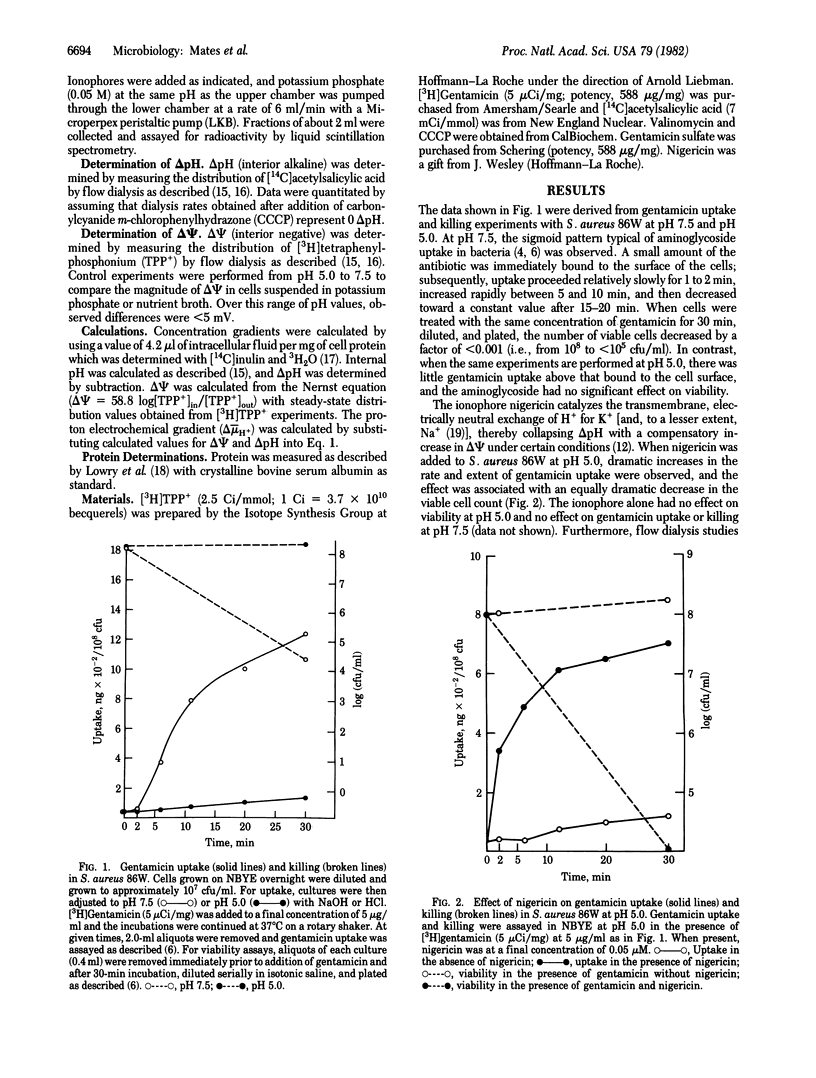
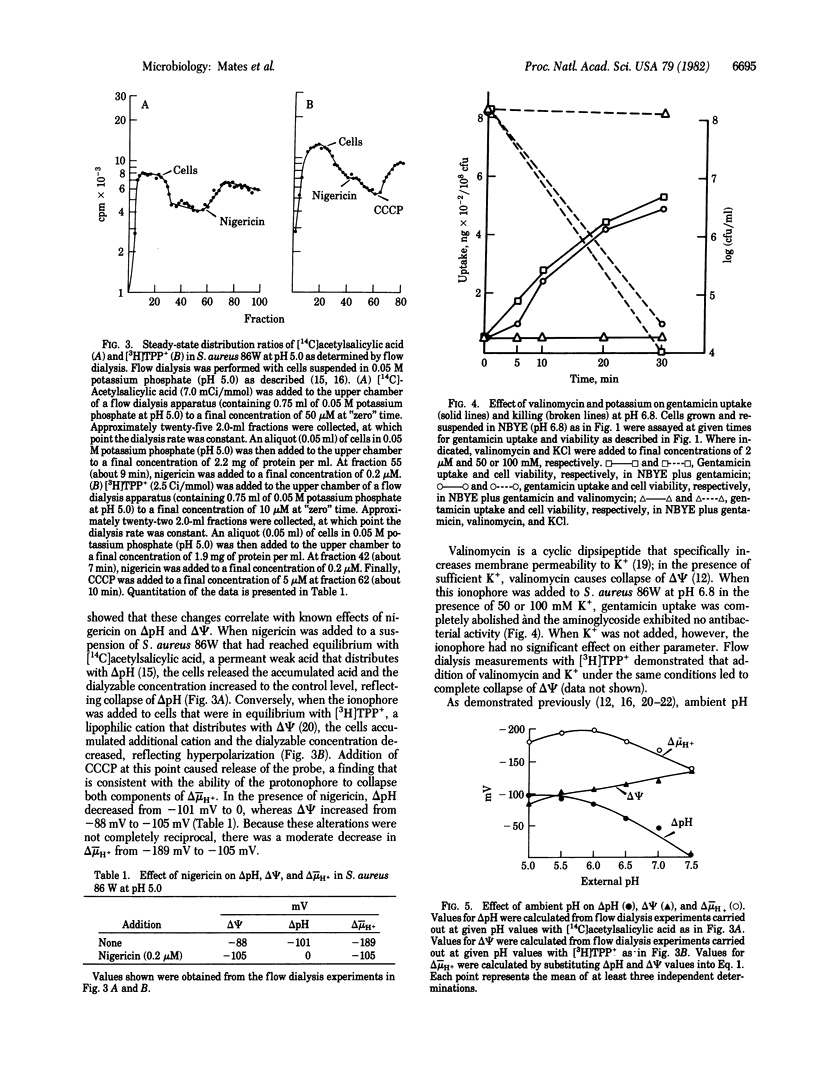
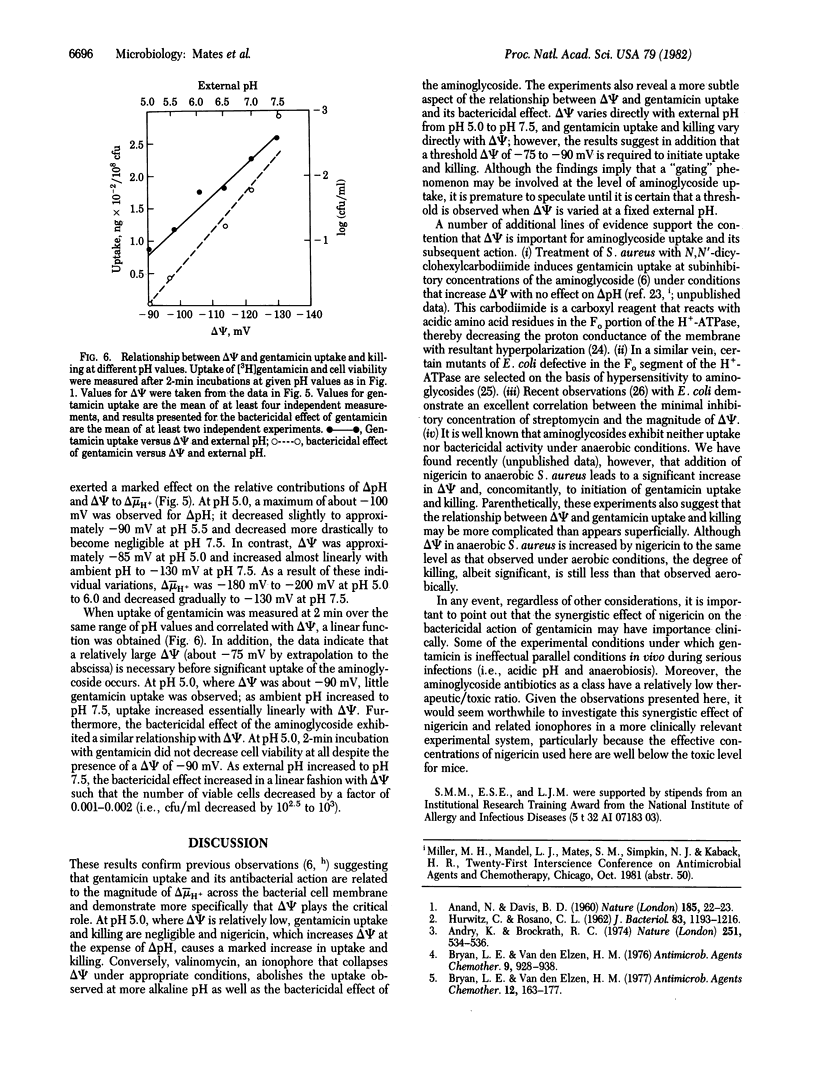
At pH 5.0, the electrical potential (delta psi, interior negative) across the plasma membrane of Staphylococcus aureus exhibits a minimum of -85 to -90 mV; the pH gradient (delta pH, interior alkaline) across the membrane approximates a maximum of about -100 mV. Under these conditions, uptake of the aminoglycoside gentamicin is negligible, and viability of the organism is not impaired by the antibiotic. In contrast, at pH 7.5, at which delta psi is about -130 mV and delta pH is 0, gentamicin uptake is observed and the drug markedly decreases viability. Dramatically, when the ionophore nigericin is added at pH 5.0, gentamicin uptake is induced, there is a striking decrease in viability, and the effect is associated with an increase in delta psi at the expense of delta pH. Consistently, valinomycin, which dissipates delta psi in the presence of potassium, abolishes gentamicin uptake and killing. In addition, from pH 5.0 to pH 7.5, there is a direct relationship between the magnitude of delta psi and both gentamicin uptake and its bactericidal effect. However, a threshold delta psi of -75 to -90 mV is apparently necessary to initiate uptake and killing. These observations provide a strong indication that delta psi plays a critical role in the uptake and antibacterial action of gentamicin and suggest that nigericin-like ionophores may be clinically useful in synergy with aminoglycosides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D. Damage by streptomycin to the cell membrane of Escherichia coli. Nature. 1960 Jan 2;185:22–23. doi: 10.1038/185022a0. [DOI] [PubMed] [Google Scholar]
- Ahmad M. H., Rechenmacher A., Böck A. Interaction between aminoglycoside uptake and ribosomal resistance mutations. Antimicrob Agents Chemother. 1980 Nov;18(5):798–806. doi: 10.1128/aac.18.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andry K., Bockrath R. C. Dihydrostreptomycin accumulation in E. coli. Nature. 1974 Oct 11;251(5475):534–536. doi: 10.1038/251534a0. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damper P. D., Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981 Dec;20(6):803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie P., Bryan L. E., Pickard M. A. Effect of enzymatic adenylylation on dihydrostreptomycin accumulation in Escherichia coli carrying an R-factor: model explaining aminoglycoside resistance by inactivating mechanisms. Antimicrob Agents Chemother. 1978 Oct;14(4):569–580. doi: 10.1128/aac.14.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- Friedberg I., Kaback H. R. Electrochemical proton gradient in Micrococcus lysodeikticus cells and membrane vesicles. J Bacteriol. 1980 May;142(2):651–658. doi: 10.1128/jb.142.2.651-658.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ C., ROSANO C. L., LANDAU J. V. Kinetics of loss of vibility of Escherichia coli exposed to streptomycin. J Bacteriol. 1962 Jun;83:1210–1216. doi: 10.1128/jb.83.6.1210-1216.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V. Induction of streptomycin uptake in resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1979 Feb;15(2):177–181. doi: 10.1128/aac.15.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller M. H., Wexler M. A., Steigbigel N. H. Single and combination antibiotic therapy of Staphylococcus aureus experimental endocarditis: emergence of gentamicin-resistant mutants. Antimicrob Agents Chemother. 1978 Sep;14(3):336–343. doi: 10.1128/aac.14.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. H., Wexler M. A., Steigbigel N. H. Single and combination antibiotic therapy of Staphylococcus aureus experimental endocarditis: emergence of gentamicin-resistant mutants. Antimicrob Agents Chemother. 1978 Sep;14(3):336–343. doi: 10.1128/aac.14.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir M. E., Wallace B. J. Isolation of mutants of Escherichia coli uncoupled in oxidative phosphorylation using hypersensitivity to streptomycin. Biochim Biophys Acta. 1979 Aug 14;547(2):218–229. doi: 10.1016/0005-2728(79)90005-7. [DOI] [PubMed] [Google Scholar]
- Patel L., Schuldiner S., Kaback H. R. Reversible effects of chaotropic agents on the proton permeability of Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3387–3391. doi: 10.1073/pnas.72.9.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. pH-dependent changes in proton:substrate stoichiometries during active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Sep 20;16(19):4270–4275. doi: 10.1021/bi00638a022. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]



