Abstract
Background
Interventions to increase brown adipose tissue (BAT) volume and activation are being extensively investigated as therapies to decrease the body weight in obese subjects. Noninvasive methods to monitor these therapies in animal models and humans are rare. We investigated whether contrast ultrasound (CU) performed in mice could detect BAT and measure its activation by monitoring BAT blood flow. After validation, CU was used to study the role of uncoupling protein 1 (UCP1) and nitric oxide synthases in the acute regulation of BAT blood flow.
Methods and Results
Blood flow of interscapular BAT was assessed in mice (n=64) with CU by measuring the signal intensity of continuously infused contrast microbubbles. Blood flow of BAT estimated by CU was 0.5±0.1 (mean±SEM) dB/s at baseline and increased 15-fold during BAT stimulation by norepinephrine (NE, 1 μg·kg−1·min−1). Assessment of BAT blood flow using CU was correlated to that performed with fluorescent microspheres (R2=0.86, p<0.001). To evaluate whether intact BAT activation is required to increase BAT blood flow, CU was performed in UCP1-deficient (UCP1−/−) mice with impaired BAT activation. Norepinephrine infusion induced a smaller increase in BAT blood flow in UCP1−/− mice than in wild-type mice. Finally, we investigated whether NOS played a role in acute NE-induced changes of BAT blood flow. Genetic and pharmacologic inhibition of NOS3 attenuated the NE-induced increase in BAT blood flow.
Conclusions
These results indicate that CU can detect BAT in mice, and estimate BAT blood flow in mice with functional differences in BAT.
Keywords: brown adipose tissue, imaging, contrast ultrasound, uncoupling protein, nitric oxide synthase
Brown adipose tissue (BAT) can be activated by the sympathetic nervous system or thyroid hormone to produce heat, and is a major contributor to nonshivering thermogenesis.1 The activation of BAT induces an increase in lipid and glucose oxidation by brown adipocytes, augmenting their mitochondrial respiration and oxygen consumption. In activated brown adipocytes, mitochondrial respiration becomes uncoupled from adenosine triphosphate (ATP) production, a process mediated by uncoupling protein (UCP) 1. Uncoupling allows protons, produced by the oxidation of lipids and glucose, to re-enter the mitochondrial matrix, while generating heat instead of ATP (for a review, see2).
Brown adipose tissue has been identified in human infants and in rodents for several decades. In rodents, it is present in large depots primarily located between the scapulae. Recently, several studies employing 18F-fluorodeoxyglucose positron-emission tomography scans coupled with computed tomography (FDG-PET-CT) have confirmed the presence of metabolically active BAT in adult humans.3–7 Stimulation of BAT to increase energy expenditure may potentially be used for anti-obesity therapies (for a review, see1). Detecting BAT and monitoring its activation state is therefore necessary for the development of BAT-targeted therapies.
BAT is a highly vascularized tissue,8 and both acute and chronic activation of BAT are associated with increased blood flow to the tissue.9–13 Suppressing the increase in blood flow associated with BAT activation impairs thermogenesis.11 However, it is unknown whether decreased activation of BAT will result in reduced blood flow to the tissue. This question can be approached by investigating the blood flow response to BAT activation in mice deficient in UCP1 (UCP1−/−). These mice have an impaired activation of BAT with a reduced capacity to uncouple mitochondrial respiration from ATP synthesis in response to stimulation.14 Of note, other UCPs such as UCP2 are upregulated in the BAT of these animals.14,15
The increase of BAT blood flow associated with BAT activation can be mediated directly or indirectly by the sympathetic nervous system. Indeed, sympathetic nervous system stimulation can induce vasodilatation, either directly through cyclic adenosine monophosphate (cAMP) or through an interaction of cAMP and nitric oxide (NO).16–19 Nitric oxide plays an important role in BAT-related metabolism and mitochondrial biogenesis of brown adipocytes.20 The most prominent pathway for NO production is via NO synthase (NOS) enzymes. In brown adipocytes, NOS3 is the predominantly expressed isoform.21 Both NOS122 and NOS316,23 can mediate vasodilation in mice, and both might have vasodilatory effects in BAT.
We hypothesized that BAT could be detected in vivo by imaging its blood flow. Microspheres have been used in rodents to assess blood flow of BAT,9–13 but this method is terminal and cannot be applied to humans. Contrast ultrasound (CU) is a noninvasive technique that estimates microvascular blood flow by visualization and quantification of intravenously-infused echogenic microbubbles.24 When microbubbles are destroyed by high-energy ultrasound pulses, the time course of their replenishment in a given tissue can be fitted to an exponential curve. The product of the peak signal intensity (A) and the slope of the replenishment (β) of this curve is an estimate of the tissue’s blood flow. Contrast ultrasound has been validated in the noninvasive estimation of myocardial blood flow, both in mice23 and in humans.25 In a recent study, CU was used to assess microvascular blood volume in muscle and white adipose tissue of humans and rats.26
The present study investigated whether CU could be used to detect BAT and its activation by measuring BAT blood flow in mice, both at baseline and after stimulation with norepinephrine (NE). The estimation of BAT blood flow using CU was validated with that obtained using microspheres. To determine whether BAT activation was required for the increased BAT blood flow detected by CU, we compared wild-type (WT) and UCP1−/− mice. Finally, we investigated whether or not NOS-dependent NO signaling plays a role in the increase in BAT blood flow induced by NE. The blood flow response to NE was measured in mice deficient in NOS1 (NOS1−/−) or NOS3 (NOS3−/−) and in animals in which NOS was inhibited by NG-nitro-L-arginine methylester (L-NAME).
Methods
Protocol
All animal studies were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital, Boston, MA. Two- to three-month-old male C57BL6 wild-type (WT, n=47), B6.129S4-Nos1tm1Plh (NOS1−/−, n=4), B6.129P2-Nos3tm1Unc (NOS3−/−, n=8) mice (Jackson Laboratory, Bar Harbor, ME), and two-month-old male B6.129-Ucp1tm1Kz (UCP1−/−, n=5) mice14 were studied. UCP1−/− mice and their WT control mice were housed at a room temperature of 26°C for 2 weeks before performing the experiments. Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg).27,28 After intubation, animals were mechanically ventilated (FiO2 1.0, 10 μl/g, 120 breaths/min), and fluid-filled catheters were surgically inserted into the left carotid artery and right jugular vein for continuous measurement of invasive hemodynamic parameters and administration of infusions, respectively. Mice were placed in a prone position for imaging of interscapular BAT. In one group of WT mice (n=4), infusions were administered using a less invasive technique via a 31G catheter placed in a tail vein. Because no surgical preparation was performed in the tail-vein experiments, mice were anesthetized with lower concentrations of ketamine (80 mg/kg) and xylazine (8 mg/kg). In all experiments, core body temperature was kept constant at 37°C with a DC Temperature Control System (FHC, Bowdoin, ME).
Acquisition of Contrast Ultrasound
Perflutren lipid microbubbles (Definity®; Lantheus Medical Imaging Inc., North Billerica, MA) were diluted 1:10 in a 0.9% saline solution and infused at a rate of 20 μl/min into the right jugular vein or the tail vein. CU was performed with a 14-MHz linear transducer (Sequoia C512, Siemens, Mountain View, CA). Both scapulae were located and used as guiding anatomical landmarks, and interscapular BAT was identified by localization of Sulzer’s vein via CU.29 Ten high-energy ultrasound frames (mechanical index 1.80, frame rate 30 Hz) were used to destroy the contrast microbubbles, and the replenishment time course of the contrast microbubbles in the BAT was recorded for 10 seconds in real-time mode (mechanical index 0.24). Similar acquisitions were obtained in the right kidney and right quadriceps femoris muscle. After the CU acquisitions at baseline, NE (Bedford Laboratories, Bedford, OH) was infused intravenously at 1 μg·kg−1·min−1. The effect of NE was verified by an increase in systemic blood pressure and heart rate after 10 min, and CU was repeated.
One group of WT mice (n=7) received an intravenous bolus injection of 50 mg/kg L-NAME30 after performing CU at baseline conditions, 10 min before infusion of NE. After the bolus injection, L-NAME was continuously infused throughout the experiment at 10 mg·kg−1·min−1.
Histology of BAT
After completion of the CU imaging, mice were euthanized, and the BAT was dissected. In a subgroup of animals, 5 μm thick paraffin-embedded slides of BAT were obtained at 1 mm intervals and stained using hematoxylin & eosin.
Analysis of Contrast Ultrasound
Regions of interest (ROIs) were traced manually within the BAT, kidney cortex, and muscle, excluding the big vessels. Average signal intensity for each ROI was automatically determined on each frame (Syngo ACQ, Siemens). A curve of signal intensity over time was obtained for each ROI and fitted to an exponential function: y=A·(1–e−β;t), where y represents the signal intensity, β is the initial slope of the curve, and A the plateau intensity. Blood flow was estimated by the product Aβ as described previously.23,24 Two to three curves were averaged for each organ under each condition. A goodness-of-fit coefficient (R2 of the fit) was obtained for each curve, and an arbitrary threshold of 0.8 was set. All curves with a goodness-of-fit of less than 0.8 were excluded.
Echocardiographic Assessment of Cardiac Output
To estimate cardiac output, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in supine position. Cardiac output was assessed before and after NE infusion using M-Mode echocardiography31 in WT mice (n=6), WT mice treated with L-NAME (n=4), and UCP1−/− mice (n=4).
Assessment of Organ Blood Flow by Fluorescent Microspheres
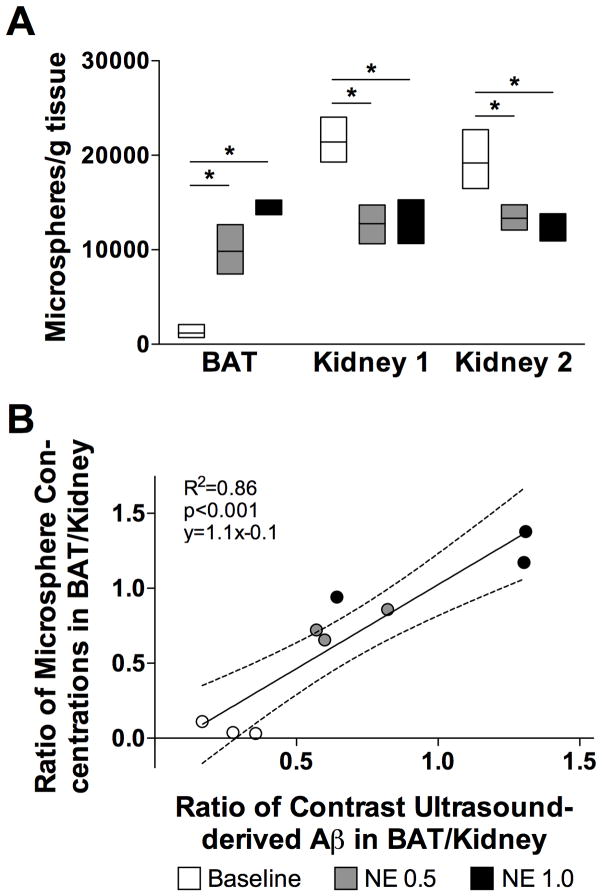
In separate experiments, blood flow to BAT and kidneys was estimated concurrently using FluoSpheres® polystyrene microspheres (15 μm; Invitrogen, Carlsbad, CA) and CU. Either saline solution (n=3) or NE (0.5 (n=3) or 1 (n=3) μg·kg−1·min−1) was infused intravenously at a rate of 10 μl/min. After 30 min, CU of BAT and right kidney was acquired. Following CU, 1·106 microspheres were injected into the left carotid artery. Mice were euthanized two minutes after the injection, and tissues were harvested. Tissue samples were processed using a previously validated protocol.32 Briefly, tissue samples were placed in 1.5 ml Eppendorf tubes for one week at room temperature and for an additional 48 h in 2N ethanolic KOH at 60°C. After centrifugation at 2000 g for 20 min, supernatants were discarded, and pellets were incubated in 0.25% Tween 80 (Fisher Scientific, Pittsburgh, PA) for 5 min. Centrifugation was repeated, and samples were washed with distilled water. Finally, microsphere pellets were dissolved in 2-ethoxyethylacetate (Sigma-Aldrich) and centrifuged at 2000 g for 10 min. Fluorescence was measured in the supernatant at wavelengths of 427 and 468 nm using a SpectraMax M5 microplate reader (Molecular Devices Inc., Sunnyvale, CA). The absolute concentration of microspheres in each sample was obtained by comparing relative fluorescence units to a standard curve of known microspheres concentrations. The ratio of the microspheres concentration in BAT to that in the right kidney was calculated.
Quantitation of mRNA Levels
BAT was dissected and snap frozen in liquid nitrogen. RNA was extracted from tissues using Trizol (Invitrogen, Carlsbad, CA), and cDNA was synthesized using MMLV-RT (Promega, Madison, WI). Real-time amplification of transcripts was detected using a Mastercycler ep Realplex (Eppendorf, Hamburg, Germany). The relative expression of target transcripts was normalized to levels of 18S RNA. Primer pairs were used to detect transcripts encoding UCP1 (CAAAAACAGAAGGATTGCCGAAA, TCTTGGACTGAGTCGTAGAGG), UCP2 (TCTCCTGAAAGCCAACCTCA, CTACGTTCCAGGATCCCAAG), and UCP3 (CCGATTTCAAGCCATGATACGC, GGCATCCATAGTCCCTCTGTATT).
Statistical Analysis
Statistical analysis was done with JMP statistical software (SAS Institute, Cary, NC). Data are expressed as mean±SEM. A two-way ANOVA for repeated measurements with selective post-hoc comparison versus WT mice was used to assess the effect of genotype or treatment on the blood flow response to NE. If the interaction of NE and genotype was significant, unpaired Student’s t-tests were used to compare ultrasound parameters between genotypes or treated mice at the same NE concentration.
Linear regression analysis was used to determine the correlation coefficient (R2) between BAT blood flow estimated by CU and fluorescent microspheres. In all experiments a probability value of less than 0.05 was considered significant.
The interobserver reliability of CU measurements was performed by two independent observers (M.C. and M.S.-C.) in 10 mice. To measure intraobserver reliability, a single observer (M.C.) repeated the measurements on the same loops several weeks after the first measurement set. Intraobserver and interobserver reliabilities were assessed using intraclass correlation coefficients (ICCs).
Results
BAT Blood Flow Assessed by CU: Feasibility, Detection Range, and Variability
Precise localization of the BAT was confirmed by histology in the first 5 mice studied (Figure 1A and B). To examine the feasibility of using CU to measure BAT blood flow, a total of 424 contrast microbubbles replenishment curves in 55 animals were analyzed. Sixty-five curves (15%) had a goodness-of-fit <0.8 and were not used, however, no animals had to be excluded from the analysis. No saturation of the CU signal intensity was noted in the tissues investigated either before or after NE infusion. After destruction of contrast microbubbles with high-energy frames, the signal intensity in the tissues returned to the pre-destruction plateau (Figure 2A and 2B).
Figure 1.
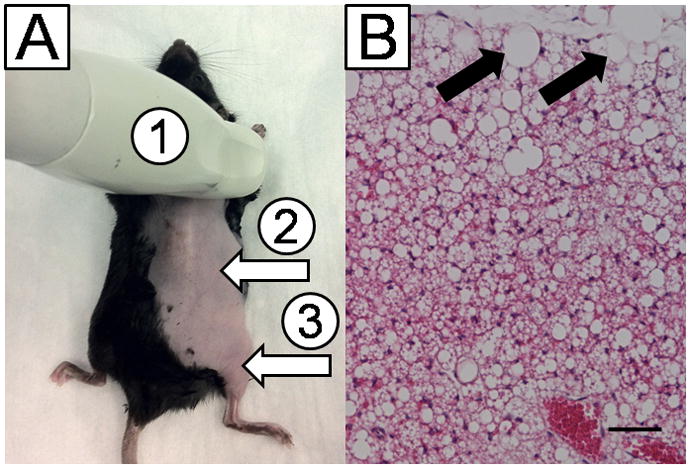
Localization of brown adipose tissue (BAT). A: Contrast ultrasound (CU) was performed in (1) interscapular BAT, (2) right kidney, and (3) right quadriceps femoris muscle of mice. The picture shows the placement and positioning of the ultrasound probe during acquisition of BAT images (1). White arrows represent the site and orientation of the CU acquisition plane for (2) kidney and (3) muscle. B: Hematoxylin & eosin staining of surgically removed BAT. Black arrows point to white adipose tissue at the edge of the specimen. The black bar represents 100 μm.
Figure 2.
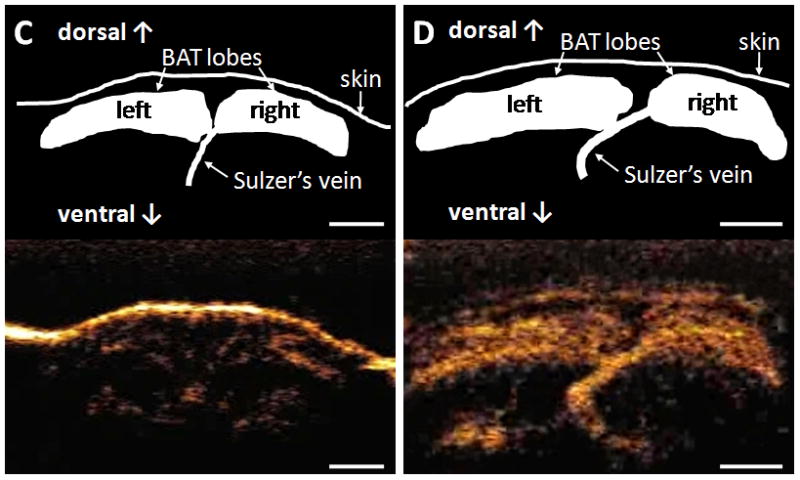
Replenishment curves of contrast microbubbles, and schematic drawings and representative still-frames of brown adipose tissue (BAT) acquired by contrast ultrasound in wild-type mice. A and B: Representative replenishment curves of contrast microbubbles at baseline (A) and during infusion of 1 μg·kg−1·min−1 norepinephrine (B). C and D: Schematic drawing and representative still-frame of interscapular BAT at baseline (C) and during infusion of 1 μg·kg−1·min−1 norepinephrine (D). White bars represent 2.5 mm.
In WT mice, when contrast microbubbles were administered via the jugular vein, baseline blood flow of BAT estimated by Aβ was 0.5±0.1 dB/s (Table). Infusion of 1 μg·kg−1·min−1 NE induced a 15-fold increase in BAT blood flow (p=0.026, Table and Figure 2C and 2D). Similar results were observed in WT mice infused via the tail vein (Table). The ICCs for the intraobserver and interobserver reliability of Aβ were 0.99 and 0.96, respectively.
Table 1.
Table Blood flow of brown adipose tissue (BAT), kidney, and quadriceps femoris muscle estimated by contrast ultrasound in 2-month-old wild-type mice.
|
|
|
|||
|---|---|---|---|---|
| Jugular vein (n=8) | Tail vein (n=4) | |||
| Baseline | NE | Baseline | NE | |
| BAT (dB/s) | 0.5±0.1 | 7.5±1.6* | 0.8±0.1 | 11.2±2.4* |
| Kidney (dB/s) | 10.4±1.9 | 6.3±0.9* | 9.2±2.9 | 8.6±1.6 |
| Muscle (dB/s) | 0.3±0.1 | 0.4±0.1 | 0.4±0.2 | 0.4±0.01 |
Infusions of contrast microbubbles and 1 μg·kg−1·min−1 norepinephrine (NE) were administered either via the jugular vein or tail vein.
p<0.05 Baseline differs from NE.
Data are depicted as mean values±SEM.
Correlation of CU-estimated Blood Flow to Microspheres-derived Blood Flow
Organ blood flow estimated by CU was compared to that obtained using microspheres. All tissues contained more than 500 microspheres/mg. The microsphere concentrations in BAT were higher in mice that received an infusion of 0.5 or 1 μg·kg−1·min−1 of NE than in mice that received an infusion of saline (Figure 3A). Conversely, both doses of NE resulted in reduced microsphere concentrations in the kidney. The concentration of microspheres was similar in both kidneys, indicating that the microspheres were evenly distributed in the circulation after injection into the left carotid artery. The ratio of BAT to right kidney blood flow, as measured by CU, closely correlated with that obtained using microspheres (R2=0.86, p<0.001; Figure 3B).
Figure 3.
Blood flow in brown adipose tissue (BAT) and kidney was assessed by contrast ultrasound (CU) and fluorescent microspheres. Baseline values are depicted in white (n=3). Values obtained at norepinephrine (NE) concentrations of 0.5 μg·kg−1·min−1 (n=3) and 1 μg·kg−1·min−1 (n=3) are depicted in grey and black, respectively. Data are shown as mean values±SEM. * p<0.05, NE differs from baseline. A: Microspheres-derived BAT and kidney blood flow. B: Correlation of CU-estimated and microspheres-derived BAT-to-kidney blood flow ratios.
NE-induced BAT Blood Flow is Impaired in UCP1−/− Mice
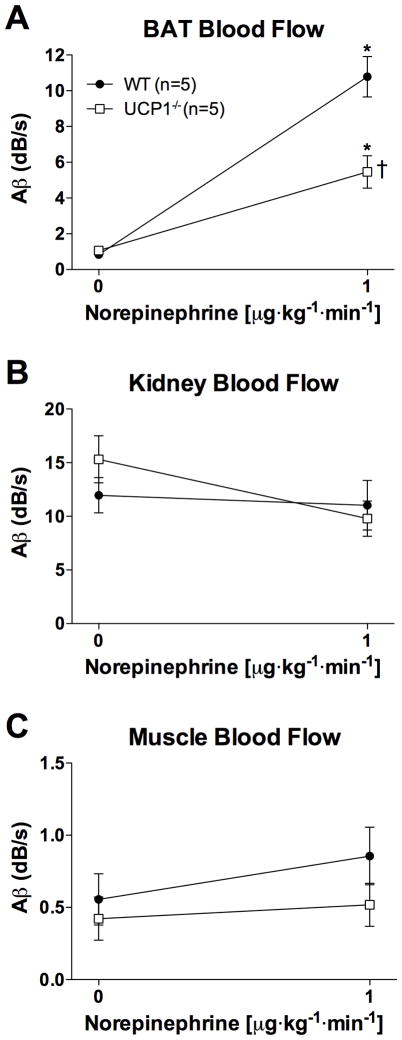
In order to investigate whether intact activation is required to increase BAT blood flow, CU studies were performed in UCP1−/− mice. Blood flow of BAT at baseline was similar between WT and UCP1−/− mice housed at thermoneutral conditions (0.8±0.1 vs 1.1±0.1 dB/s, respectively). NE infusion increased BAT blood flow both in WT and UCP1−/− mice (p=0.003 and 0.015, respectively; Figure 4A). The increase in BAT blood flow, however, was markedly blunted in the UCP1−/− mice when compared to WT mice (5-fold vs 14-fold, respectively, p=0.026, Figure 4A). Blood flow in kidney (Figure 4B) and muscle (Figure 4C) did not significantly differ between WT and UCP1−/− mice (p=0.261 and 0.557, respectively).
Figure 4.
Blood flow in (A) brown adipose tissue (BAT), (B) kidney, and (C) in the quadriceps femoris muscle estimated by contrast ultrasound before and during infusion of 1 μg·kg−1·min−1 norepinephrine (NE) in wild-type (WT) mice and in mice deficient in uncoupling protein 1 (UCP1−/−). Data are depicted as mean values±SEM. * p<0.05, BAT blood flow at NE 1 μg·kg−1·min−1 differs from baseline BAT blood flow. † p<0.05, BAT blood flow in UCP1−/− mice differs from BAT blood flow in WT mice.
Real-time PCR of BAT in WT and UCP1−/− Mice
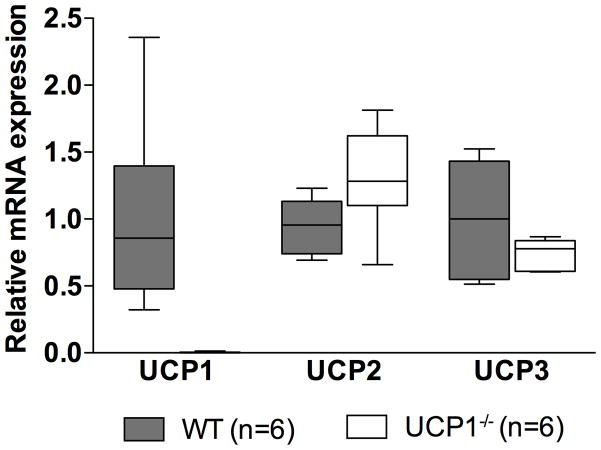
To account for possible compensatory changes in the expression of other uncoupling proteins in UCP1−/− mice, relative mRNA expression of UCP1, UCP2, and UCP3 was measured in the BAT of both WT and UCP1−/− mice. As expected, mRNA expression of UCP1 was not detectable in UCP1−/− mice (Figure 5). mRNA expression levels of UCP2 and UCP3 did not differ in BAT from WT and UCP1−/− mice (p=0.077 and 0.185, respectively).
Figure 5.
Relative uncoupling protein (UCP) mRNA levels in mice deficient in UCP1 (UCP1−/−). Data are depicted as mean values±SEM.
NOS-dependent Regulation of BAT Blood Flow Response
Infusion of 1 μg·kg−1·min−1 NE increased CU-derived BAT blood flow in both NOS1−/− and NOS3−/− mice, as well as in WT mice treated with the NOS inhibitor L-NAME (ANOVA, p<0.001, Figure 6A). The NE-induced increase in BAT blood flow in NOS3−/− mice and L-NAME-treated mice was lower than that in WT mice (p=0.034 and 0.016, respectively), whereas the NE-induced increase in BAT blood flow in NOS1−/− mice did not differ from WT mice (p=0.456). In contrast to NOS1−/− and NOS3−/− mice, kidney blood flow was lower in WT mice treated with L-NAME than in untreated WT mice (p=0.018). Blood flow to the kidney did not change in any of the groups during infusion of 1 μg·kg−1·min−1 NE (Figure 6B, p=0.964). Muscle blood flow was not significantly influenced by genotype or treatment with NE (Figure 6C, p=0.134).
Figure 6.
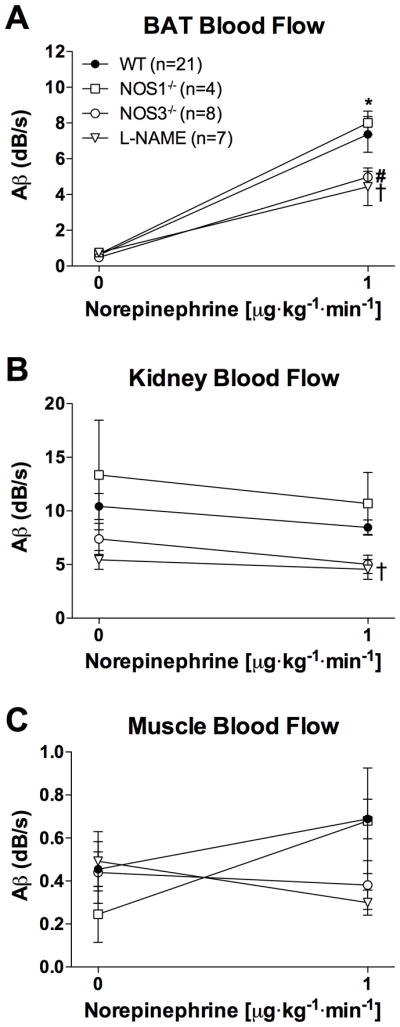
Blood flow in (A) brown adipose tissue (BAT), (B) kidney, and (C) quadriceps femoris muscle estimated by contrast ultrasound before and during infusion of 1 μg·kg−1·min−1 norepinephrine (NE). Mice deficient in nitric oxide synthase 1 (NOS1−/−) or 3 (NOS3−/−), as well as animals treated with NG-nitro-L-arginine methylester (L-NAME) are compared to wild-type (WT) mice. Data are depicted as mean values±SEM. * p<0.001, BAT blood flow at NE 1 μg·kg−1·min−1 differs from baseline BAT blood flow in all groups. † p<0.05, BAT and kidney blood flow in mice treated with L-NAME differs from BAT and kidney blood flow in WT mice. # p<0.05, BAT blood flow in NOS3−/− mice differs from BAT blood flow in WT mice.
Effect of NE on Cardiac Output of Mice
To exclude the possibility that variations in cardiac output were responsible for the differences in the BAT blood flow responses observed between mouse strains, cardiac output was estimated by echocardiography. Continuous infusion of 1 μg·kg−1·min−1 NE increased cardiac output in WT and UCP1−/− mice, and in WT mice treated with L-NAME (p<0.001). Cardiac output in WT mice (6.4±0.5 ml/min at baseline vs 12.5±1.0 ml/min) and UCP1−/− mice (7.8±0.9 ml/min at baseline vs 12.1±2.2 ml/min) increased to a similar degree, whereas cardiac output increased to a lesser degree in WT mice treated with L-NAME (7.0±1.0 ml/min at baseline vs 8.8±0.9 ml/min; p=0.03 compared to untreated WT animals).
Discussion
The present study describes and validates CU as a novel noninvasive method that is able to identify and localize BAT and characterize the regulation of its blood flow in mice. Contrast ultrasound detected increases in BAT blood flow associated with NE-induced BAT stimulation. Although stimulation of BAT by NE was accompanied by an increase in BAT blood flow in UCP1−/− mice, this increase was markedly blunted compared to the increase observed in WT animals. The response of BAT blood flow after stimulation by NE was also measured in mice with reduced NOS-dependent NO synthesis. The increase in blood flow of BAT was reduced in NOS3−/− mice and mice treated with L-NAME when compared to WT animals.
Detection of BAT blood flow by CU was feasible in all mice studied. Interobserver and intraobserver variabilities in CU-estimated BAT blood flow were similar to those reported for CU-estimated myocardial blood flow in mice.23 Continuous infusion of contrast microbubbles yielded a constant concentration in the organs imaged (plateau), and the length of the loop acquisition allowed the replenishment of contrast microbubbles to the plateau after their destruction by high-energy ultrasound pulses. Both conditions are necessary to fit an exponential curve to the contrast microbubble signal intensity over time. Despite the high magnitude of changes in BAT blood flow, which was far greater than that of myocardial blood flow before and after vasodilation,23 curves were analyzable both at low and high BAT blood flow. Contrast ultrasound was feasible using a jugular venous line for infusion, as well as the less invasive approach of infusion via the tail vein.
Early studies of BAT in rodents measured its blood flow with non-survival approaches.9,13,33 Using radioactive microspheres, Foster et al demonstrated a 13-fold increase of BAT blood flow in rats infused with NE,9 similar to that we found in mice both with microspheres and CU. Of note, the magnitude of the NE-induced increase in BAT blood flow appears comparable to that detected with the more physiologic stimulus of cold exposure.34–36 In addition, Virtanen et al reported a 15-fold increase in uptake of FDG into supraclavicular BAT after cold exposure in humans,5 and Carter et al observed a 15- to 16-fold activation of BAT in mice exposed to cold.34,35 These data indicate that, irrespective of species and mode of BAT activation, the degree of metabolic activation appears to be of similar magnitude as the blood flow increase of BAT.
Activation of BAT by the sympathetic nervous system results in increased blood flow to the tissue, which is necessary in order to provide a sufficient supply of oxygen, lipids, and glucose to BAT. Additionally, the increased blood flow helps dissipate the heat produced in BAT throughout the rest of the body.2 One objective of this study was to investigate whether the changes in BAT blood flow are coupled to the changes in BAT activation. Contrast ultrasound revealed that BAT blood flow during infusion of NE was lower in UCP1−/− mice than in WT mice. UCP1−/− mice demonstrate intact lipolysis and oxidative capacity at baseline, but treatment with NE fails to increase oxygen consumption and thermogenesis in these animals.37 Thus, our findings demonstrate that when BAT activation is decreased, as in UCP1−/− mice, BAT blood flow is reduced.
In UCP1−/− mice, 1 μg·kg−1·min−1 NE still induced a 5-fold increase in blood flow, which corresponds to approximately 30% of the increase in BAT blood flow observed in WT mice. One possible explanation for the NE-induced increase in BAT blood flow in UCP1−/− mice is that it is due to an increase in cardiac output. Since the increase of cardiac output in UCP1−/− mice was markedly lower than the increase in blood flow of these mice (55% vs 500%, respectively), it is unlikely that changes in cardiac output can fully account for the augmented BAT blood flow. One limitation of our study, however, is that the estimation of cardiac output using M-Mode echocardiography, although easy to obtain and to interpret, relies on several geometrical assumptions.
Another possible explanation for the increase in BAT blood flow noted in NE-treated UCP1−/− mice is that there is a concomitant increase in BAT oxygen consumption mediated by other UCP proteins. Several investigators have reported that UCP2 mRNA levels are increased in the BAT of UCP1−/− mice, suggesting a compensatory increase in UCP synthesis.14,15 In contrast, the current study did not reveal increased UCP2 gene expression in UCP1−/− mice, possibly due to their housing at thermoneutral conditions. Finally, it is conceivable that a limited vasodilatory response to NE in BAT occurs even though there is no increase in oxygen consumption and thermogenesis. Such dissociation of the vascular and thermogenic response has been reported with chronic BAT activation. Both increased expression of vascular endothelial growth factor and angiogenesis are preserved in BAT of UCP1−/− mice chronically exposed to cold.38,39
Nitric oxide plays an important role in mitochondrial biogenesis and function of BAT.20 The most prominent pathway for NO production is via NOS enzymes. Both NOS122 and NOS316,23 can mediate vasodilation induced by beta-adrenergic stimulation. Our data suggest that NOS3-dependent NO production is involved in the acute regulation of BAT blood flow. NE infusion increased BAT blood flow in both NOS3−/− mice and mice treated with the NOS inhibitor L-NAME, but the increase in BAT blood flow was less than that observed in WT mice (32% and 40%, respectively). Nagashima et al previously reported that L-NAME completely abolished the NE-induced increase in BAT blood flow in rats.11 The doses of both NE and L-NAME reported by Nagashima et al, however, were markedly higher (12-fold and 10-fold, respectively) than those used in the present experiments, and may have impaired BAT blood flow by decreasing the cardiac output.40
In summary, the current findings validate CU as a noninvasive method to estimate BAT blood flow in vivo in mice. Furthermore, the data suggest that CU can detect changes in blood flow induced by stimuli that activate BAT. Our data imply that BAT blood flow and activation are at least partially coupled, and that NOS3-dependent NO production plays a role in induction of BAT blood flow by NE. Contrast ultrasound could be used to monitor the effect of anti-obesity therapies that modulate BAT function. Furthermore, if translated into humans, CU could be performed serially, and thus be applied in a longitudinal follow-up of BAT-modulating therapies.
Acknowledgments
The authors thank Drs. Randall Mynatt and Leslie Kozak (Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA) for generously providing UCP1−/− mice, and Dr. Rajeev Malhotra (Massachusetts General Hospital, Boston, MA) for assistance in statistical analysis.
Sources of Funding
This work was supported by NIH grant R21-DK092909 (to M.S.-C.), by a Scientist Development Grant 10SDG2610313 from the American Heart Association (to E.S.B.), and by the Fondation LeDucq (K.D.B.). Definity® was generously provided by Lantheus Medical Imaging Inc.
Footnotes
Disclosures
None.
References
- 1.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 3.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 6.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol-Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 8.Lever JD, Nnodim JO, Symons D. Arteriovenous anastomoses in interscapular brown adipose tissue in the rat. J Anat. 1985;143:207–210. [PMC free article] [PubMed] [Google Scholar]
- 9.Foster DO, Frydman ML. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol. 1978;56:110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- 10.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 11.Nagashima T, Ohinata H, Kuroshima A. Involvement of nitric oxide in noradrenaline-induced increase in blood flow through brown adipose tissue. Life Sci. 1994;54:17–25. doi: 10.1016/0024-3205(94)00573-7. [DOI] [PubMed] [Google Scholar]
- 12.Ma SW, Foster DO. Brown adipose tissue, liver, and diet-induced thermogenesis in cafeteria diet-fed rats. Can J Physiol Pharmacol. 1989;67:376–381. doi: 10.1139/y89-061. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell NJ, Stock MJ. Influence of noradrenaline on blood flow to brown adipose tissue in rats exhibiting diet-induced thermogenesis. Pflugers Arch. 1981;389:237–242. doi: 10.1007/BF00584784. [DOI] [PubMed] [Google Scholar]
- 14.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 15.Matthias A, Jacobsson A, Cannon B, Nedergaard J. The bioenergetics of brown fat mitochondria from UCP1-ablated mice. Ucp1 is not involved in fatty acid-induced de-energization (“uncoupling”) J Biol Chem. 1999;274:28150–28160. doi: 10.1074/jbc.274.40.28150. [DOI] [PubMed] [Google Scholar]
- 16.Al Zubair K, Bexis S, Docherty JR. Relaxations to beta-adrenoceptor subtype selective agonists in wild-type and NOS-3-KO mouse mesenteric arteries. Eur J Pharmacol. 2008;587:216–223. doi: 10.1016/j.ejphar.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Rebich S, Devine JO, Armstead WM. Role of nitric oxide and cAMP in beta-adrenoceptor-induced pial artery vasodilation. Am J Physiol. 1995;268:H1071–1076. doi: 10.1152/ajpheart.1995.268.3.H1071. [DOI] [PubMed] [Google Scholar]
- 18.Akimoto Y, Horinouchi T, Shibano M, Matsushita M, Yamashita Y, Okamoto T, Yamaki F, Tanaka Y, Koike K. Nitric oxide (NO) primarily accounts for endothelium-dependent component of beta-adrenoceptor-activated smooth muscle relaxation of mouse aorta in response to isoprenaline. J Smooth Muscle Res. 2002;38:87–99. doi: 10.1540/jsmr.38.87. [DOI] [PubMed] [Google Scholar]
- 19.Brawley L, Shaw AM, MacDonald A. Role of endothelium/nitric oxide in atypical beta-adrenoceptor-mediated relaxation in rat isolated aorta. Eur J Pharmacol. 2000;398:285–296. doi: 10.1016/s0014-2999(00)00319-8. [DOI] [PubMed] [Google Scholar]
- 20.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi-Utsumi K, Gao B, Ohinata H, Hashimoto M, Yamamoto N, Kuroshima A. Enhanced gene expression of endothelial nitric oxide synthase in brown adipose tissue during cold exposure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R623–626. doi: 10.1152/ajpregu.00310.2001. [DOI] [PubMed] [Google Scholar]
- 22.Capettini LS, Cortes SF, Lemos VS. Relative contribution of eNOS and nNOS to endothelium-dependent vasodilation in the mouse aorta. Eur J Pharmacol. 2010;643:260–266. doi: 10.1016/j.ejphar.2010.06.066. [DOI] [PubMed] [Google Scholar]
- 23.Raher MJ, Thibault H, Poh KK, Liu R, Halpern EF, Derumeaux G, Ichinose F, Zapol WM, Bloch KD, Picard MH, Scherrer-Crosbie M. In vivo characterization of murine myocardial perfusion with myocardial contrast echocardiography: validation and application in nitric oxide synthase 3 deficient mice. Circulation. 2007;116:1250–1257. doi: 10.1161/CIRCULATIONAHA.107.707737. [DOI] [PubMed] [Google Scholar]
- 24.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 25.Vogel R, Indermuhle A, Reinhardt J, Meier P, Siegrist PT, Namdar M, Kaufmann PA, Seiler C. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J Am Coll Cardiol. 2005;45:754–762. doi: 10.1016/j.jacc.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 26.Sjoberg KA, Rattigan S, Hiscock N, Richter EA, Kiens B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. Am J Physiol Heart Circ Physiol. 2011;301:H450–458. doi: 10.1152/ajpheart.01174.2010. [DOI] [PubMed] [Google Scholar]
- 27.Ohlson KB, Lindahl SG, Cannon B, Nedergaard J. Thermogenesis inhibition in brown adipocytes is a specific property of volatile anesthetics. Anesthesiology. 2003;98:437–448. doi: 10.1097/00000542-200302000-00025. [DOI] [PubMed] [Google Scholar]
- 28.DosSantos RA, Alfadda A, Eto K, Kadowaki T, Silva JE. Evidence for a compensated thermogenic defect in transgenic mice lacking the mitochondrial glycerol-3-phosphate dehydrogenase gene. Endocrinology. 2003;144:5469–5479. doi: 10.1210/en.2003-0687. [DOI] [PubMed] [Google Scholar]
- 29.Rauch JC, Hayward JS. Topography and vascularization of brown fat in a small nonhibernator (deer mouse, Peromyscus maniculatus) Can J Zool. 1969;47:1301–1314. doi: 10.1139/z69-203. [DOI] [PubMed] [Google Scholar]
- 30.Mattson DL, Meister CJ. Renal cortical and medullary blood flow responses to L-NAME and ANG II in wild-type, nNOS null mutant, and eNOS null mutant mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R991–997. doi: 10.1152/ajpregu.00207.2005. [DOI] [PubMed] [Google Scholar]
- 31.Tournoux F, Petersen B, Thibault H, Zou L, Raher MJ, Kurtz B, Halpern EF, Chaput M, Chao W, Picard MH, Scherrer-Crosbie M. Validation of noninvasive measurements of cardiac output in mice using echocardiography. J Am Soc Echocardiogr. 2011;24:465–470. doi: 10.1016/j.echo.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Oosterhout MF, Willigers HM, Reneman RS, Prinzen FW. Fluorescent microspheres to measure organ perfusion: validation of a simplified sample processing technique. Am J Physiol. 1995;269:H725–733. doi: 10.1152/ajpheart.1995.269.2.H725. [DOI] [PubMed] [Google Scholar]
- 33.Heim T, Hull D. The blood flow and oxygen consumption of brown adipose tissue in the new-born rabbit. J Physiol. 1966;186:42–55. doi: 10.1113/jphysiol.1966.sp008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter EA, Bonab AA, Hamrahi V, Pitman J, Winter D, Macintosh LJ, Cyr EM, Paul K, Yerxa J, Jung W, Tompkins RG, Fischman AJ. Effects of burn injury, cold stress and cutaneous wound injury on the morphology and energy metabolism of murine brown adipose tissue (BAT) in vivo. Life Sci. 2010;89:78–85. doi: 10.1016/j.lfs.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter EA, Bonab AA, Paul K, Yerxa J, Tompkins RG, Fischman AJ. Association of heat production with 18F-FDG accumulation in murine brown adipose tissue after stress. J Nucl Med. 2011;52:1616–1620. doi: 10.2967/jnumed.111.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty acid-induced thermogenesis. J Biol Chem. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 38.Fredriksson JM, Nikami H, Nedergaard J. Cold-induced expression of the VEGF gene in brown adipose tissue is independent of thermogenic oxygen consumption. FEBS Lett. 2005;579:5680–5684. doi: 10.1016/j.febslet.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 39.Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, Cannon B, Cao Y. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Maling HM, Highman B. Exaggerated ventricular arrhythmias and myocardial fatty changes after large doses of norepinephrine and epinephrine in unanesthetized dogs. Am J Physiol. 1958;194:590–596. doi: 10.1152/ajplegacy.1958.194.3.590. [DOI] [PubMed] [Google Scholar]