Abstract
Heart failure is increasing in incidence throughout the world, especially in industrialized countries. Although the current therapeutic modalities have been successful in stabilizing the course of heart failure, morbidity and mortality remain quite high and there remains a great need for innovative breakthroughs that will offer new treatment strategies for patients with advanced forms of the disease. The past few years have witnessed a greater understanding of the molecular underpinnings of the failing heart, paving the way for novel strategies in modulating the cellular environment. As such, gene therapy has recently emerged as a powerful tool offering the promise of a new paradigm for alleviating heart failure. Current gene therapy research for heart failure is focused on exploring potential cellular targets and preclinical and clinical studies are ongoing toward the realization of this goal. Efforts also include the development of sophisticated viral vectors and vector delivery methods for efficient transduction of cardiomyocytes.
Keywords: Heart failure, Gene therapy, Calcium cycling proteins, SERCA2a, Phospholamban, inhibitor 1, Protein phosphatase 1, Adeno-associated virus, Neutralizing antibodies, Pharmacologic therapy
Introduction
Heart failure (HF) is a clinical syndrome of many etiologies in which the heart fails to pump the required amount of blood to provide the various organs with their metabolic needs. A major cause of morbidity, the number of HF cases in the United States in 2008 was 5.8 million, and HF was a contributing cause of 283,000 deaths in 2006. About 50% of those diagnosed with HF will die within 5 years [1, 2]. Recently, a deeper understanding of molecular and cellular changes characterizing the failing heart has made targeting by gene transfer a clinical possibility. In this review, we will discuss the three main factors involved for successful gene therapy: 1) selection of appropriate molecular targets; 2) successful vector design; and 3) targeted delivery methods. Specifically, we will discuss our experience in cardiac gene therapy in the context of calcium cycling and the reuptake of calcium by sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA2a) pump into the sarcoplasmic reticulum (SR).
Selection of Targets
Excitation–Contraction Coupling and Relaxation
Cardiomyocyte ultrastructure
In the cardiomyocyte, the sarcolemma extensively invaginates to form extensive T-shaped tubules that are in intimate contact with the interdigitated contractile myofibrils actin and myosin. T-tubules are also in very close proximity with the SR and intermyofibrillar mitochondria (IMFM), which are organelles of calcium storage and release. The SR forms a cistern tightly opposing the T-tubules. Located on the SR membrane, the ryanodine receptor (RYR) is an important protein that allows for the release of calcium stored in the SR into the cytosol. The L-type calcium channels (LTCC) are located on the T-tubules, which allows for the entry of Ca2+ into the cytosol in a voltage-dependent manner. SERCA2a protein is a crucial pump also located on the SR and is responsible for the reuptake of calcium into the SR, where it is stored, linked to proteins calsequestrin, calnexin, and calreticulin.
Calcium cycles during contraction–relaxation
Calcium has a central role in the regulation of the phases of the cardiac cycle. In the contraction phase, waves of depolarization spread down the transverse tubules, opening up LTCC (Fig. 1) and permitting the entry of small amounts of calcium from the extracellular space into the cytosol. This flux of calcium ions activates RYRs, inducing the release of larger amounts of calcium from the SR. In the cytosol, the released calcium binds to troponin (Tn), causing tropomyosin to expose the myosin head attachment sites on the actin myofibril. This enables myosin and actin to interact and leads to cardiomyocyte contraction. Cardiomyocyte relaxation phase is initiated with the activation of SERCA2a pump by high levels of cytosolic calcium. In the relaxation phase, calcium is extruded from the cytoplasm, with 75% being pumped into the SR by SERCA2a and 25% into the extracellular space by the sodium calcium exchanger (NCX) found on the sarcolemma [3, 4].
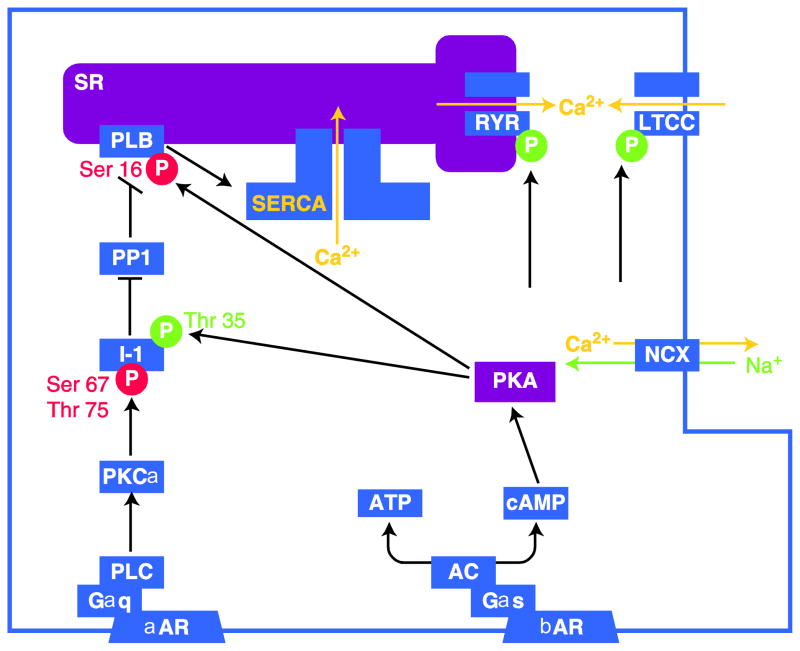
Fig. 1.
Proteins involved in calcium cycling. Schematic showing the augmentation of calcium cycling and cardiac function through the β-adrenergic pathway and decrease in calcium cycling and cardiac function through the α-adrenergic pathway. PLB inhibits SERCA. PLB phosphorylation by PKA at Ser16 relieves the inhibitory function of PLB and increases SERCA activity and Ca2+ uptake into SR. PLB is also regulated by PP1. Dephosphorylation of PLB via PP1 reverses inhibition of SERCA. I-1 inhibits the function of PP1. I-1 is activated by phosphorylation at Thr35 by PKA, while phosphorylation by PKCα at Ser67 and Thr75 inhibits I-1. Arrow heads show either activation or phosphorylation of the target; bar heads show either deactivation or dephosphorylation of target. Green encircled P represents phosphorylation to increase target function. Red encircled P represents phosphorylation to decrease target function
αAR, α-adrenergic receptor; AC, adenylyl cyclase; ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; βAR, β-adrenergic receptor; Ca2+, calcium ion; cAMP, cyclic adenosine monophosphate; Gαs, G protein αs subunit; Gαq, G protein αq subunit; I-1, protein phosphatase-1 inhibitor 1; LTCC, L-type calcium channel; Na+, sodium ion; NCX, sodium calcium exchanger; P, phosphate; PKA, protein kinase A; PKCα, protein kinase C; PLB, phospholamban; PLC, phospholipase C; PP1, protein phosphatase 1; RYR, ryanodine receptor; SERCA, sarcoplasmic endoplasmic reticulum calcium ATPase; SR, sarcoplasmic reticulum
The activity of SERCA2a is controlled by the protein phospholamban (PLB). PLB has two regulatory sites: one site is phosphorylated by protein kinase A (PKA) at position serine 16 in response to β-adrenergic stimulation; the other is activated by calcium and calmodulin. Another cytosolic protein implicated in calcium cycling is protein phosphatase 1 (PP1), which indirectly affects SERCA2a function by activating PLB. PP1 is itself regulated by the protein inhibitor 1 (I-1) [5].
During sympathetic stimulation, there is augmentation of calcium cycling and cardiac contraction by phosphorylation of the proteins involved, including LTCC, RYR, Tn, PLB, and I-1. Direct PKA phosphorylation of PLB leads to release of its inhibition of SERCA2a, enhancing cardiac contraction. Moreover, PKA phosphorylation of I-1 at Thr35 leads to I-1 inhibition of PP1 and cessation of PP1 dephosphorylation of PLB, thus further increasing activity of SERCA2a. Recently, it was shown that protein kinase C-α (PKC-α) phosphorylation of I-1 at Ser67 and Thr75 reduces I-1 inhibition of protein phosphatase, leading to enhanced activity of the PP1 and causing depressed cardiac function [6••].
Pathophysiology in Heart Failure
Congestive HF (CHF) is a debilitating clinical syndrome associated with the inability of the heart to properly pump blood to the vasculature. As a result, there is activation of the various neurohormonal pathways, including the renin-angiotensin-aldosterone system (RAAS) and sympathetic activation, in an attempt to restore adequate blood flow. At the cardiomyocyte level, CHF is associated with diffuse changes, including inefficient energy production and utilization as well as alterations in signal transduction mechanisms and proteins. Changes in the expression profiles and activity of proteins occur at the level of the extracellular matrix, sarcolemma, cytosol, and in multiple organelles including the SR and mitochondria. The aforementioned alterations of proteins result in abnormal calcium handling and cycling during the cardiac cycle. Moreover, CHF is associated with changes in shape and size due in part to cardiomyocyte death in the form of necrosis, apoptosis, and autophagy [7]. We will focus on the changes of the proteins involved in calcium channel cycling, which have been best characterized as potential targets for future gene therapy.
Proteins Involved in Calcium Cycling
SERCA2a and phospholamban
In HF, the central perturbation of normal calcium cycling is the decrease in levels of SERCA2a expression, leading to a decrease in systolic calcium, prolonged calcium reuptake, and elevated end-diastolic calcium levels [8, 9]. In addition, the remaining SERCA2a is rendered dysfunctional in failing hearts by oxidative stress, nitration, and other forms of post-translational modifications. Moreover, there is an increase in the levels of the dephosphorylated form of regulatory PLB leading to further inhibition and attenuation of SERCA2a activity. Intracellular calcium levels are further increased in diastole by PKA hyperphosphorylation of RYR channels, increasing the probability of an open channel and a calcium leak into the cytosol.
Overexpression of SERCA2a levels by cardiomyocyte gene delivery has led to the restoration of the abnormal calcium transients leading to improved cardiac contractility, reduction of the frequency of arrhythmias, and improved oxygen utilization in rodent and large animal models with HF [10].
Attenuating the inhibition of SERCA2a by dephosphorylated PLB with pseudophosphorylated PLB gene transfer has resulted in improved SERCA2a activity and cardiac function in a sheep HF model [11]. However, the initial enthusiasm generated by these findings has been thwarted by the discovery of PLB-null genotypes that display a dilated cardiomyopathy phenotype [12].
I-1
In HF, total and dephosphorylated I-1 levels are markedly decreased, leading to impaired inhibition of PP1 and, ultimately, attenuation of SERCA2a activity by active PLB [13].
It has been shown that introduction of a constitutively active form of phosphorylated I-1 levels in HF rats leads to enhanced SR calcium transport and improved cardiac contractility. Moreover, I-1 gene transfer has resulted in partial reversal of cardiac remodeling associated with HF [14].
S100A1
Found predominantly in the myocardium, S100A1 is another regulatory protein belonging to the S100 family of calcium-binding proteins. This protein has a role regulating SERCA2a and RYR activity. The level of S100A1 protein was found to be diminished in HF [15]. Restoration of S100A1 levels through gene delivery in large animal HF models was found to improve cardiac function parameters.
The Adrenergic System
HF leads to a compensatory activation of the adrenergic system in an attempt to restore cardiac output and blood flow. However, extended activation leads to the attenuation of the adrenergic response. This is evident through changes in its downstream signaling pathway. Changes include downregulation of β-adrenergic receptors (β-ARs) and functional decoupling between β-ARs and G proteins through β-AR phosphorylation. The latter is due to increases in β-adrenergic receptor kinase-1 (βARK1) activity and increased Gi-protein function. As such, multiple interventions at different levels of the adrenergic pathway have been attempted with gene therapy to revitalize response to stimulation. Direct and intracoronary β2-AR gene delivery to the myocardium resulted in augmented cardiac performance in non-HF rabbits [16, 17]. Moreover, overexpression of a βARK1-inhibiting protein, βARKct, led to the reversal of left ventricle (LV) dysfunction 3 weeks post–myocardial infarction (MI) in a rabbit MI model [18]. Furthermore, augmentation of the adrenergic system by activation of adenylyl cyclase VI (ACVI) in ACVI transgenic mice with severe CHF phenotype 5 weeks post-MI resulted in improved LV contractile function [19].
Gene Therapy and Cell Death
As mentioned, cell death in HF takes place in the form of necrosis, apoptosis, and autophagy.
Apoptosis and autophagy serve as programmed cell death, and are involved in normal organ development and response to cellular insult. Some of the gene therapy treatments aimed at improving function in HF also have resulted in limiting cardiomyocyte death. Overexpression of a key antiapoptotic protein, Bcl2, with adenovirus-mediated gene transfer led to decreased apoptosis and ventricular remodeling in a regional ischemia/reperfusion model in rabbits [20]. Increased I-1 activity in transgenic mice significantly decreased necrotic and apoptotic cell death in an ischemia/reperfusion-induced injury model in mice [5]. It was found that elevated β2 receptor in HF had an antiapoptotic function through a Gi-mediated pathway concentration [21]. Moreover, addition of S100A1 to the extracellular medium of neonatal cardiomyocytes was shown to inhibit apoptosis upon exposure to oxidative stress [22].
Choice of Vectors in Gene Therapy
Vessels of gene delivery are classified into two main categories: nonviral and recombinant viral vectors. This review will focus on viral vectors. Briefly, nonviral vectors include plasmid DNA, liposome-DNA complexes, and polymer-DNA complexes [23, 24]. The advantages of nonviral vectors include the ease of vector production, the reduced limitation on the expression cassette size, and the relatively minimal biosafety risks. Their limitations include low transfection efficiency and transient effect due to their intracellular degradation. This makes nonviral vectors applicable in instances where transient, short-lived expression of particular genes is desired, such as the transient expression of angiogenic factors by a modest number of gene-modified cells to obtain the desired angiopathic phenotype. In contrast, in cardiovascular disease states, such as HF, a widespread and sustained transgene expression is needed to achieve the desired therapeutic effects. For these disease states, viral vectors are the most suitable due to their high gene transfer efficiency and, for specific vectors, long-term transgene expression.
Adenoviral Vectors
Recombinant adenoviral vectors are used mainly in preclinical small animal model studies. Adenoviral vectors can be produced in high titers and are very efficient in transducing the myocardium in vivo [25]. However, the duration of transgene expression is limited to 1 week. A major limitation for the use of adenoviral vectors in translational research is the intense immune and inflammatory response that they evoke.
Lentivirus Vector
Lentivirus vectors transduce mitotically quiescent cells through genomic integration. Positive attributes of this virus are the ability to confer long-term stable transgene expression and higher packaging capacity in comparison to recombinant adeno-associated viruses (r-AAVs). However, clinically lentiviruses are less desirable due to the potential pathogenicity of the parental virus, which is based on the human immunodeficiency virus type 1 (HIV-1) [26]. Modifications, such as deletion of all accessory proteins from the packaging system, separation of packaging elements into multiple plasmids, and the use of a chimeric 5′-long-term repeat (LTR) and a self-inactivating 3′-LTR in the vector plasmid, have been made to make the virus more clinically suitable [27, 28].
Adeno-associated Virus
The wild-type adeno-associated virus (AAV) is a single-stranded nonenveloped virus that belongs to the Parvoviridae family and Dependovirus genus, requiring helper viruses for replication. The AAV is a naturally occurring, nonpathogenic, nonintegrating virus that has a 4.8-kb gene consisting of two open readings frames (ORFs) that are flanked by inverted terminal repeats (ITRs). The right ORF codes for three structural proteins, VP1, VP2, and VP3, and the left ORF codes for four regulatory proteins, Reps78, Reps68, Reps52, and Reps40 [29, 30]. To date, 12 different AAV serotypes have been identified, with serotypes AAV1, AAV8, and AAV9 showing preferential tropism to the cardiomyocyte [31]. There have been r-AAVs produced containing a therapeutic gene of interest, with the appropriate promoter, polyadenylation signal flanked by ITRs. Vectors of r-AAVs have been used in gene therapy for genetic disorders such as hemophilia, cystic fibrosis, and hereditary emphysema [32, 33]. The favorable clinical attributes of r-AAVs include lack of pathogenicity, low cytotoxicity, and a limited host immune response. The r-AAV vector has been shown to have stable long-term gene expression, and studies involving large animal models with single rAAV2 injection into the muscle and liver have demonstrated sustained expression for more than 3 years. Major limitations of the r-AAV vector systems include the ability to produce high titers of consistently pure and bioactive viral stocks, the limited packaging of capacity of a 4.8-kb plasmid, and the potential for preexisting neutralizing antibodies (Nabs) in human populations. Currently, the research involving AAVs has shifted from studying their wild-type AAVs to manipulating the viral capsid and designing new r-AAVs with greater specificity and greater tropism to the desired tissue. Peptide insertion complimentary to specific cell surface receptors or proteins at tolerant capsid sites has resulted in designing r-AAVs with 500-fold transduction efficiency in myeloid leukemia cells [34]. Other methods, such as DNA family shuffling, have led to the production of chimeric r-AAVs that are highly specific for skeletal muscle tissue and for cardiomyocytes [35••].
Delivery Methods
The ideal gene delivery method is one that transduces with the highest possible efficiency while targeting the largest possible number of cardiomyocytes. A number of different delivery methods are available for laboratory testing on both small and large animals. In small animals, the primary methods of delivery include tail vein injection, direct intramyocardial injection, and a catheter-based technique, which we have developed. In this approach, the aorta and the pulmonary artery are clamped for 30 seconds while a catheter is inserted into the LV apex. The catheter is then advanced beyond the aortic valve, delivering a high concentration of the viral vector to the coronary vasculature. This method allows for efficient and homogenous transgene delivery to the right and left ventricles of the heart [36]. Gene delivery methods for larger animals include pericardial delivery, surgical delivery, and catheter-based myocardial delivery. Pericardial delivery has low transduction efficiency with distribution limited mainly to the epicardium. Transduction efficiency can be improved by coadministration of proteolytic enzymes leading to the disruption of cellular and extracellular barriers to the myocardium [37]. Surgical gene transfer methods also have been described. Animals have been placed in bypass with anterograde injection of the vector, resulting in high efficiency of transduction. More recently, a complete surgical isolation of the heart in situ with retrograde viral administration was described [38]. In this technique, while on cardiopulmonary bypass, the heart is isolated with cross-clamping of the aorta and the pulmonary artery. The viral solution is then delivered in a retrograde fashion into the coronary sinus and allowed to circulate in the heart for 30 minutes while on cardiopulmonary bypass, resulting in efficient cardiomyocyte transduction. Although successful in large animals, the translation of this technique to patients with CHF may be limited [38].
Percutaneous catheter–based gene delivery to the myocardium in vivo involves administration through multiple routes, including the coronary arteries, the endocardium, and the coronary veins. Intracoronary delivery using a slow infusion as compared to bolus infusion is superior and offers, on average, a 50% transduction efficiency. Transduction efficiency is affected by a number of factors, such as animal species, the pharmacological agents used to permeabilize vasculature, and vector-related titer variability. In comparison, percutaneous endocardial delivery results in a more intense focal gene delivery of the myocardium and is suitable for therapies involving angiogenesis and, to a lesser extent, focal arrhythmia therapy [39]. Retrograde infusion of vector via the coronary veins is a novel catheter-based technique for myocardial gene delivery that has been shown to be superior to anterograde coronary injections methods. The retrograde infusion is more advantageous than anterograde intracoronary delivery method in that it bypasses diseased arteries that are likely to impede vector delivery [40]. Because retrograde infusion delivery required the stoppage of coronary blood flow, it may not be suitable for patients with severe HF. More recently, we developed a percutaneous and clinically applicable catheter-based gene delivery method that allowed slow selective anterograde myocardial gene transfer, resulting in a transduction efficiency of about 60%. This method has proven to be translatable in the human cardiac catheterization laboratory and was the one chosen for the SERCA2a gene therapy clinical trials.
Immune Response
Nabs to the AAV present a major impediment for the use of AAVs in gene therapy. The presence of Nabs in humans has impacted the efficiency of gene transfer and resulted in variability in the response to gene therapy between different individuals and across populations. Studies conducted from different populations showed the highest prevalence of Nabs to the AAV2 serotype closely followed by AAV1. In comparison, a lower prevalence of Nabs was found to AAV serotypes 5 to 9. The compliment system also was implicated in the activation of humoral immunity and development of Nabs [41•, 42]
T cell response and subsequent cytotoxic T lymphocyte (CTL) activation due to transient AAV capsid protein expression in transduced cells is also another factor impacting success of gene therapy [43]. It was found that the route of administration of the vector and also dosage played a role in the magnitude of CTL-mediated cytolytic immune response to transduced cells [44, 45]. In clinical trials, an enzyme-linked immunosorbent spot (ELISPOT) assay is employed to evaluate potential T cell response development against AAV capsid proteins.
Permeabilizing Agents in Gene Therapy
Adjuncts, including vasodilators and permeabilizing agents, have been incorporated in the protocols of gene therapy, resulting in augmented uptake and transduction of the desired gene. These agents include nitroglycerin, nitroprusside, serotonin, bradykinin, histamine-papaverine, substance P, adenosine, and vascular endothelial growth factor (VEGF). Their use in the setting of HF must be approached with caution considering their effects on systemic blood pressure. Nitroglycerin was employed in the clinical trial of AAV1.SERCA2a gene transfer in HF patients [46, 47].
Clinical Trials
Following years of extensive preclinical studies evaluating the effect of SERCA2a upregulation monitoring its toxicology profile, the first phase 1 trial in humans, CUPID (Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease), was launched in 2008. The goal of the trial was to restore SERCA2a enzyme in patients with HF by gene transfer of the SERCA complementary DNA (cDNA) employing a recombinant AAV vector (AAV1.SERCA2a), with dosage ranging from 1.4 × 1011 to 3 × 1012 deoxyribonuclease (DNase)-resistant particles (DRPs) per patient. As mentioned, the vector was slowly introduced by anterograde epicardial coronary artery infusion (AECAI) through a percutaneous femoral access. All patients in phase 1 (and phase 2 as well) were required to have implantable cardioverter-defibrillators (ICDs) out of concern about possible proarrhythmic state, brought about by inhomogeneous SERCA2a expression. At 6 months, the phase 1 trial demonstrated an acceptable safety profile. It also identified improvements in the parameters adopted for the study: symptomatic; functional characteristics; biomarker; and LV function/remodeling parameters. Although phase 1 trial demonstrated biological efficacy, it was an open-label trial, making the results hard to interpret [10, 48]. The safety profile of phase 1 led to the approval of a double-blind, placebo-controlled, randomized phase 2 trial of AAV1.SERCA2a.
Phase 2 of the CUPID trial enrolled 39 patients to receive one of three escalating doses (low dose: 6 × 1011 of AAV1 DRP; middle dose: 3 × 1012 DRP; and high dose: 1 × 1013 DRP) of mediated intracoronary delivery of SERCA2a gene versus the placebo. At 6 months, patients reported improvement or stabilization in a number of parameters, including symptoms (New York Heart Association classification and Minnesota Living With Heart Failure Questionnaire), functional tests (6-minute walk test and maximal oxygen consumption), biomarker levels (N-terminal pro-B-type natriuretic peptide), LV function/remodeling (LV ejection fraction), and LV end-systolic volume. Additionally, after 12 months of receiving a single infusion, patients in the high-dose cohort versus placebo had an 88% risk reduction (HR 0.12; P = 0.003) of major cardiovascular events, including number of HF-related hospitalizations, episodes of worsening of HF, need for LV assist device (LVAD) or cardiac transplant, and death. Furthermore, the mean duration of hospitalization in the high-dose group during the 12-month period was 0.4 days per patient compared with 4.5 days per patient in the placebo group. Even though larger studies are needed to establish AAV1.SERCA2a as a treatment modality for advanced HF, the CUPID trial clearly validated that SERCA2a has a central role in the pathogenesis of HF [49••].
Currently, the effects of SERCA2a are being investigated in two clinical trials. The first trial involves patients with advanced HF having received LVAD at least 1 month before treatment and who will receive either AAV6.SERCA2a or saline. This trial is being conducted in the United Kingdom. A second phase 2, single-center, double-blinded, randomized, placebo-controlled trial in patients with severe HF is also underway at the Institute of Cardiology Pitié-Salpêtrière in Paris, France.
Clinical trials are also underway for the intracoronary administration of ACVI through an adenovirus-5–mediated vector (Ad5.hAC6) in a dose-escalating fashion ranging from 3.2 × 109 to 3.2 × 1012 viral particles in six dose groups using a 3:1 randomization fashion with phosphate-buffered saline (buffered saline to be used as control).
Conclusions
Gene therapy holds the promise of providing a novel treatment modality for HF. It offers focused and complimentary therapy to the organ of interest, limiting undesirable systemic side effects while ensuring a permanent therapeutic state. The therapeutic modulation of calcium cycling proteins has served as the basis for these gene therapy techniques. Specifically, extensive studies in animals have shown that SERCA2a is at the core of calcium cycling and is downregulated in HF, and restoration of this critical protein improves and reverses HF.
Recently, the CUPID clinical trial has conclusively shown that AAV1.SERCA2a gene delivery in patients with advanced HF has a positive biological outcome and has clearly demonstrated that SERCA2a is an important therapeutic target offering the potential of an innovative treatment of patients with severe HF.
Footnotes
Disclosures
R.G. Kratlian: none. Roger J. Hajjar is a scientific founder of Celladon Inc., which is developing AAV1.SERCA2a for the treatment of heart failure.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87(4):275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 4.Katz AM, Lorell BH. Regulation of cardiac contraction and relaxation. Circulation. 2000;102(20 Suppl 4):IV69–74. doi: 10.1161/01.cir.102.suppl_4.iv-69. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaou P, Rodriguez P, Ren X, et al. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104(8):1012–1020. doi: 10.1161/CIRCRESAHA.108.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47(3):365–371. doi: 10.1016/j.yjmcc.2009.05.010. This is a comprehensive review article discussing the role of protein phosphatase-1 inhibitor-1 (I-1) in heart failure. It also discusses the effects of differential phosphorylation of I-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhalla NS, Saini-Chohan HK, Rodriguez-Leyva D, et al. Subcellular remodelling may induce cardiac dysfunction in congestive heart failure. Cardiovasc Res. 2009;81(3):429–438. doi: 10.1093/cvr/cvn281. [DOI] [PubMed] [Google Scholar]
- 8.Gwathmey JK, Copelas L, MacKinnon R, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61(1):70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt U, Hajjar RJ, Helm PA, et al. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30(10):1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 10.Hajjar RJ, Zsebo K, Deckelbaum L, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14(5):355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Kaye DM, Preovolos A, Marshall T, et al. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50(3):253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 12.Haghighi K, Kolokathis F, Pater L, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111(6):869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Armouche A, Pamminger T, Ditz D, et al. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61(1):87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Pathak A, del Monte F, Zhao W, et al. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res. 2005;96(7):756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 15.Rohde D, Ritterhoff J, Voelkers M, et al. S100A1: a multifaceted therapeutic target in cardiovascular disease. J Cardiovasc Transl Res. 2010;3(5):525–537. doi: 10.1007/s12265-010-9211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurice JP, Hata JA, Shah AS, et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary beta2-adrenergic receptor gene delivery. J Clin Invest. 1999;104(1):21–29. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah AS, Lilly RE, Kypson AP, et al. Intracoronary adenovirus-mediated delivery and overexpression of the beta(2)-adrenergic receptor in the heart : prospects for molecular ventricular assistance. Circulation. 2000;101(4):408–414. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- 18.Shah AS, White DC, Emani S, et al. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103(9):1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 19.Lai NC, Tang T, Gao MH, et al. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51(15):1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee S, Stewart AS, Bish LT, et al. Viral gene transfer of the antiapoptotic factor Bcl-2 protects against chronic postischemic heart failure. Circulation. 2002;106(12 Suppl 1):I212–217. [PubMed] [Google Scholar]
- 21.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100(22):2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 22.Most P, Boerries M, Eicher C, et al. Extracellular S100A1 protein inhibits apoptosis in ventricular cardiomyocytes via activation of the extracellular signal-regulated protein kinase 1/2 (ERK1/2) J Biol Chem. 2003;278(48):48404–48412. doi: 10.1074/jbc.M308587200. [DOI] [PubMed] [Google Scholar]
- 23.Felgner PL. Nonviral strategies for gene therapy. Sci Am. 1997;276(6):102–106. doi: 10.1038/scientificamerican0697-102. [DOI] [PubMed] [Google Scholar]
- 24.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 25.Isner JM. Myocardial gene therapy. Nature. 2002;415(6868):234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 26.Klages N, Zufferey R, Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol Ther. 2000;2(2):170–176. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- 27.Galimi F, Noll M, Kanazawa Y, et al. Gene therapy of Fanconi anemia: preclinical efficacy using lentiviral vectors. Blood. 2002;100(8):2732–2736. doi: 10.1182/blood-2002-04-1245. [DOI] [PubMed] [Google Scholar]
- 28.Galimi F, Verma IM. Opportunities for the use of lentiviral vectors in human gene therapy. Curr Top Microbiol Immunol. 2002;261:245–254. doi: 10.1007/978-3-642-56114-6_13. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyostio SR, Owens RA, Weitzman MD, et al. Analysis of adeno-associated virus (AAV) wild-type and mutant Rep proteins for their abilities to negatively regulate AAV p5 and p19 mRNA levels. J Virol. 1994;68(5):2947–2957. doi: 10.1128/jvi.68.5.2947-2957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter PJ, Samulski RJ. Adeno-associated viral vectors as gene delivery vehicles. Int J Mol Med. 2000;6(1):17–27. doi: 10.3892/ijmm.6.1.17. [DOI] [PubMed] [Google Scholar]
- 32.Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16(5):541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- 33.Flotte TR. Adeno-associated virus-based gene therapy for inherited disorders. Pediatr Res. 2005;58(6):1143–1147. doi: 10.1203/01.pdr.0000189226.03684.fe. [DOI] [PubMed] [Google Scholar]
- 34.Michelfelder S, Lee MK, de Lima-Hahn E, et al. Vectors selected from adeno-associated viral display peptide libraries for leukemia cell-targeted cytotoxic gene therapy. Exp Hematol. 2007;35(12):1766–1776. doi: 10.1016/j.exphem.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 35••.Wang J, Faust SM, Rabinowitz JE. The next step in gene delivery: Molecular engineering of adeno-associated virus serotypes. J Mol Cell Cardiol. 2011;50(5):793–802. doi: 10.1016/j.yjmcc.2010.10.017. This article reviews the future direction of AAV construction, discussing different methods for engineering viruses exclusive for the desired organ or tissue. [DOI] [PubMed] [Google Scholar]
- 36.Hajjar RJ, Schmidt U, Matsui T, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95(9):5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fromes Y, Salmon A, Wang X, et al. Gene delivery to the myocardium by intrapericardial injection. Gene Ther. 1999;6(4):683–688. doi: 10.1038/sj.gt.3300853. [DOI] [PubMed] [Google Scholar]
- 38.Bridges CR, Gopal K, Holt DE, et al. Efficient myocyte gene delivery with complete cardiac surgical isolation in situ. J Thorac Cardiovasc Surg. 2005;130(5):1364. doi: 10.1016/j.jtcvs.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Koransky ML, Robbins RC, Blau HM. VEGF gene delivery for treatment of ischemic cardiovascular disease. Trends Cardiovasc Med. 2002;12(3):108–114. doi: 10.1016/s1050-1738(01)00158-x. [DOI] [PubMed] [Google Scholar]
- 40.Boekstegers P, von Degenfeld G, Giehrl W, et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7(3):232–240. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 41•.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381–390. doi: 10.1086/595830. This article addresses the prevalence of adeno-associated virus antibodies in different world populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 43.Mingozzi F, Meulenberg JJ, Hui DJ, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brockstedt DG, Podsakoff GM, Fong L, et al. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol. 1999;92(1):67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- 45.Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111(9):1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donahue JK, Sasano T, Kelemen K. Gene therapy approaches to ventricular tachyarrhythmias. J Electrocardiol. 2007;40(6 Suppl):S187–191. doi: 10.1016/j.jelectrocard.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10(8):828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaski BE, Jessup ML, Mancini DM, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15(3):171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Jessup M, Greenberg B, Mancini D, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in Patients With Advanced Heart Failure. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.022889. This article discusses the results of phase 2 of the CUPID clinical trial in patients with severe heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]