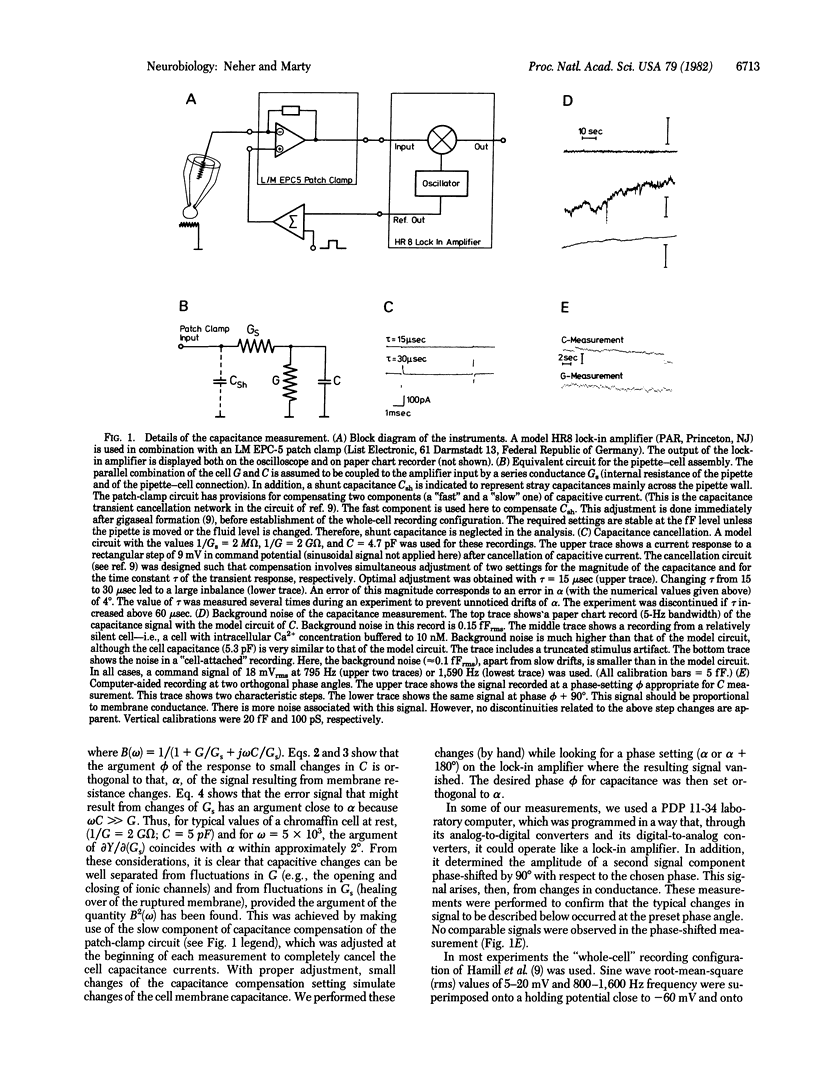
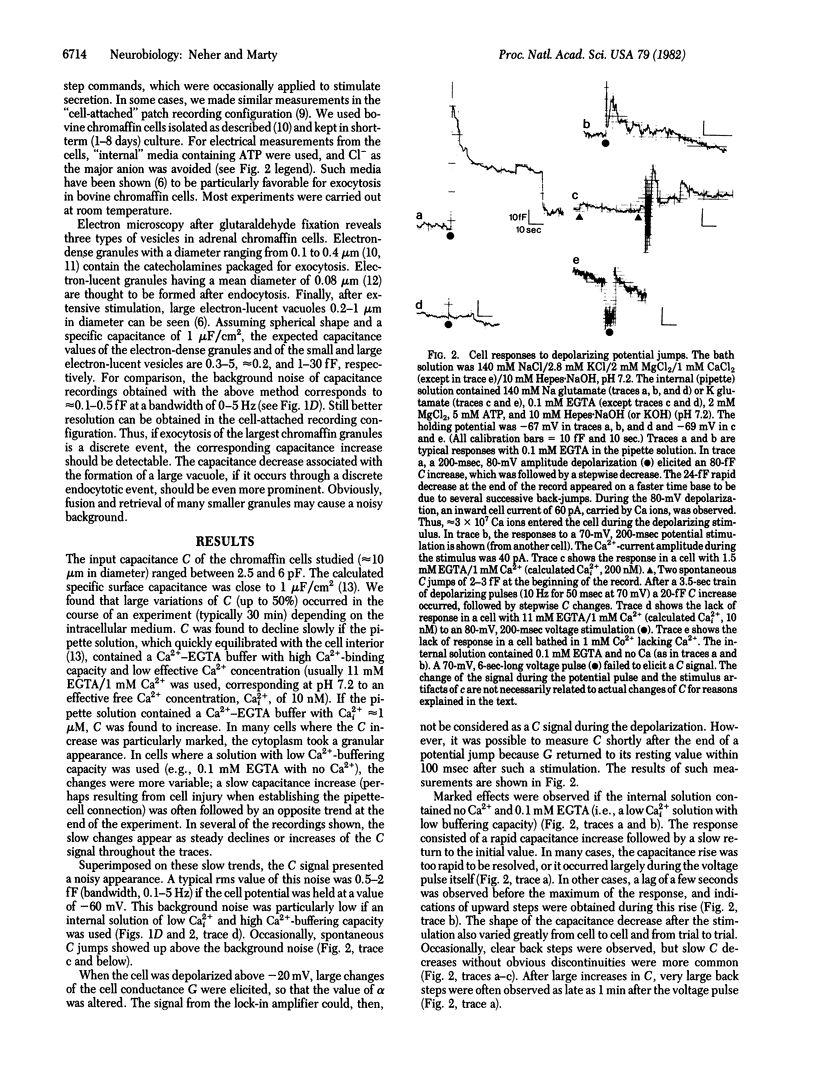
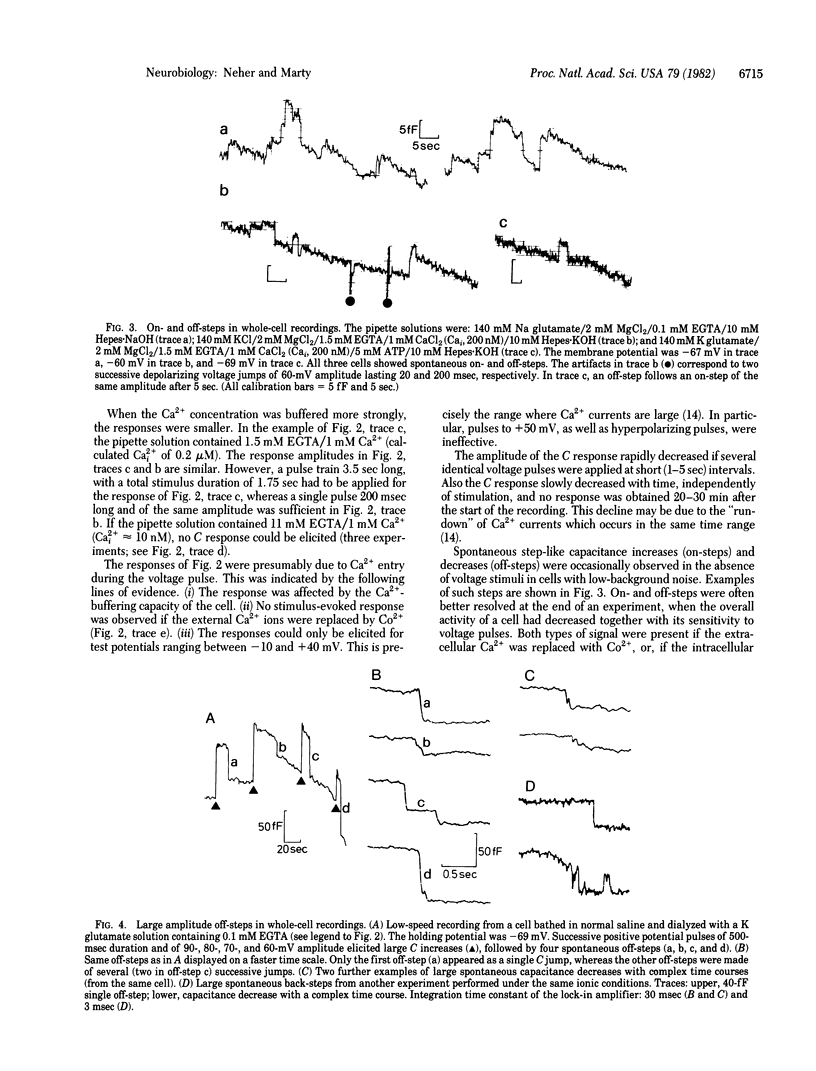
Abstract
The capacitance of the surface membrane of small adrenal chromaffin cells was measured with patch-clamp pipettes. Continuous and discrete changes of capacitance were observed. They were interpreted as changes of surface area connected to exocytotic or endocytotic processes. Most of the measurements were performed in the "whole-cell" recording configuration [Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85-100], which allows the intracellular Ca2+ concentration to be controlled. With an internal solution highly buffered to low values of Ca2+ concentration (10 nM), the surface capacitance usually decreased and could not be markedly changed by electrical stimulation. At low buffering capacity and medium Ca2+ concentrations (0.1-1 microM), the capacitance measurement showed large fluctuations and discrete steps, reflecting both capacitance decrease and increase. A large transient increase of capacitance could be induced by electrical stimulation under these conditions. It was linked to Ca2+ currents through the membrane. Relatively large (2-6 x 10(-14) F) steps of capacitance decrease were common after extensive stimulation. The size distribution of step-like capacitance changes is well compatible with the idea that steps of capacitance increase reflect individual events of exocytosis of chromaffin granules, whereas steps of the opposite polarity reflect the formation of vesicles or vacuoles by endocytosis.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams S. J., Holtzman E. Secretion and endocytosis in insulin-stimulated rat adrenal medulla cells. J Cell Biol. 1973 Feb;56(2):540–558. doi: 10.1083/jcb.56.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Benedeczky I., Smith A. D. Ultrastructural studies on the adrenal medulla of golden hamster: origin and fate of secretory granules. Z Zellforsch Mikrosk Anat. 1972;124(3):367–386. doi: 10.1007/BF00355037. [DOI] [PubMed] [Google Scholar]
- Case R. M. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978 May;53(2):211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E. D., SABATINI D. D. Submicroscopic analysis of the secretory process in the adrenal medulla. Fed Proc. 1960 Dec;19(Suppl 5):70–78. [PubMed] [Google Scholar]
- Fenwick E. M., Fajdiga P. B., Howe N. B., Livett B. G. Functional and morphological characterization of isolated bovine adrenal medullary cells. J Cell Biol. 1978 Jan;76(1):12–30. doi: 10.1083/jcb.76.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M. Common mechanisms of hormone secretion. Annu Rev Pharmacol Toxicol. 1977;17:27–47. doi: 10.1146/annurev.pa.17.040177.000331. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Griesser G. H., Lindmar R., Löffelholz K., Wolf U. Establishment, characterization and fibre outgrowth of isolated bovine adrenal medullary cells in long-term cultures. Neuroscience. 1980;5(8):1445–1460. doi: 10.1016/0306-4522(80)90006-8. [DOI] [PubMed] [Google Scholar]