Abstract
Background
We previously reported in a cross-sectional study that patients who were in periodontal maintenance programs and were taking vitamin D and calcium supplementation had a trend for better periodontal health compared with patients not taking supplementation. The objective of the present study was to determine, for the same group of subjects, whether there was a difference in periodontal health over a one–year period.
Methods
Fifty-one patients enrolled in maintenance programs from two dental clinics were recruited. Twenty-three were taking vitamin D (≥400 international units/day) and calcium (≥1000mg/day) supplementation, and twenty-eight were not taking supplementation. All subjects had ≥2 interproximal sites with ≥3 mm clinical attachment loss. For mandibular-posterior teeth, these clinical parameters were recorded: gingival index, plaque index, probing depth, attachment loss, bleeding upon probing, calculus index and furcation involvement. Photostimulable-phosphor, posterior bitewing radiographs were taken to assess alveolar bone. Daily vitamin D and calcium intakes were estimated by nutritional analysis. Data were collected at baseline, 6 months, and 12 months.
Results
Clinical parameters improved with time in both groups (p<0.01). When clinical measures were considered collectively, the results were borderline significant at baseline (p=0.061), significant at 6 months (p=0.049) but not significant at 12 months (p=0.114). After adjusting for covariates, the effect of supplements was significant at baseline (p=0.037), borderline at 6 months (p=0.058) and not significant at 12 months (p=0.142)
Conclusion
Calcium and vitamin D supplementation has a modest positive effect on periodontal health, and consistent dental care improves clinical parameters of periodontal disease regardless of such supplements. Calcium and vitamin D supplementation has a modest positive effect on periodontal health, and consistent dental care improves clinical parameters of periodontal disease regardless of such supplements. Our findings raise the possibility that vitamin D, perhaps at higher doses, may positively impact on periodontal disease severity.
Keywords: vitamin D, calcium, chronic periodontitis, alveolar bone
INTRODUCTION
Vitamin D and calcium are fundamental for bone mineralization and for the prevention of osteoporosis. Vitamin D plays an important role in calcium homeostasis, promoting calcium absorption in the intestine and stimulating osteoblasts to enable normal bone growth and preservation. Severe vitamin D deficiency causes mineralization defects (osteomalacia), but chronically low intake of vitamin D and calcium leads to negative calcium balance and bone loss, and it is reasonable to expect this effect to occur in alveolar bone as it does in other bones of the body. A number of epidemiological studies have reported a positive association between low bone mass or osteoporosis and alveolar bone loss and tooth loss.1-9 This suggests that low bone mass is a risk factor for the development and progression of periodontal disease.4, 6
Besides its role in bone/calcium homeostasis, the biologically active form of vitamin D, 1α,25-dihydroxyvitamin has been demonstrated to be a potent immunomodulator due to its anti-inflammatory effect through inhibition of cytokine production by immune cells and stimulation of monocytes/macrophages to secrete peptides with potent antibiotic activity .9-14 In fact, the immunomodulatory effect of vitamin D has been linked to modulation of bacterial-mediated infections, with low levels of vitamin D being associated with an increased risk of infectious diseases.15 Vitamin D may, therefore, be beneficial for the treatment of periodontal disease, an inflammatory condition involving bacteria and host interactions, during which host-defense cells activated by bacterial release of inflammatory mediators result in the destruction of supporting periodontal tissues, including connective tissue and alveolar bone.16
The effects of vitamin D and calcium supplementation on periodontal disease have not been completely clarified. Although a number of early studies suggested that vitamin D and calcium supplementation reduced tooth loss and alveolar ridge resorption, most of these studies included heterogeneous populations or did not directly measure periodontal disease status.17-23 In other studies, it was reported that low calcium intake resulted in alveolar bone loss in periodontal disease patients and that supplementation led to decreased inflammation, reduction in pocket depth, tooth mobility, and decreased crestal alveolar bone loss.24-27 Other investigators were not, however, able to reproduce these results.28 Drawbacks of all these studies are that information on methods and results are limited. More recently, analyses of the Third National Health and Nutrition Examination Survey (NHANES III) that included 12,000 adults revealed associations between periodontal health and the vitamin D and calcium intakes of subjects.29, 30 This suggests that lower dietary intakes of vitamin D and calcium result in poorer periodontal health in a dose-dependent fashion.
Recent studies have identified a high prevalence of vitamin D and calcium insufficiency in the general population for which the current recommended intakes are respectively 400-600 IU and 1000-1200 mg daily for those > than 50 years of age.31 It is estimated that one billion people worldwide have vitamin D deficiency,32, 33 and in the NHANES III cohort the majority of subjects had serum levels of 25(OH)D (the best indicator of vitamin D status) far below 80 nmol/l,29 which many investigators consider to be a threshold for vitamin D sufficiency.34 Although the vast majority of epidemiological studies have found beneficial effects of vitamin D and calcium supplementation, the use of oral supplementation is low and varies greatly.
We previously reported the results of a cross-sectional study designed to determine whether subjects who were diagnosed with periodontal disease in periodontal maintenance programs and were taking vitamin D and calcium supplements had better indices of periodontal health relative to patients not taking such supplements. Despite relatively modest differences in estimated vitamin D status, all clinical, periodontal parameters including attachment loss, alveolar crest-height, probing depth, gingival index and furcation involvement were better in subjects who took vitamin D and calcium oral supplementation relative to non-takers.35 The objective of the present study was to determine, in the same study population, whether such differences persisted after one year.
MATERIALS AND METHODS
Study Design
Our Health-Insurance-Portability-and-Accountability-Act compliant study was approved by the Institutional Review Boards at Washington University School of Medicine, Southern Illinois University School of Dental Medicine, and Saint Louis University Center for Advanced Dental Education. This pilot, observational clinical trial was conducted with subjects who were recruited from patients enrolled in the periodontal-disease maintenance program at the Graduate Periodontics clinic at the Center for Advanced Dental Education of Saint Louis University, and in the recall program at Southern Illinois University School of Dental Medicine. Subjects had previously filled out institutional-review-board approved questionnaires to determine their levels of oral supplementation.36 All subjects signed informed-consent documents for participation in the study. Individuals were asked to bring their bottles of oral supplementation to their baseline appointments and were asked how long they had been taking oral supplements.
Inclusion and exclusion criteria
The enrollment goal was to have 23 subjects in each of two groups: one group in which subjects had been taking vitamin D (≥400 international units/day) and calcium (≥1000 mg/day) supplementation for more than 18 months (“takers”) and the other group in which subjects had been taking neither vitamin D nor calcium supplementation and had dietary intakes (see Nutrient Analysis) of calcium <1000 mg/day and of vitamin D < 400 international units/day (“non-takers”). Patients enrolled were postmenopausal women (≥ 5 years since last menstrual period) and men age 50 to 80. Furthermore, they had to have moderate to severe chronic periodontal disease (≥ 2 interproximal sites with 3 mm or greater clinical attachment loss)37 and a minimum of two mandibular and one maxillary posterior teeth.
The following exclusion criteria were used: 1) periodontal surgery within the last year; 2) scaling and root planing as part of initial periodontal therapy within the past six months; 3) history of diabetes; 4) history of diseases, conditions or use of medications that might affect bone and mineral metabolism and/or periodontal health; 5) treatment with fluorides for more than 2 weeks within 24 months of enrollment; 6) treatment with estrogen within the last 6 months; 7) treatment with biphosphonates in the past 12 months or lifetime exposure to biphosphonates for more than 3 years; 8) body mass indexes (wt/ht2) < 18.5 or ≥ 33.9) behavioral eating disorders; and 10) current treatment with antibiotics.
Nutrient analysis
Each subject's food intake was analyzed for vitamin D and calcium. This was done using a semi quantitative food frequency questionnaire1 (FFQ, the Brief Block 2000, Block Dietary Data Systems), which was originally based on the second NHANES-II data and revised using NHANES-III food-intake data.38, 39 The food frequency questionnaires were analyzed by Block Dietary Data Systems (Berkeley, CA). The results were interpreted by a registered dietitian (CAS) at Washington University's General Clinical Research Center.
Clinical assessment
Plaque Index40, Gingival Index,40 Calculus Index41 probing depth, cementoenamel-junction-gingival-margin distance (CEJ-GM, attachment loss), bleeding upon probing and furcation involvement42 were recorded. A University of North Carolina (UNC) #15 probe2 as used for the measurements. Clinical measurements were taken at six sites (buccal, lingual, mesiolingual, mesiobuccal, distolingual, distobuccal) for each mandibular posterior tooth. All clinical data were entered into computerized forms at chairside, and measurements were taken at baseline, 6 months and 1 year.
All probing depth and CEJ-GM measurements were made twice for each patient and the average recorded for each site. If the two measurements at a site varied by more than 1 mm, the measurement for that site was repeated until two consecutive identical measurements were obtained.43 One masked examiner (NMG) performed all clinical procedures at both institutions. She was calibrated for intra and inter-examiner error. The calibration protocol involved measuring five representative subjects twice by the operator and the reference standard examiner. The operator was considered calibrated for the gingival index once she achieved at least 80% intra- and inter-examiner exact reproducibility plus 95% intra- and inter-examiner reproducibility within ± 1 index unit. For clinical attachment loss and probing depth, calibration was based on: 1) 85% and 90% intra-examiner reproducibility within ± 1 mm respectively; 2) at least 95% intra-examiner reproducibility within ± 2 mm for both parameters; and 3) at least 75% inter-examiner agreement for probing depth within ± 1 mm and at least 60% inter-examiner agreement for clinical attachment loss within ± 1 mm. For bleeding on probing, the examiner and the reference standard examiner observed each other and discussed and evaluated the criteria for this variable.
Radiographic assessment
Photostimulable-phosphor bitewing radiographs of the mandibular posterior teeth were taken at baseline, 6 months and at the end of one year. Details concerning radiographic methods, measurements, and measurement precision are presented elsewhere.44-46 In brief, subjects were rigidly attached to the x-ray tube by means of a vacuum coupling device and custom cross-arch bite plates with occlusal registration. Custom software and NIH ImageJ47 were used for image registration and measurement. Cementoenamel-junction-alveolar-crest-height (CEJ-AC) measurements were made at the mesial and distal of posterior teeth. Measurements of change in alveolar crest height and x-ray absorbance (radiodensity) were made from subtraction images.
Periodontal treatment
Subjects received periodontal maintenance therapy at 3 month intervals. Treatment consisted of scaling and root planing according to patient needs, polishing of all teeth, reinforcement of oral hygiene procedures, and a general dental examination.
Study subjects
Fifty-one patients enrolled in the periodontal disease maintenance programs were recruited between June 2007 and February 2008. Twenty-three subjects were included in the taker group and 28 subjects in the non-taker group. Of all study subjects, 24 were enrolled at Saint Louis University and 27 at Southern Illinois University. The enrollment goal was to have 23 subjects in each group, and that goal was achieved. After dietary analysis, it was determined that five of the subjects in the non-taker group had dietary intakes of vitamin D and/or calcium that exceeded the exclusion criteria for this group. Five additional subjects were, therefore, enrolled in the non-taker group. Data on all 28 subjects in the non-taker group were entered into analyses. Data were collected at baseline, at 6 months, and at the end of one year. Only 2 subjects dropped out, one of whom was replaced. Table 1 contains demographic data on age, gender, race, smoking history, and alcohol consumption for both groups.
Table 1.
Demographics
| Taker (N=23) (%) | Non-taker (N=28) n (%) | |
|---|---|---|
| Age, mean (std) | 64 (6.4) | 62 (8.6) |
| Gender, N (%)* | ||
| Male | 6 (26%) | 16 (57%) |
| Female | 17 (74%) | 12 (43%) |
| Race, N (%) | ||
| African American | 2 (9%) | 6 (21%) |
| Asian | 0 (0%) | 1 (4%) |
| Caucasian | 20 (87%) | 19 (68%) |
| Hispanic/Latino | 1 (4%) | 2 (7%) |
| Smoking, N (%) | ||
| Smoker | 1 (4%) | 1 (4%) |
| Non-smoker | 16 (70%) | 20 (71%) |
| Past smoker | 6 (26%) | 7 (25%) |
| Alcohol, N (%) | ||
| < 1 glass/month | 12 (52%) | 15 (54%) |
| 2-30 glass/month | 10 (43%) | 10 (36%) |
| >30 glass/month | 1 (4%) | 3 (11%) |
P < 0.05
Statistical analyses
The normality assumption was checked graphically and logarithmic transformation was performed when necessary. Plots of measurements at baseline, 6 months, and 12 months were created and percentage differences between takes and non-takers determined. The Student T test was used to compare the differences between takers and non-takers for all clinical and radiological measurements. Repeated-measures models were used to assess the effect of vitamin D and calcium consumption over time on the collective measurements of attachment loss, bleeding, gingival index, pocket depth, and furcation measurements. These analyses were also performed adjusting for the covariates of race, smoking, alcohol use, and gender. Our study was powered (80%, two-sided, α = 0.05) to detect over one year a 0.2 mm difference in radiographic alveolar crest height between groups. We used SAS (version 9.2, SAS Institute, Cary, NC) for all statistical analyses.
RESULTS
As expected by the design, the two groups significantly differed in total daily intake of vitamin D and calcium (P<0.0001). For subjects who did not take oral supplementation, the mean daily calcium intake was 642 mg (95% confidence interval: 505-779), and the mean daily vitamin D intake was 156 IU (117-195). For subjects who did take oral supplementation, the mean daily calcium intake was 1769 mg (1606-1933), and the mean daily vitamin D intake was 1049 IU (781-1317).
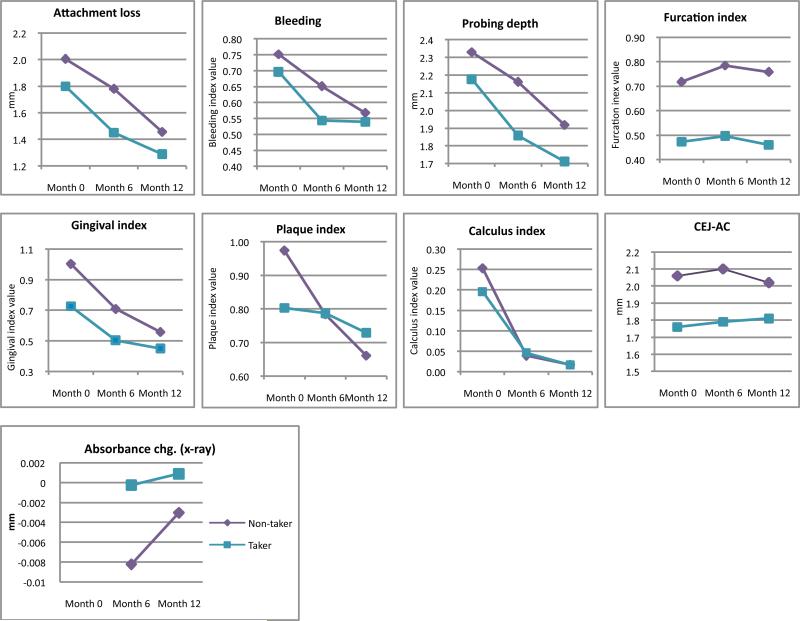
Overall, all the clinical parameters measured were worse in the non-takers at baseline and mostly remained worse throughout the study (Fig. 1). On average, periodontal parameters were 23.0%, 19.9%, and 15.6% better in takers compared with non-takers at baseline, 6 months, and 12 months, respectively (Table 2). Because of relatively large standard deviations, none of the results for the clinical and radiographic measurements were statistically significant in univariate analyses. When considered collectively, the clinical measurements of attachment loss, bleeding, gingival index, pocket depth, and furcation were borderline different between the 2 groups at baseline (p=0.061) and significant at 6 months (p=0.049), but not significant at 12 months (p=0.114). Adjusting for 4 covariates (race, smoking, alcohol, and gender, all not significantly different between groups) changed the results only marginally, with the effect of taking supplements significant at baseline (p=0.037), borderline at 6 months (p=0.058), and not significant at 12 months (p=0.142). Collectively, the 5 clinical measures of periodontal disease decreased significantly through time in both takers (p<0.0001) and non-takers (p=0.002), as well as in both groups combined (p<0.0001).
Figure. 1.
Month 0 (baseline), Month 6, and Month 12 measurements: attachment loss, bleeding, probing depth, gingival index, plaque index, furcation index, calculus index, radiographic CEJ-AC distance, radiographic crest height, and absorbance.
Table 2.
Percent differences* in periodontal measurements between takers and non-takers at baseline, 6 months, and 12 months. Positive percentages indicate better periodontal health in takers.
| Periodontal Parameters | Baseline | 6 months | 12 months |
|---|---|---|---|
| Probing depth | 7.03% | 16.32% | 12.08% |
| Attachment loss | 11.51% | 22.84% | 13.02% |
| CEJ-AC distance | 17.05% | 17.32% | 11.60% |
| Bleeding | 7.90% | 19.83% | 5.13% |
| Gingival Index | 38.03% | 40.49% | 23.98% |
| Plaque Index | 21.27% | -0.39% | -9.33% |
| Furcation | 51.77% | 57.99% | 64.70% |
| Calculus Index | 29.33% | -15.36% | 3.54% |
| Mean | 22.99% | 19.88% | 15.59% |
| Standard deviation | 15.80% | 22.54% | 22.02% |
[(mean measurement for non-takers minus mean measurements for taker)/mean measurements for taker)] × 100
Radiographic measured changes in alveolar crest height at 6 and 12 months were less than 0.01 mm with similar differences between groups; however, by X ray absorbance takers had denser bone at 6 and 12 months than non-takers and the difference was close to being significant (P=0.07).
DISCUSSION
Our results indicate that strictly following a periodontal maintenance program improves periodontal health regardless of standard calcium and vitamin D supplementation. During the study we, however, observed significant or borderline improvements in clinical parameters in patients who took oral supplementation compared with those who did not, and this may have clinical relevance with regard to periodontal health. Previous reports have suggested that vitamin D may reduce the susceptibility to gingival inflammation through anti-inflammatory effects,20, 48, 49 and one study demonstrated an inverse linear association between 25(OH)D and bleeding on probing,48 which may be in agreement with our results for which bleeding on probing and inflammation were lower at baseline in takers and remained lower throughout periodontal maintenance therapy. Because of the inclusion of different co-variates, the results of baseline statistical testing that we report here are slightly different those that we reported previously.35
Different factors can be attributed to the lack of consistent statistically significant differences between the groups. Estimates of mean alveolar crest height loss per year range from 0.05 to 0.11 mm in the general population, with most estimates being 0.1 mm loss per year.50-52 We recruited patients with moderate periodontal disease, which we defined for our study as patients with ≥ 2 sites with ≥ 3 mm of clinical attachment loss.37 Because our intent was for the subjects to have moderate periodontal disease, we thought it reasonable to assume that the rate of loss for these patients might be 2 to 3 times the background rate of 0.1 mm loss per year. We, thus, expected that these patients would lose 0.2 to 0.3 mm of crest height per year. In our previous study, periodontally healthy women who received only oral calcium and vitamin D supplementation for 3 years experienced a mean 0.10 mm increase (standard deviation = 0.23) in crest height.53 In our pilot-data study, we did not think it was reasonable to expect that vitamin D and calcium supplementation would result in bone gain; but we did anticipate that in those patients taking supplementation their alveolar crest heights would remain stable and those not taking supplementation would lose ~ 0.2 mm of crest height. As it turned out, at baseline, alveolar bone and attachment loss were only ~2 mm in non-takers and ~1.8 mm in takers. Our subjects were medically healthy, and many had been enrolled in periodontal-maintenance programs for at least six months and received regular treatments before being recruited. Even though the average ages for the takers and non-takers were 64 and 62, they had lost little alveolar bone over their lifetimes; so little change would be expected over a one-year period (particularly for subjects who were relatively periodontally healthy and undergoing periodontal maintenance therapy), and little change in alveolar bone was detected. In studies of the effects of vitamin D and calcium supplementation on periodontal health, it is essential for investigators to assure that their subjects have severe enough periodontal disease to detect an effect. Even though our subjects met the inclusion criteria, most exhibited only localized previously treated moderate attachment loss. Our unintentional inclusion of subjects with minimal attachment loss and bone loss is a weakness of our pilot study.
We realize another limitation of our study was the number of subjects taking relatively low amounts of vitamin D, even though we made concerted efforts to recruit takers with high intakes of vitamin D. Although the difference in vitamin D intake between the two groups is apparently large (893 IU/day), such a difference would be predicted to raise serum levels by only ~15.6 nmol/l, based on the assumption that 40 IUs of vitamin D supplementation raises 25(OH)D levels by ~0.70 nmol/l.54 It is, therefore, likely that the vitamin D supplements used by our enrollees were not sufficient to normalize serum 25(OH)D (80 nmol/l) in all of these individuals, considering that a large proportion of the population is vitamin D insufficient or deficient.20, 39 Studies in which there have been large differences in intakes between the treatment and control groups have been successful in determining the beneficial effects of vitamin D and calcium supplementation in contrast to trials in which the intake differences have been small.55, 56 Such was the case in a recent publication in which the investigators concluded that increased 25(OH)D levels might be directly associated with worse periodontal inflammation and aggressive periodontitis.57 The major weakness of that study was 25(OH)D levels for the aggressive-periodontitis, chronic-periodontitis, and control-group subjects were below the threshold level (80 nmol/l) for 25(OH)D; therefore no effect of 25(OH)D would be expected and none was found. With cross-sectional or cohort studies, it would be exceedingly difficult or impossible to find enough subjects who have moderate or severe chronic periodontitis and different enough levels of 25(OH)D to be able to detect a statistically significant effect of 25(OH)D. The best way to detect vitamin D effects is through randomized, controlled trials in which the treatment-arm subjects receive 2,000 to 10,000 IU/day and serum levels of 25(OH)D are monitored to assure that treatment-arm subjects achieve 25(OH)D levels of 125 to 175 nmol/L.10
In conclusion, because vitamin D did not have a consistently statistically significant effect on periodontal health, the data from this pilot study can neither support nor refute recommendations for higher doses of Vitamin D supplementation; however, our findings do not deny the possibility that Vitamin D supplements may reduce the severity of the periodontal disease if used at higher doses than those currently included in supplements and support the rationale for randomized clinical trials on the effects of vitamin D on periodontitis.
One-sentence summary: Although the findings support the possibility that vitamin D supplementation may improve clinical and radiographic parameters in patients with periodontitis, these data can neither support nor refute recommendations for higher doses of Vitamin D supplementation.
Acknowledgments
This publication was made possible by Grant Number R21 DE016918-01A2 from the National Institute of Dental and Craniofacial Research, Grant Number M01RR00036 General Clinical Research Center from the National Center for Research Resources (NCRR), a component of the National Institute of Health (NIH), and Grant Number UL1 RR024992 from the NCRR, a component of the NIH and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIDCR, NCRR, or NIH.
Footnotes
The authors report no financial relationships related to any products involved in this study.
Brief Block 2000, Block Dietary Data Systems, Berkeley, CA, USA
Hu-Friedy, Chicago Il, USA
REFERENCES
- 1.Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol. 1997;26:3–15. doi: 10.1038/sj.dmfr.4600226. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoat M. The association between osteoporosis and oral bone loss. Journal of Periodontology. 2005;76:2125–2132. doi: 10.1902/jop.2005.76.11-S.2125. [DOI] [PubMed] [Google Scholar]
- 3.Jeffcoat MK, Reddy MS. Alveolar bone loss and osteoporosis: Evidence for a common mode of thearpy using the bisphoshonate alendronate. In: Davidovitch Z, Norton L, editors. The Biologic Mechanism of Tooth Resorption and Replacement by Implants. Harvard Society for the Advancement of Orthodontics; Boston: 1996. pp. 365–373. [Google Scholar]
- 4.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporosis International. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 5.Southard KA, Southard TE, Schlechte JA, Meis PA. The relationship between the density of the alveolar processes and that of post-cranial bone. J Dent Res. 2000;79:964–969. doi: 10.1177/00220345000790041201. [DOI] [PubMed] [Google Scholar]
- 6.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–1498. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- 7.Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67:1076–1084. doi: 10.1902/jop.1996.67.10s.1076. [DOI] [PubMed] [Google Scholar]
- 8.Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women.[see comment]. Journal of Periodontology. 2005;76:2116–2124. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]
- 9.Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13:719–764. doi: 10.1210/edrv-13-4-719. [DOI] [PubMed] [Google Scholar]
- 10.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opinion on Pharmacotherapy. 2008a;9:107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 11.Cannell JJ, Vieth R, Willett W, et al. Cod liver oil, vitamin A toxiicty, frequent respiratory infections, and the vitamin D defiency epidemic. Annals of Otology, Rhinology and Laryngology. 2008c;117:864–870. doi: 10.1177/000348940811701112. [DOI] [PubMed] [Google Scholar]
- 12.Dale BA, Kimball JR, Krisanaprakornkit S, et al. Localized antimicrobial peptide expression in human gingiva. Journal of Periodontal Research. 2001;36:285–294. doi: 10.1034/j.1600-0765.2001.360503.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response.[see comment]. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 14.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infection & Immunity. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 16.Cochran DL. Inflammation and bone loss in periodontal disease. Journal of Periodontology. 2008;79:1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 17.Baxter JC. The nutritional intake of geriatric patients with varied dentitions. J Prosthet Dent. 1984;51:164–168. doi: 10.1016/0022-3913(84)90253-1. [DOI] [PubMed] [Google Scholar]
- 18.Habets LL, Bras J, Borgmeyer-Hoelen AM. Mandibular atrophy and metabolic bone loss. Endocrinology, radiology and histomorphometry. Int J Oral Maxillofac Surg. 1988a;17:208–211. doi: 10.1016/s0901-5027(88)80035-3. [DOI] [PubMed] [Google Scholar]
- 19.Habets LL, Bras J, van Merkesteyn JP. Mandibular atrophy and metabolic bone loss. Histomorphometry of iliac crest biopsies in 74 patients. Int J Oral Maxillofac Surg. 1988b;17:325–329. doi: 10.1016/s0901-5027(88)80013-4. [DOI] [PubMed] [Google Scholar]
- 20.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001a;111:452–456. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 21.Renner RP, Boucher LJ, Kaufman HW. Osteoporosis in postmenopausal women. J Prosthet Dent. 1984;52:581–588. doi: 10.1016/0022-3913(84)90352-4. [DOI] [PubMed] [Google Scholar]
- 22.Wical KE, Brussee P. Effects of a calcium and vitamin D supplement on alveolar ridge resorption in immediate denture patients. J Prosthet Dent. 1979;41:4–11. doi: 10.1016/0022-3913(79)90347-0. [DOI] [PubMed] [Google Scholar]
- 23.Wical KE, Swoope CC. Studies of residual ridge resorption. II. The relationship of dietary calcium and phosphorus to residual ridge resorption. J Prosthet Dent. 1974;32:13–22. doi: 10.1016/0022-3913(74)90094-8. [DOI] [PubMed] [Google Scholar]
- 24.Groen JJ, Duyvensz F, Halsted JA. Diffuse alveolar atrophy of the jaw (non-inflammatory form of paradental disease) and pre-senile osteoporosis. Geront Clin. 1960;2:68–86. doi: 10.1159/000244610. [DOI] [PubMed] [Google Scholar]
- 25.Krook L, Lutwak L, Whalen JP, Henrikson PA, Lesser GV, Uris R. Human periodontal disease. Morphology and response to calcium therapy. Cornell Vet. 1972;62:32–53. [PubMed] [Google Scholar]
- 26.Lutwak L, Krook L, Henrikson PA, et al. Calcium deficiency and human periodontal disease. Isr J Med Sci. 1971;7:504–505. [PubMed] [Google Scholar]
- 27.Lutwak L, Singer FR, Urist MR. UCLA conference: Current concepts of bone metabolism. Ann Intern Med. 1974;80:630–644. doi: 10.7326/0003-4819-80-5-630. [DOI] [PubMed] [Google Scholar]
- 28.Uhrbom E, Jacobson L. Calcium and periodontitis: clinical effect of calcium medication. J Clin Periodontol. 1984;11:230–241. doi: 10.1111/j.1600-051x.1984.tb02213.x. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 30.Nishida M, Grossi SG, Dunford RG, Ho AW, Trevisan M, Genco RJ. Calcium and the risk for periodontal disease. J Periodontol. 2000;71:1057–1066. doi: 10.1902/jop.2000.71.7.1057. [DOI] [PubMed] [Google Scholar]
- 31.Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. Journal of the American Dietetic Association. 2004;104:980–983. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 32.Glerup H, Mikkelsen K, Poulsen L, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. Journal of Internal Medicine. 2000;247:260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. Resurrection of vitamin D deficiency and rickets. Journal of Clinical Investigation. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis International. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 35.Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of the effects of vitamin D and calcium oral supplementation on Periodontitis. Journal of Periodontology. 2009;80(9):1433–1439. doi: 10.1902/jop.2009.090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon D, Hildebolt CF, Miley DD, et al. Calcium and vitamin D use among adults in periodontal-disease maintenance programs. British Dental Journal. 2009;206:627–631. doi: 10.1038/sj.bdj.2009.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Block G. A revision of the block dietary questionnaire and database, based on NHANES III data, submitted to the “Brief Communications” section of the American Journal of Epidemiology--June of 1998. 1998. Unpublished.
- 39.Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139:1190–1196. doi: 10.1093/oxfordjournals.aje.a116965. [DOI] [PubMed] [Google Scholar]
- 40.Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 41.Greene JC. The oral hygiene index-development. Periodontology. 1967;38:625–629. [PubMed] [Google Scholar]
- 42.Glickman I. Clinical Periodontology. Saunders; Philadelphia: 1953. [Google Scholar]
- 43.Osborn J, Stoltenberg J, Huso B, Aeppli D, Pihlstrom B. Comparison of measurement variability using a standard and constant force periodontal probe. J Periodontol. 1990;61:497–503. doi: 10.1902/jop.1990.61.8.497. [DOI] [PubMed] [Google Scholar]
- 44.Couture RA, Dixon D, Hildebolt C. A precise receptor-positioning device for subtraction radiography, based on cross-arch stabilization. Dento Maxillo Facial Radiology. 2005;34:231–236. doi: 10.1259/dmfr/22285074. [DOI] [PubMed] [Google Scholar]
- 45.Couture RA, Hildebolt CF. Precise image-receptor calibration and monitoring of beam quality with a step wedge. Dentomaxillofac Radiol. 2002;31:56–62. doi: 10.1038/sj/dmfr/4600659. [DOI] [PubMed] [Google Scholar]
- 46.Hildebolt C, Couture R, Garcia N, et al. Alveolar bone measurement precision for phosphor-plate projection images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e96–e107. doi: 10.1016/j.tripleo.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institutes of Health, USA ImageJ.
- 48.Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. American Journal of Clinical Nutrition. 2005;82:575–580. doi: 10.1093/ajcn.82.3.575. [DOI] [PubMed] [Google Scholar]
- 49.Hildebolt C. Effect of Vitamin D and Calcium on Periodontitis. Journal of Periodontology. 2005;76:1576–1587. doi: 10.1902/jop.2005.76.9.1576. [DOI] [PubMed] [Google Scholar]
- 50.Albandar JM. A 6-year study on the pattern of periodontal disease progression. J Clin Periodontol. 1990;17:467–471. doi: 10.1111/j.1600-051x.1990.tb02346.x. [DOI] [PubMed] [Google Scholar]
- 51.Selikowitz HS, Sheiham A, Albert D, Williams GM. Retrospective longitudinal study of the rate of alveolar bone loss in humans using bite-wing radiographs. J Clin Periodontol. 1981;8:431–438. doi: 10.1111/j.1600-051x.1981.tb00892.x. [DOI] [PubMed] [Google Scholar]
- 52.Socransky SS, Haffajee AD, Goodson JM, Lindhe J. New concepts of destructive periodontal disease. J Clin Periodontol. 1984;11:21–32. doi: 10.1111/j.1600-051x.1984.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 53.Civitelli R, Pilgram TK, Dotson M, et al. Hormone/estrogen replacement therapy improves alveolar and postcranial bone density in postmenopausal women. Archives of Internal Medicine. 2002;162:1409–1415. doi: 10.1001/archinte.162.12.1409. [DOI] [PubMed] [Google Scholar]
- 54.Heaney RP. Long-latency deficiency disease: insights from calcium and vitamin D. Am J Clin Nutr. 2003;78:912–919. doi: 10.1093/ajcn/78.5.912. [DOI] [PubMed] [Google Scholar]
- 55.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials.[see comment]. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 56.Boonen S, Vanderschueren D, Haentjens P, Lips P. Calcium and vitamin D in the prevention and treatment of osteoporosis - a clinical update. Journal of Internal Medicine. 2006;259:539–552. doi: 10.1111/j.1365-2796.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- 57.Liu K, Meng H, Tang X, et al. Elevated plasm calcifediol is associated with aggressive periodontitis. J Periodontol. 2009;80:1114–1120. doi: 10.1902/jop.2009.080675. [DOI] [PubMed] [Google Scholar]