Abstract
The control and execution of movement could potentially be altered by the presence of stroke-induced weakness if muscles are incapable of generating sufficient power. The purpose of this study was to identify compensatory strategies during a forward (sagittal) reaching task for twenty persons with chronic stroke and ten healthy age-matched controls. We hypothesized that the paretic anterior deltoid would be maximally activated (i.e., saturated) during a reaching task and that task completion would require activation of additional muscles, resulting in compensatory movements out of the sagittal plane. For reaching movements by control subjects, joint motion remained largely in the sagittal plane and hand trajectories were smooth and direct. Movement characteristics of the non-paretic arm of stroke subjects were similar to control subjects except for small increases in the abduction angle and the percentage that anterior deltoid was activated. In contrast, reaching movements of the paretic arm of stroke subjects were characterized by increased activation of all muscles, especially the lateral deltoid, in addition to the anterior deltoid, with resulting shoulder abduction power and segmented and indirect hand motion. For the paretic arm of stroke subjects, muscle and kinetic compensations increased with impairment severity and weaker muscles were used at a higher percentage of their available muscle activity. These results suggest that the inability to generate sufficient force with the typical agonists involved during a forward reaching task may necessitate compensatory muscle recruitment strategies to complete the task.
Keywords: Upper extremity, arm, motor control, biomechanics, EMG, rehabilitation
INTRODUCTION
The upper extremity remains severely impaired in over 50% of persons with stroke despite intensive and prolonged rehabilitation (Nakayama et al. 1994). Reaching function is critical to daily activities (Granger et al. 1986) and, in persons with stroke, its performance is strongly correlated with upper extremity impairment (e.g., Levin 1996; Lum et al. 1999; Kamper et al. 2002).
Reaching is a task of motor abundance as the number of joints available for movement and their coordination is unrestricted (Bernstein 1967). Despite the infinite number of possible movement patterns, hand and joint motions are similar across the healthy population (Kaminski et al. 1995). Joint abundance provides the functional ability to control movements adaptively to account for both environmental factors and internal constraints, such as injury (Latash 1996). Impairments that may potentially constrain reaching performance following stroke include altered intersegmental dynamics (Beer et al. 2000), impaired sensation (Zackowski et al. 2004), spasticity (Levin, 1996), and muscle weakness (Mercier and Bourbonnais, 2004). It is known that constraints from stroke impairments can result in the need for additional degrees of freedom at other joints such as movement compensations of the trunk during reaching (Cirstea and Levin 2000).
The gravitational forces during reaching to an elevated target are particularly challenging for individuals with stroke (Reinkensmeyer et al. 1999). We believe these demands would require compensations such as altered muscle activation. In fact, Trombly et al. (1992) have suggested that individuals with stroke use a higher fraction of their maximum muscle activity with their paretic (versus non-paretic) arm during reaching. As their results were inconclusive with a small sample (n=5), we propose to test if paretic muscles approach saturation (i.e., muscle activity approaches capacity). We also postulated that a saturation of muscle activation is related to muscle weakness and that saturation would require recruitment of additional muscles, resulting in movements out of the sagittal plane. Such a hypothesis is driven by clinical observations that individuals with stroke often use stereotypical out-of-plane reaching movement strategies (Carr and Shepherd, 1999).
Dynamic analyses in the lower extremity have elucidated a number of compensatory kinematic and kinetic movement strategies during gait following stroke (de Quervain et al 1996). For example, increased hip abduction power (i.e., hip hiking) of the paretic limb can compensate for deficits in ankle plantarflexion push-off in order to preserve functional gait (Kim and Eng 2004). Such non-sagittal compensations have yet to be analyzed for reaching movements. We hypothesized that age-matched controls would perform reaching movements primarily in the sagittal plane because task appropriate muscles are used substantially below capacity. Conversely, we hypothesized that the paretic arm of individuals with stroke would demonstrate saturation of their muscle activity during the reaching activity and require recruitment (compensation) from other muscles resulting in out-of-plane components during the task.
METHODS
Participants
Twenty older adults (Age: Mean=60.9, SD=6.1, Range=49–72 years; Sex: 13 males and 7 females) were recruited from the community with the following inclusion criteria: 1) a minimum of one year post-stroke, 2) present with hemiparesis secondary to first cerebrovascular accident (CVA), 3) able to provide informed consent, 4) able to follow one and two step commands and 5) able to voluntarily flex/abduct their shoulder 45 degrees and extend their elbow 120 degrees. None of the subjects with stroke presented with hemispatial neglect (Schenkenberg et al. 1980). Ten right-handed healthy adults of similar age (Mean=61.0, SD=9.0, Range=51–77 years) and gender (6 males and 4 females) were recruited from the community to serve as the control group. Musculoskeletal or neurological conditions (in addition to the CVA for the stroke participants) that would affect arm function were exclusion criteria for all participants. The characteristics of the participants with stroke are described in Table 1. To identify stroke-induced changes to reaching performance, we used the non-dominant arm of healthy subjects for comparison (‘control condition’). As our central purpose was to identify the effect of stroke on reaching performance, we used only the non-dominant arm of control subjects for comparison. Motor control of the dominant arm is superior to the non-dominant arm in healthy individuals (Sainburg and Kalakanis, 2000) so this represented a conservative approach of identifying stroke related deficits. Local university and hospital ethics committees approved the study protocol.
Table 1.
Characteristics of Chronic Stroke Participants
| Code | Sex | Age (yrs) | Time since stroke (yrs) | FM Motor Score (/66) | MAS Score (/4) | Dominant/Paretic Side | Injury Location/Type (Taken from chart review) |
|---|---|---|---|---|---|---|---|
| SR01 | M | 57 | 6 | 19 | 1 | R/R | Sub-cortical/Hemorrhagic |
| SR02 | F | 64 | 4 | 64 | 1 | R/R | Cortical/Hemorrhagic |
| SR03 | M | 67 | 5 | 59 | 0 | R/L | Sub-cortical/Hemorrhagic |
| SR04 | M | 66 | 4 | 64 | 1 | R/R | Cortical/Hemorrhagic |
| SR05 | F | 60 | 3 | 26 | 1+ | R/L | Sub-cortical/Ischemic |
| SR08 | M | 59 | 5 | 18 | 3 | R/R | Sub-cortical/Ischemic |
| SR09 | F | 59 | 2 | 38 | 3 | R/L | Sub-cortical/Ischemic |
| SR10 | M | 57 | 8 | 62 | 0 | L/L | Sub-cortical/Ischemic |
| SR11 | M | 59 | 1 | 25 | 1 | R/R | Sub-cortical/Ischemic |
| SR12 | M | 58 | 5 | 36 | 1 | R/R | Cortical/Ischemic |
| SR13 | M | 63 | 11 | 41 | 1 | L/R | Cortical/Ischemic |
| SR14 | F | 67 | 2 | 62 | 0 | R/L | Cortical/Hemorrhagic |
| SR15 | F | 69 | 2 | 34 | 1+ | R/R | Sub-cortical/Ischemic |
| SR16 | M | 50 | 1 | 57 | 0 | R/L | Sub-cortical/Hemorrhagic |
| SR17 | M | 61 | 5 | 15 | 1 | R/R | Sub-cortical/Ischemic |
| SR18 | M | 72 | 4 | 18 | 4 | R/L | Sub-cortical/Ischemic |
| SR19 | M | 56 | 3 | 14 | 1+ | L/R | Cortical/Hemmorrhagic |
| SR20 | F | 57 | 7 | 55 | 1 | R/R | Cortical/Ischemic |
| SR21 | F | 49 | 1 | 44 | 2 | R/R | Sub-cortical/Hemorrhagic |
| SR22 | M | 66 | 7 | 13 | 1 | R/R | Sub-cortical/Ischemic |
|
| |||||||
| 13M/7F | 60.9±6.1 | 4.3±2.6 | 38.2±19.0 | 1.3±1.1 | 17 R (3L)/13 R (7L) | 7 Cortical/13 Sub-cortical | |
Motor impairment of the paretic arm in participants with stroke was assessed by the upper extremity motor component of the Fugl-Meyer scale (Fugl-Meyer et al. 1975) and by the Modified Ashworth Scale (MAS) for hypertonia (Bohannon and Smith 1987) (0 = no increase in muscle tone and 4 = rigid). Twelve participants with stroke were evaluated a second time two to three days following the first assessment to establish inter-session reliability.
Experimental Setup
Participants sat in a chair with their arm relaxed and their hand resting on their ipsilateral thigh (i.e., shoulder adducted, elbow in mid-flexion, and forearm pronated) and reached to a shoulder height target (i.e., glenohumeral joint). Active range of motion was assessed while the subject was in the experimental setup and the target (3x3 cm square) was located just inside the workspace limits of the paretic arm of the subjects with stroke or the non-dominant arm of the control subjects. Thus, the target distance was identical for the paretic and non-paretic arm of the subjects with stroke. The horizontal forward distance (shoulder to finger tip) was a mean of 66.3 cm (min=59.4, max=74.7) for the control group and 56.2 cm (min=39.5, max=76.9) for the stroke group.
Participants performed five unconstrained reaching movements to the target. For each trial, participants started in a relaxed state and were instructed: “At the sound of the tone (an auditory cue), reach and touch the target at as fast as possible.” Participants touched the target with their pointing finger (index finger tip or index distal interphalangal joint if unable to extend the interphalangal joint). Crossing lap belts (extending from just above the shoulder to the opposite hip) supported the torso to prevent trunk and hip movement.
Participants also performed three isometric maximal voluntary contractions (MVC) of upper extremity movements (shoulder flexion, extension, abduction, adduction, internal and external rotation and elbow flexion and extension) in mid-range using the isometric mode of a dynamometer system (KinCom, Chattanooga, TN). The isometric protocol has been described in detail previously (McCrea et al. 2003).
Data Collection
Three non-collinear infrared emitting diodes (IREDs) were placed on each upper limb segment (hand, forearm, and upper arm) and registered relative to anatomical landmarks. IRED movements were tracked by an optoelectronic sensor (Northern Digital Optotrak) at 60 Hz and then filtered using a second-order Butterworth low-pass filter at 10Hz. All movements were represented in terms of the right arm (left arm movements were reflected across the mid-sagittal plane). Movement initiation and cessation were identified from the tangential velocity profile of an IRED attached to the pointer (i.e., tip of index finger). Movement began and terminated when the tangential velocity rose above and fell below 5% of the peak velocity (relative to zero baseline).
Muscle activation was recorded during the reaching movement by surface electrodes (self-adhesive, silver, silver-chloride pellet electrodes with 7mm diameter, fixed interelectrode distance of 30 mm, Kendall Meditrace, Chicopee, MA) placed on the anterior and lateral heads of the deltoid, the long head of the triceps, the biceps brachium, and the brachioradialis. Electromyograms (EMGs) were collected (Bortec Electronics, Calgary, Canada), converted analog to digital (600 Hz) (AT-MIO-64E-3, National Instruments, Austin, TX), and low-pass filtered at 100 Hz. EMG was also recorded during MVCs, along with the peak torque. The isometric protocol has been reported previously (McCrea et al. 2003).
Neuromuscular Force and Activity
EMG profiles were converted to a time base of 0 to 100% of movement. For each muscle, the mean EMG during reaching was normalized by the EMG at MVC to quantify saturation (i.e., % muscle utilization). The peak torque during the MVC was also recorded to provide an indication of muscle strength.
Joint Configuration and Mechanics
Right-handed coordinate systems (direction of axes in anatomical position: X=medial-lateral, Y= posterior-anterior, Z=inferior-superior) were embedded within each segment and rigid body calculations were used to track their global position and location on a frame-by-frame basis. Local joint angles, velocities, moments, and powers were described as the relative motion of the distal segment to the more proximal segment. The observed joint angles were computed using an Euler sequence: rotation 1 about the X-axis is a pure flexion/extension movement, rotation 2 about the Y’-axis is an adduction/abduction movement and rotation 3 about the Z’’ axis is a pure rotation about the longitudinal internal-external movement. These angles are referred to as α, β, and γ and correspond approximately to clinical descriptions of flexion/extension, ad/abduction, and in/external rotation angles; they have previously been used to describe motions in the upper extremity (Prokopenko et al. 2001). Euler sequences describe the rotations of a set of three joints in a fictitious mechanism that represents the three degrees of rotational freedom allowed at the shoulder joint. At each point in time, the humerus’ current orientation was represented by an Euler angle sequence (note that Euler angles represent instantaneous orientation and do not imply that the humerus has gone through any particular temporal sequence of rotations).
Limb inertias were estimated by using anthropometric tables by Yeadon and Morlock (1989). Muscle joint moments were calculated by a recursive Newton-Euler method (Meglan 1991). Mechanical powers were computed as the dot product of joint moments and velocities and represented about each rotational axis (i.e., flexor/extensor power, adductor/abductor power, and internal/external rotation power). For peak powers, positive power represents a generation of energy resulting from a concentric contraction while negative power represents absorption of energy resulting from an eccentric contraction. Moment and power analyses during reaching have previously been shown to be sensitive indicators of neurological impairment (e.g., Riener and Straube 1997).
Preliminary inspection of profiles indicated that, regardless of arm condition (paretic, non-paretic, control), reaching tasks were executed without rotations of the wrist or forearm (in part, because our task did not require a specific hand orientation). Subsequent analyses, therefore, were restricted to flexion-extension of the elbow and the three rotational axes of the shoulder. To facilitate comparisons between subjects, all profiles were converted to a time base that extended from 0 to 100% of movement; kinetic profiles (i.e., powers and moments) were additionally normalized by subject mass. From individual profiles we calculated mechanical effort (peak powers) and change in arm positioning (change in joint angles). For each trial, reaching strategies were determined by analyzing the patterns of joint trajectories (or bursts in the case of power profiles).
Hand Path & Trajectory
Kinematic descriptors of pointer movement were derived from path and trajectory profiles of each trial. Path directness, the ratio of the direct to the actual path length (Bastian et al. 1996), quantified the ability to execute a straight-line path. Segmentation and skewness parameters described the tangential velocity profile. Segmentation is a count of velocity peaks and is proposed to relate to the number of motor commands required to execute a movement (Krebs et al. 1999). More specifically, our segmentation algorithm identified peaks as local maxima separated by at least 100 ms (i.e., less than 10 Hz). A statistical definition of skewness (Zar 1999) measured the central tendency of the velocity profile. A velocity profile becomes asymmetric and positively skewed (i.e., peaks occur earlier) when accuracy requirements necessitate a controlled deceleration.
Analyses
Analyses were performed on the mean values (across trials) of hand path/trajectory, joint configuration, peak power, and muscle activation parameters. The parameters indicated generally excellent relative [intra-class correlations, ICC(1,1) (Shrout and Fleiss 1979)] and absolute [standard error of measurement, SEM, expressed as a percentage of the mean (Eliasziw et al. 1994)] reliability across two testing sessions. The exception (removed from further analyses because of poor reliability) was brachioradialis activation of the paretic arm. For other parameters, ICCs ranged from 0.74 to 0.96 (mean 0.87) in the paretic arm and from 0.64 to 0.96 (mean 0.80) in the non-paretic arm; SEMs ranged from 1.1 to 31.6 (mean 15.3) % in the paretic arm and from 4.1 to 33.5 (mean 16.9) % in the non-paretic arm. All subsequent analyses used parameter values from the first day.
Multivariate analyses of variance (MANOVAs) were used to assess the effect of arm condition (paretic, non-paretic, control) on hand path/trajectory, joint configuration, kinetic, and muscle utilization parameters with post-hoc analyses of variance (ANOVAs) and Duncan’s comparison tests to identify differences among arm conditions. The combined use of multivariate and univariate analyses allowed for global protection of type I error while maintaining the ability to identify differences within individual parameters (Zar 1999); multivariate F and Wilks-Lambda were calculated for MANOVAs (Zar 1999).
To determine the contribution of muscle strength, we correlated the percent of muscle utilization over the reaching task with the peak normalized isometric torque of their primary joint actions (e.g., anterior deltoid muscle utilization with shoulder flexion torque). In the paretic condition, Pearson correlations evaluated relations of motor impairment (Fugl-Meyer) against each parameter.
RESULTS
Muscle Activation
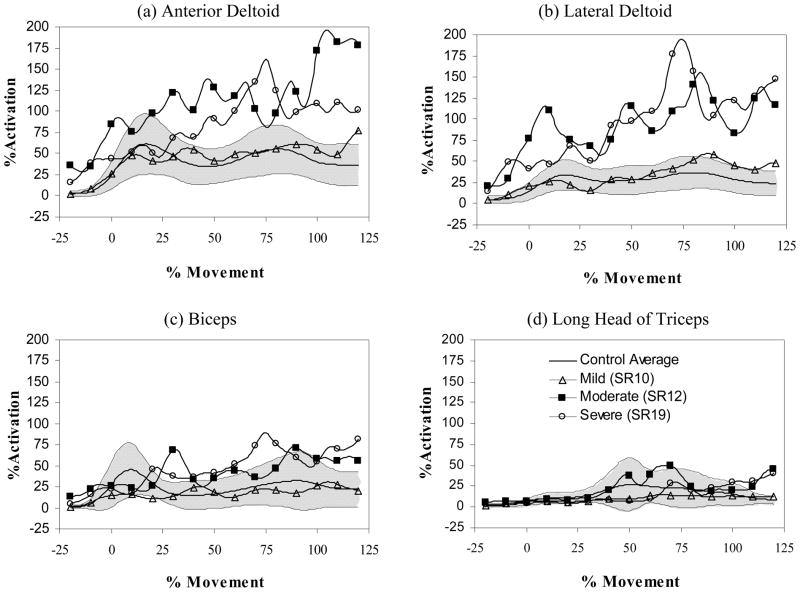
Muscle activity patterns were similar across the control subjects and unaltered by ensemble averaging. (i.e., the defining characteristics of the curve still occurred at specific times and with similar amplitudes) (see Figure 1). Consistent with previous studies of reaching in three dimensions (Flanders 1991) and the sagittal plane (Flanders et al. 1996), deltoid activity was characterized by phasic bursts occurring early and late in movement and by increased activity at movement termination; the magnitude of the EMG activities was largest for the anterior head and smallest for the lateral head. The same activation pattern was shared by biceps brachii. Triceps (long head) activity was characterized by a single burst occurring midway throughout movement. Activation patterns for the non-paretic arm condition were similar to those of the control condition.
Figure 1.
Normalized muscle activation profiles for the (a) anterior deltoid, (b) lateral deltoid, (c) biceps, and (d) triceps (long head). The percent of muscle utilization is shown on the vertical axis and normalized movement time on the horizontal axis. Profiles are shown for mild (open triangle), moderate (filled square), and severe (open circle) impairments of the paretic arm (single trial profiles from individual subjects are shown compared to the control average (N=10 subjects)) compared to the control average (solid line) and range (1 standard deviation – shaded region). Notice how muscle utilization patterns saturate and become irregular with increases in impairment. Mild, moderate and severe corresponded to approximately the lower, middle and upper third of the Fugl-Meyer range of scores for the paretic arm condition.
The EMG patterns in the control and non-paretic arm conditions, however, were not present in the paretic arm condition. Activation patterns were highly variable across participants and included pattern abnormalities such as segmented activation, co-activation, prolonged firing, and changes in recruitment and burst timing. There was a significant effect of arm condition on the relative muscle utilization (Wilks’ Lambda statistic 0.544, p<0.05, F(8,88)=3.12, p =.005). The relative muscle utilization was greater within the paretic arm condition for all muscles compared to the control conditions; most notable was the doubling of utilization in the anterior and lateral heads of the deltoid. Utilization was also correlated to increasing impairment severity within the paretic condition (see Table 2).
Table 2.
Percent of Muscle Activity
| Parameter | p | Control (n=10) | Non-paretic (n=20) | Paretic (n=20) | |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | FM Correlation | ||
| Anterior Deltoida | 0.008 | .443 (.152 to .733) | .617 (.434 to .908) | 1.023 (.800 to 1.246) | −0.677** |
| Lateral Deltoida | <0.001 | .300 (.083 to .518) | .347 (.170 to .525) | .844 (.678 to 1.011) | −0.548** |
| Biceps Brachialisa | 0.001 | .204 (.070 to .338) | .299 (.189 to .408) | .511 (.408 to .614) | −0.331 |
| Triceps Long Head | 0.240 | .189 (.014 to .363) | .239 (.097 to .381) | .361 (.227 to .495) | 0.085 |
P-values provided for the significance level of the ANOVA comparing the three arm conditions (control, non-paretic, paretic)
Post-hoc multiple comparison test significantly differentiated descriptor associated with the paretic arm from all other arm groups. Significance of Correlations:
p<.05,
p<.01.
Moderate to strong negative relationships were identified between peak torque (strength assessment) and muscle utilization values of the paretic arm condition, indicating that weak muscles are used at a higher proportion of their capacity during movement. More specifically, significant relationships existed between the peak torque and muscle utilization values for movements to lift the arm against gravity (biceps muscle utilization versus peak elbow flexion torque: r= −0.624, p=0.007; anterior deltoid muscle utilization versus peak shoulder flexion torque: r=−0.514, p=0.035; lateral deltoid muscle utilization versus peak shoulder abduction torque: r=−0.633, p=0.006). However, these relationships were not significant for the non-paretic or control arm conditions.
Kinetics
Shoulder moment of the control condition was predominantly flexor. In the early phase of movement, this moment was primarily due to joint acceleration and then asymptotically approached a value that was largely determined by the gravitational moment of the arm. The moment at the elbow was always flexor, increasing during elbow flexion (peak at ~ 15–30% of movement time) and decreasing during elbow extension. Moment profiles of the less affected arm condition were qualitatively similar to those of the arm of the control condition. In the more affected arm condition, there was a reduced flexor moment that coincided with a large increase in abduction moment; this compensation was particularly evident early in the movement profile. The flexor-extensor moment pattern of the elbow was similar to the control and non-paretic arm conditions, but more variable. Internal-external moment profiles of all arm conditions were bi-phasic (first internal then external) but were small in magnitude.
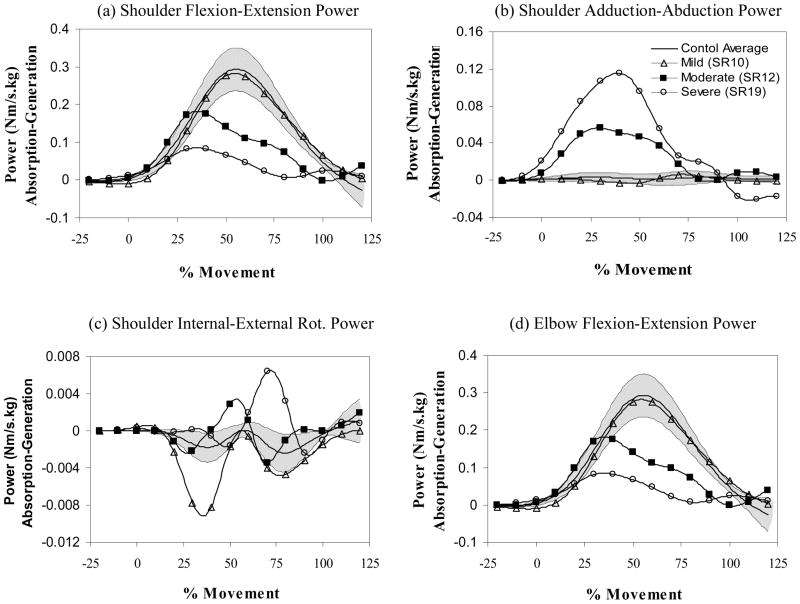
Shoulder power in the non-paretic and control conditions (see Figure 2) was primarily flexor and concentric (generation) in origin with a peak occurring slightly after the midpoint of movement. Elbow power was bi-phasic and, as expected, primarily along the flexion-extension axis; this power was initially concentric (generation) while the elbow flexed and eccentric (absorption) as the elbow extended under the force of gravity. There was a large increase in abductor generation power for the paretic arm condition that coincided with a large reduction in flexor generation power; 15 of the 20 subjects with stroke demonstrated greater than the maximum abductor power observed in control subjects. Elbow power remained bi-phasic in the paretic arm but with substantially reduced generation and absorption peaks.
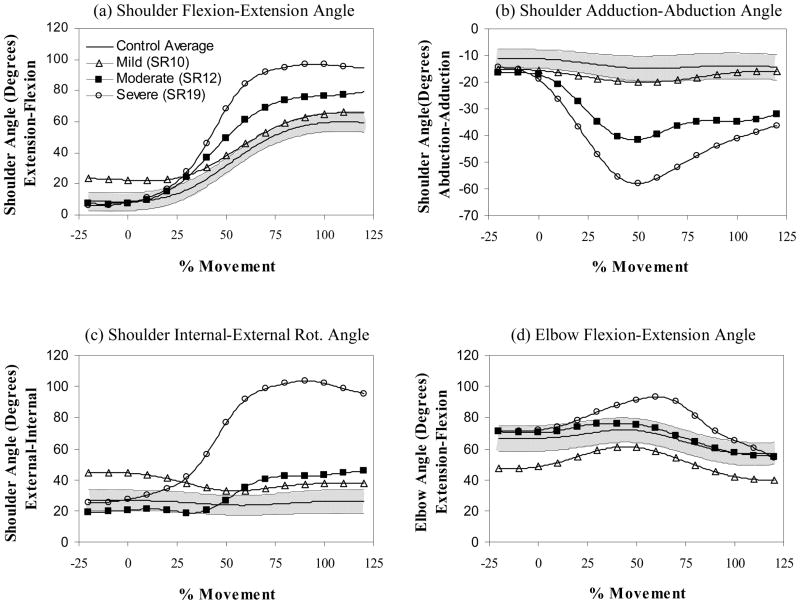
Figure 2.
Joint path profiles for (a) shoulder flexion-extension, (b) shoulder adduction-abduction, (c) shoulder internal-external rotation, and (d) elbow flexion-extension. Joint angle is shown on the vertical axis and normalized movement time on the horizontal axis. Profiles are shown for mild (open triangle), moderate (filled square), and severe (open circle) impairments of the paretic arm (single trial profiles from individual subjects are shown compared to the control average (N=10 subjects)) (solid line) and range (1 standard deviation – shaded region). Notice how the profiles become progressively more abducted and internally rotated with impairment. Mild, moderate and severe corresponded to approximately the lower, middle and upper third of the Fugl-Meyer range of scores for the paretic arm condition.
The MANOVA included peak power variables which substantially contributed to the total power (shoulder flexor generation, shoulder abductor generation and elbow flexor absorption). These peak power variables were significantly affected by arm condition (Wilks’ Lambda statistic of 0.501, p < 0.05; F(6, 90)=4.33, p < 0.001) and highly correlated with impairment level (see Table 3). The shifting of power generation in the shoulder from flexor to abductor was particularly noticeable: in the paretic condition, abductor power was 14.6% of the total generated power (abductor plus flexor power) while the abductor power was only 1.6% of the total generated power in the control condition.
Table 3.
Power Absorption and Generation
| Peak Powers (Nm/kgsec) | p | Control (n=10) | Non-paretic (n=20) | Paretic (n=20) | |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | FM Correlation | ||
| Shoulder Flexor (Concentric)a | 0.002 | .306 (.254 to .359) | .278 (.240 to .316) | .198 (.160 to .236) | 0.702** |
| Shoulder Abductor (Concentric)a | 0.003 | .005 (−.007 to .017) | .010 (.001 to .019) | .029 (.020 to .038) | −0.701** |
| Elbow Flexor (Eccentric)a | 0.006 | −4.184 (−5.466 to −2.903) | −3.379(−4.309 to −2.450) | −1.756 (−2.686 to −.827) | −0.665** |
P-values provided for the significance level of the ANOVA comparing the three arm conditions (control, non-paretic, paretic)
Post-hoc multiple comparison test significantly differentiated descriptor associated with the paretic arm from all other arm groups. Significance of Correlations:
p<.05,
p<.01.
Note that generation and absorption powers have been described in terms of eccentric/concentric to facilitate interpretation.
Joint Configurations
In the control arm condition, the upper arm was raised using primarily shoulder flexion (see Figure 3). Shoulder flexion was consistently coupled with small increases (0–15°) in the abduction angle early in movement and decreases (~0–10°) in the abduction angle late in movement. Internal-external rotation was always small, variable in direction and not generally coupled with shoulder flexion. Elbow movement was consistently bi-phasic with slight flexion (5–15°) occurring early and extension (10–40°) near the end of the movement. Movements of the non-paretic arm were qualitatively similar to those of the control condition.
Figure 3.
Power profiles for planes defined by (a) shoulder flexion-extension, (b) shoulder adduction-abduction, (c) shoulder internal-external rotation, and (d) elbow flexion-extension. Joint power is shown on the vertical axis and normalized movement time on the horizontal axis. Power generation is positive and absorption is negative. Profiles are shown for mild (open triangle), moderate (filled square), and severe (open circle) impairments of the paretic arm (single trial profiles from individual subjects are shown compared to the control average (N=10 subjects)) compared to the control average (solid line) and range (1 standard deviation – shaded region). Notice how power shifts from the shoulder flexion-extension plane to the adduction-abduction plane as impairment increases. Mild, moderate and severe corresponded to approximately the lower, middle and upper third of the Fugl-Meyer range of scores for the paretic arm condition.
Elbow movements of the paretic arm were also bi-phasic but unlike the control and non-paretic arm conditions, there were prominent abduction and internal rotation movements; the paretic arm of 19 of the 20 subjects with stroke had greater than the maximum abduction angle observed in control subjects. While the absolute timing and magnitude of abduction and internal rotations varied in the paretic arm condition, events characterizing these movements and those of the elbow were coupled. Abduction peaked with elbow flexion early in movement (i.e., at less than 50% of task movement time) before decreasing slightly midway through movement. Internal rotation was initiated at the same time as elbow extension and increased gradually until the end of movement.
The net angular change (i.e., initial to final) for shoulder flexion and elbow extension were pre-specified by the required end point position of the task and, therefore, not analyzed. Changes in abduction and internal rotation angles were affected by arm condition (Wilks’ Lambda statistic of 0.519, p < 0.05, F (4,92)=5.19, p < 0.001) and highly correlated to severity of impairment in the paretic arm (see Table 4). There was also a significant increase in maximum abduction angle for the non-paretic arm condition versus the control condition.
Table 4.
Joint Configurations
| Peak Joint Angle (Degrees) | p | Control (n=10) | Non-paretic (n=20) | Paretic (n=20) | |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | FM Correlation | ||
| S. Adduction (+)/Abduction (−)a,b | 0.001 | −.71(−4.74 to 3.31) | −5.03 (−7.96 to −2.11) | −10.62 (−13.54 to −7.70) | .816** |
| S. Internal (+)/External (−) Rota | 0.009 | −.797 (−9.164 to 7.570) | −2.259 (−8.329 to 3.811) | 10.689 (4.619 to 16.759) | −.627** |
P-values provided for the significance level of the ANOVA comparing the three arm conditions (control, non-paretic, paretic)
Post-hoc multiple comparison test significantly differentiated descriptor associated with the paretic arm from all other arm groups;
Significantly differentiated descriptor associated with the non-paretic arm from control arm group. Significance of Correlations:
p<.05,
p<.01.
Hand Path & Trajectory
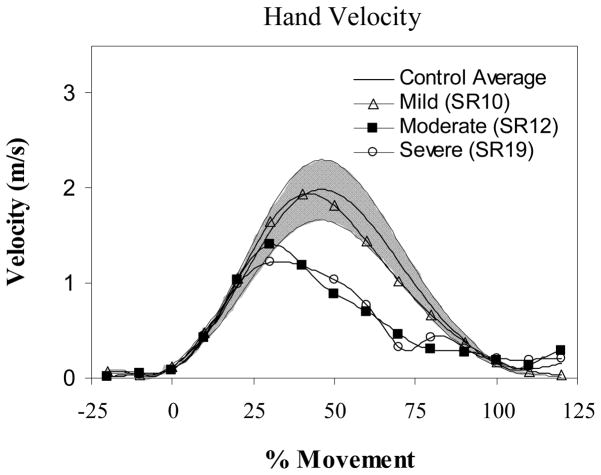
In both the control and non-paretic arm conditions (but not the paretic) the path was slightly curved and the velocity profile was substantially bell shaped (see Figure 4). Kinematic descriptors of the hand path, particularly the directness parameter, were significantly affected by arm condition (Wilks’ Lambda statistic of 0.565, p < 0.05; F (6,90) =3.47, p=.002) and correlated with the severity of impairment for the paretic arm condition (see Table 5).
Figure 4.
Tangential velocity profile of the hand for mild (open triangle), moderate (filled square), and severe (open circle) impairments of the paretic arm (single trial profiles from individual subjects are shown compared to the control average (N=10 subjects)) (solid line) and range (1 standard deviation – shaded region). Velocity is shown on the vertical axis and normalized movement time on the horizontal axis. Notice how the profile becomes progressively more skewed and more segmented as impairment increases. Mild, moderate and severe corresponded to approximately the lower, middle and upper third of the Fugl-Meyer range of scores for the paretic arm condition.
Table 5.
Hand Kinematics
| Parameter (Dimensionless) | p | Control (n=10) | Non-paretic (n=20) | Paretic (n=20) | |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | FM Correlation | ||
| Directnessa | <0.001 | .991 (.967 to 1.015) | .988 (.970 to 1.005) | .936 (.919 to .954) | .854** |
| Segmentationa | 0.039 | 1 (Exact) | 1 (Exact) | 1.377 (1.154 to 1.600) | −.518* |
| Skewnessa | 0.100 | −.031 (−3.932 to 3.932) | −.456 (−3.330 to 2.419) | 3.724 (.849 to 6.598) | .042 |
P-values provided for the significance level of the ANOVA comparing the three arm conditions (control, non-paretic, paretic)
Post-hoc multiple comparison test significantly differentiated descriptor associated with the paretic arm from all other arm groups. Significance of Correlations:
p<.05,
p<.01.
DISCUSSION
Sagittal plane movement strategy results in direct, non-segmented, reaching trajectory in healthy individuals
Successful completion of reaching tasks in this study required a specific positioning of the hand (i.e., target) at the end of movement but not a specific positioning of arm joints. Moreover, the central nervous system was free to use any hand and joint motions between start and end postures. With the initial posture of the upper limb in the same para-sagittal plane formed by the targets and starting position, reaching tasks could have been accomplished entirely through shoulder flexion and elbow extension. The net joint movement (i.e., stop-start) of the control subjects was consistent with this prediction. However, the elbow flexion that preceded the required elbow extension suggests that the control of joint motion is not strictly predicted by external spatial factors. Early elbow flexion is advantageous because it initially reduces the lever distance between the shoulder and arm center of arm mass which, in turn, reduces required shoulder flexor moments, powers, and total energy. This strategy of flexing (before extending) the elbow and keeping the arm within the sagittal plane resulted in direct, non-segmented, and symmetric hand motion in the control group and is consistent with a minimization of mechanical energy, and thus, muscular consumption of metabolic energy (Hogan 1984).
Stereotypical compensatory movement strategy in the paretic arm condition
Despite the abundant mechanical degrees of freedom available in the arm and heterogeneity of the lesion type, side, location and resulting impairment of the subjects, compensatory movements were stereotypical and described by increasing abduction and internal rotation related to severity of impairment in the paretic arm. Reduced agonist recruitment capacity, as a consequence of stroke, can prevent the completion of upper extremity tasks (Gowland et al. 1992). Similarly, our results indicate that muscle weakness constrains the movement patterns available to the central nervous system because primary agonist muscles alone are not capable of generating the required execution torques. Secondly, they suggest that compensatory movements can occur via the recruitment of additional agonist muscles, thereby distributing the muscle force. In our reaching task, for example, the anterior deltoid was maximally activated (i.e., saturated) and flexor power substantially reduced, and thus, increased abductor power generation and increased activation of the lateral deltoid were necessary to achieve the task. Note that the compensation for the anterior deltoid is task-specific and is observed because the anterior deltoid is the primary mover for the reaching task; the compensation does not specifically occur because the anterior deltoid might have greater deficits than the other upper extremity muscles. This suggests that persons with stroke may also exhibit stereotypical compensations for other movements, despite the fact that patters of weakness may vary across subjects with stroke (Colebatch and Gandevia, 1989; Mercier and Bourbonnais, 2004; McCrea et al. 2003). This is supported by observations that the directional ranges of individual muscles in the paretic arm broaden following stroke (i.e., muscles are recruited for more movements) (Dewald et al. 1995).
Latash and Anson (1996) emphasized that altered motor patterns do not necessarily indicate a failure of the task, but rather, that a CNS re-organization of priorities generates an adaptive change within the redundancy of the motor system. Klatzky (1996) suggested that constraints (e.g., resulting from the motor system or physical environment) result in adjustments of movement priorities. In the reaching task, saturated anterior deltoid activity in individuals with stroke is accompanied by lateral deltoid activity which results in successful completion of the task (elevating the arm and reaching the target), albeit, the interim movement occurs out of the sagittal plane. The significant correlations between shoulder muscle strength and saturation of muscle activity provide further evidence that muscle weakness is one factor that contributes to the necessity of the compensation.
Temporal coupling between the shoulder and elbow was also evident in the paretic arm condition: shoulder abduction with elbow flexion or shoulder internal rotation with elbow extension. These inter-joint couplings are consistent with the description of flexor (elbow flexion, shoulder abduction and external rotation) and extensor (elbow extension, shoulder adduction and internal rotation) pathological synergies that emerge following stroke (Brunnstrom 1966). The inability to isolate torque generation to selected joints (Dewald and Beer 2001) is consistent with previous observations of abnormal co-activations and interjoint dynamics between the elbow and shoulder joints (Beer et al. 2004; Bourbonnais et al. 1989; Dewald et al. 1995).
A pathological reduction in the number of available synergies may also degrade the ability to execute desired hand trajectories. There were deficits in the quality of hand kinematics in the paretic arm condition, indicating that abnormal joint couplings were detrimental to reaching performance. In both control and stroke subjects (Reisman and Scholz 2003), joint motion errors co-vary in a compensatory fashion such that the planned hand path is approximately achieved. According to the Uncontrolled Manifold Hypothesis (e.g., Scholz and Schoner 1999), a neural restriction on the number of ‘goal equivalent postures’ (i.e., joint configurations which result in the same hand position) would reduce the effectiveness of this error compensation. Indeed, previous work suggests that neural restrictions are a mechanism that leads to a loss of directional hand control during reaching movements in severe motor impairment (Reinkensmeyer et al. 2002).
The stereotypical reaching strategies of the paretic arm could also result from limited joint range (i.e., contractures) as it has been shown that small reductions in joint ranges can cause large reductions in the number of potential movement synergies that can be performed (Kamper and Rymer 1999). However, participants in our study were excluded from if they had significant impairments in the active range of the shoulder and/or elbow and the passive ranges of shoulder and elbow joints were largely unimpaired in these subjects. It is also possible that factors such as comfort or joint pain (Cruse et al. 1990), particularly of the shoulder, could influence the movement planning; however, subjects reported that pain was not present during the test session.
Non-paretic arm has altered joint mechanics
Although there were significant changes to joint motion and mechanical effort (increased abduction angle and flexor-to-abductor power shift) for the non-paretic arm condition, hand motion quality was similar to the control condition. Reduced strength and rate of force generation in the non-paretic upper extremity of individuals with stroke (McCrea et al. 2003) may necessitate alternative recruitment strategies to complete the task. The combined abduction-flexion movement in the non-paretic arm condition likely facilitated a wider recruitment of muscle fibers across the deltoid so that sufficient shoulder power could be developed. The unaltered hand motion quality observed in our study, points to the ability of the CNS to exploit redundancy of the neuromuscular system.
Limitations & Conclusions
The reaching distance of the subjects with stroke was determined by the active range of the paretic arm and the mean forward distance was about 85% of the distance that the control subjects used. It is possible that this shorter distance could have promoted a shoulder abductor strategy, however, in our pilot testing we never observed a shoulder abductor strategy with the control subjects at short or far reaching distances. It should be noted that, in some cases, the magnitude of muscle activity during reaching movements exceeded 100% of the value obtained during maximum voluntary contractions. This can be attributed to the dependence of EMG amplitude on joint position and velocity (Leedham and Dowling, 1995; Nakazawa et al. 1993), (i.e., MVC is isometric, while the reaching movement is dynamic). In addition, potentiation may also occur when multiple synergistic muscles are activated during functional movements (DeLuca and Erim, 2002; Huang and Abraham, 2001). Additionally, Landau and Sahrmann (2002) demonstrated that the paretic tibialis anterior muscle of individuals with stroke had lower levels of maximal voluntary torque, but not electrically stimulated force, compared to control subjects, suggesting that the central regulation of muscle activation may be disrupted. Such observations agree with Tang and Rymer (1981) who found that the amount of EMG produced per unit force was greater in the paretic elbow flexor muscles compared to controls and they attributed this increased recruitment from a compensation to the reduced mean motor unit discharge rate that they observed in the paretic muscles. However, others have reported that the EMG-force relationship in the elbow is similar between stroke patients and controls (Fellows et al. 1994). Although individuals with stroke may not be generating a true maximum contraction, the measurement of their “MVC” is important because it provides a relative indication of the available voluntary force with which to undertake functional tasks.
This study examined the differences in reaching characteristics among arm conditions without matching reaching speeds and one may argue that such differences are related to the naturally slower movements of the paretic arm condition. To examine the potential confounding effects of reaching speed, we ran additional analyses of covariance with peak tangential hand velocity (covariate) and found that parameter differences between arm conditions remained significant.
This study shows that movement compensations following stroke are, in part, required to circumvent weakness and saturated muscle activity, which act to restrict the motor abundance of the upper extremity. Strengthening of paretic muscles may potentially limit the lost degrees of freedom and improve the quality of movement compensation. The results of this study can be generalized to individuals with chronic stroke over a broad range of motor impairment (range of score from 13 to 64 out of a possible 66 on the motor component of the upper extremity Fugl-Meyer scale). However, other impairments such as proprioception may also play a role (Mercier et al. 2004) in reaching performance and should be considered separately.
Acknowledgments
The authors would like to thank the Canadian Institutes of Health Research for operating funds and a New Investigator Award to JJE (# 63617), the Michael Smith Foundation of Health Research for a Scholar Award to JJE and the BC Neurotrauma Fund for a studentship to PHM. We’d like to thank Chihya Hung for her support during the manuscript preparation phase.
References
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Bernstein N. The coordination and regulation of movements. New York: Pergamon; 1967. [Google Scholar]
- Bohannon RW, Smith M. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Bourbonnais D, Vanden NS, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subject. Brain. 1989;112:85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- Carr JH, Shepherd RB. Reaching and Manipulation. In: Carr JH, Shepherd RB, editors. Neurological Rehabilitation, Optimizing Motor Performance. Oxford: Reed Educational and Professional Publishing Ltd; 1999. pp. 126–153. [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112:749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Cruse H, Wischmeyer E, Bruwer M, Brockfeld P, Dress A. On the cost functions for the control of the human arm movement. Biol Cybern. 1990;62:519–528. doi: 10.1007/BF00205114. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive in motor units of a synergistic muscle pair. J Neurophysiol. 2002;87:2200–2204. doi: 10.1152/jn.00793.2001. [DOI] [PubMed] [Google Scholar]
- De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78:1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Dewald JPA, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Eliasziw M, Young SL, Woodbury MG, Fryday-Field K. Statistical methodology for the assessment of interrater and intrarater reliability. Phys Ther. 1994;74:89–100. doi: 10.1093/ptj/74.8.777. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Kaus C, Ross HF, Thilmann AF. Agonist and antagonist EMG activation during isometric torque development at the elbow in spastic hemiparesis. Electroencephalogr Clin Neurophysiol. 1994;93:106–112. doi: 10.1016/0168-5597(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Flanders M. Temporal patterns of muscle activation for arm movements in three-dimensional space. J Neurosci. 1991;11:2680–2693. doi: 10.1523/JNEUROSCI.11-09-02680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders M, Pellegrini JJ, Geisler SD. Basic features of phasic activation for reaching in vertical planes. Exp Brain Res. 1996;110:67–79. doi: 10.1007/BF00241376. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. Poststroke hemiplegic patient: evaluation of physical performance. Scand J Rehab Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gowland C, deBruin H, Basmajian JV, Plews N, Burcea I. Agonist and antagonist activity during voluntary upper-limb movement in patients with stroke. Phys Ther. 1992;72:624–633. doi: 10.1093/ptj/72.9.624. [DOI] [PubMed] [Google Scholar]
- Granger CV, Hamilton BB, Sherwin FS. Guide for the use of uniform data set for medical rehabilitation. Uniform Data System for Medical Rehabilitation Project Office, Buffalo General Hospital; New York, 1403, USA: 1986. [Google Scholar]
- Hogan N. An organizing principle for a class of voluntary movements. J Neurosci. 1984;4:2745–2754. doi: 10.1523/JNEUROSCI.04-11-02745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang IS, Abraham LD. Quantitative EMG analysis to investigate synergistic coactivation of ankle and knee muscles during isokinetic ankle movement. Part 1: time amplitude analysis. J Electromyogr Kinesiol. 2001;11:319–325. doi: 10.1016/s1050-6411(01)00012-8. [DOI] [PubMed] [Google Scholar]
- Kaminski TR, Bock C, Gentile AM. The coordination between trunk and arm motion during pointing movements. Exp Brain Res. 1995;106:457–466. doi: 10.1007/BF00231068. [DOI] [PubMed] [Google Scholar]
- Kamper DG, Rymer ZW. Effects of geometric joint constraints on the selection of final arm posture during reaching: a simulation study. Exp Brain Res. 1999;126:134–138. doi: 10.1007/s002210050723. [DOI] [PubMed] [Google Scholar]
- Kamper DG, McKenna-Cole AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil. 2002;83:702–707. doi: 10.1053/apmr.2002.32446. [DOI] [PubMed] [Google Scholar]
- Kim CM, Eng JJ. Magnitude and pattern of 3D kinematic and kinetic gait profiles in persons with stroke: Relationship to walking speed. Gait Posture. 2004;20:140–146. doi: 10.1016/j.gaitpost.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzky RL. What makes a population atypical - Priorities or constraints? Behav Brain Sci. 1996;19:78. [Google Scholar]
- Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proc Natl Acad Sci U S A. 1999;96:4645–4649. doi: 10.1073/pnas.96.8.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau WM, Sahrmann SA. Preservation of directly stimulated muscle strength in hemiplegia due to stroke. Arch Neurol. 2002;59:1453–1457. doi: 10.1001/archneur.59.9.1453. [DOI] [PubMed] [Google Scholar]
- Latash M, Anson J. What are “normal movements” in atypical populations? Behav Brain Sci. 1996;19:55–106. [Google Scholar]
- Leedham JS, Dowling JJ. Force-length, torque-angle and EMG-joint angle relationships of the human in vivo biceps brachii. Eur J Appl Physiol Occup Physiol. 1995;70:421–426. doi: 10.1007/BF00618493. [DOI] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119:281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Kenney DE, Van der Loos HF. Quantification of force abnormalities during passive and active-assisted upper-limb reaching movements in post-stroke hemiparesis. IEEE Trans Biomed Eng. 1999;46:652–662. doi: 10.1109/10.764942. [DOI] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ, Hodgson AJ. Time and Magnitude of Torque Generation is Impaired in Both Arms following Stroke. Muscle Nerve. 2003;28:46–53. doi: 10.1002/mus.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meglan DW. PhD thesis in Mechanical Engineering. Ohio State University; 1991. Enhanced Analysis of Human Motion. [Google Scholar]
- Mercier C, Bertrand AM, Bourbonnais D. Differences in the magnitude and direction of forces during a submaximal matching task in hemiparetic subjects. Exp Brain Res. 2004;157:32–42. doi: 10.1007/s00221-003-1813-x. [DOI] [PubMed] [Google Scholar]
- Mercier C, Bourbonnais D. Relative shoulder flexor and handgrip strength is related to upper limb function after stroke. Clin Rehabil. 2004;18:215–221. doi: 10.1191/0269215504cr724oa. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Compensation in Recovery of Upper Extremity Function After Stroke: The Copenhagen Stroke Study. Arch Phys Med. 1994;75:852–857. doi: 10.1016/0003-9993(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Kawakami Y, Fukunaga T, Yano H, Miyashita M. Differences in activation patterns in elbow flexor muscles during isometric, concentric and eccentric contractions. Eur J Appl Physiol Occup Physio. 1993;66:214–220. doi: 10.1007/BF00235096. [DOI] [PubMed] [Google Scholar]
- Prokopenko RA, Frolov AA, Biryukova EV, Roby-Brami A. Assessment of the accuracy of a human arm model with seven degrees of freedom. J Biomech. 2001;34:177–185. doi: 10.1016/s0021-9290(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Dewald JP, Rymer WZ. Guidance-based quantification of arm impairment following brain injury: a pilot study. IEEE Trans Rehabil Eng. 1999;7:1–11. doi: 10.1109/86.750543. [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, McKenna Cole A, Kahn LE, Kamper DG. Directional control of reaching is preserved following mid/moderate stroke and stochastically constrained following severe stroke. Exp Brain Res. 2002;143:525–530. doi: 10.1007/s00221-002-1055-3. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain. 2003;126:2510–2527. doi: 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- Riener R, Straube A. Inverse dynamics as a tool for motion analysis: arm tracking movements in cerebellar patients. J Neurosci Meth. 1997;72:87–96. doi: 10.1016/s0165-0270(96)02168-1. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol. 2000;83:2661–2675. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–517. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psych Bull. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Tang A, Rymer WZ. Abnormal force--EMG relations in paretic limbs of hemiparetic human subjects. J Neurol Neurosurg Psychiatry. 1981;44:690–698. doi: 10.1136/jnnp.44.8.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly CA. Deficits of reaching in subjects with left hemiparesis: a pilot study. Am J Occup Ther. 1992;46:887–897. doi: 10.5014/ajot.46.10.887. [DOI] [PubMed] [Google Scholar]
- Yeadon MR. Morlock The appropriate use of regression equations for the estimation of segmental inertia parameters. J Biomech. 1989;22:683–689. doi: 10.1016/0021-9290(89)90018-3. [DOI] [PubMed] [Google Scholar]
- Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostistical Analysis. 4. Prentice-Hall Inc; Upper Saddle River, NJ: 1999. [Google Scholar]