Abstract
Hematopoietic stem cell transplantation (HSCT) is an important treatment option for patients with malignant and non-malignant hematologic diseases. Methods to improve transplant efficiency are being explored with the intent of improving engraftment and immune reconstitution post-HSCT. A current approach under investigation involves treatment of donor cells with inhibitors that target the protease CD26, a negative regulator of the chemokine CXCL12/SDF-1. CD26 inhibitor treatment has been shown to improve the functional response of CD34+ cord blood (CB) cells but not CD34+ G-CSF mobilized PBSC to CXCL12/SDF-1. The effect of CD26 inhibitors on un-fractionated CB, bone marrow (BM), or G-CSF mobilized peripheral blood (mobPB) MNC has not previously been evaluated. We observed that although CB had greater CD26 expression than BM or mobPB, treatment with a CD26 inhibitor (Diprotin A) resulted in increased responsiveness to SDF-1 for all three MNC sources tested. This suggests that clinical therapeutic benefit may be gained to utilizing CD26 inhibitors as a strategy to improve engraftment of un-fractionated mobPB cells as well as CB cells.
Keywords: mobilized peripheral blood stem cells, hematopoietic stem cell transplantation, CXCL12/SDF-1, CD26/DPPIV
Introduction
Hematopoietic stem cell transplantation (HSCT) has been historically performed using hematopoietic stem and progenitor cells (HSC/HPC) obtained from the bone marrow (BM). However, collection of HSC/HPC using BM is an invasive procedure. Alternative HSC/HPC sources such as mobilized peripheral blood (mobPB) or cord blood (CB) are arguably preferred do to ease of collection. Transplants in adults involving BM also exhibit prolonged neutrophil and platelet recovery compared to mobPB, which puts patients at greater risk of infection and bleeding, respectively [1]. Hence, BM is less widely utilized, and methods to improve transplant efficiency utilizing mobPB should not be overlooked.
Mobilized PB is currently used as a prominent source for HSC/HPC for adults with hematological diseases undergoing HSCT. Following mobilization, donors can easily undergo apheresis procedures in an outpatient setting in order to acquire peripheral blood stem cells (PBSC). It is known that in the absence of cytokine treatment, the peripheral blood contains very few PBSC [2]. HSC/HPC can be mobilized into the peripheral blood (PB) by various combinations of cytokine and/or growth factor treatments such as granulocyte colony-stimulating factor (G-CSF) [3], granulocyte macrophage colony-stimulating factor (GM-CSF) [4], stem cell factor (SCF) [5], flt-3 ligand (FL) [6], and interleukin-3 (IL-3) [7]. The most common cytokine used for HSC mobilization is G-CSF. However, single agent G-CSF mobilization has a quantifiable failure rate (often quoted at between 5%–30%), [8] suggesting that under certain circumstances G-CSF may not be able to produce sufficient quantities of PBSC for transplantation (< 2×106 CD34+ cells per kg body weight of the recipient).. However, G-CSF treatment in combination with the newer agent AMD3100, a CXCR4 antagonist, further increases mobilization of CD34+ cells [9]. AMD3100 currently has FDA approval for its autologous use in multiple myeloma and lymphoma patients, but not yet for allogenic use in matched donors. This limits the potential donor population. Transplantation of inadequate numbers of PBSC can lead to delayed engraftment, a prolonged period of being immuno-compromised, and decreased patient survival For donors unable to mobilize adequate numbers of PBSC in particular, it is therfore important to improve upon strategies that enhance HSC/HPC trafficking to the bone marrow (BM) during transplantation. There is also an argument for decreasing the time to platelet, neutrophil, and/or immune reconstitution in all patients.
When a matched donor is unavailable, cord blood (CB) can be used as an alternative source for HSC/HPC. Some benefits for using CB for HSCT include its availability, lower histocompatability requirement, and reduced risk of graft vs. host disease (GVHD) [10]. However, a single CB donor produces a limited number of HSC/HPC and restricts patient eligibility for HSCT. CB usually generates sufficient HSC/HPC for transplantation in children and can include adults if the CB unit contains a higher number of stem cells. More than one CB unit can be used to ensure adequate numbers of HSC/HPC but, in doing so, the patient is put at greater risk of developing GVHD [11]. Therefore, current studies investigating methods to expand CB HSCT for use in larger children and adults continue [12]. Methods for improving engraftment would also be beneficial in this clinical scenario.
Chemokines are a large family of cytokines that have a traditional role as chemo-attractants and activators of leukocytes. They have also been implicated in the regulation of leukocyte development [13]. Chemokines interact with chemokine receptors, a subfamily of G-protein seven-transmembrane receptors [14]. CXCL12, also known as stromal-cell derived factor-1 (SDF-1), acts through its chemokine receptor, CXCR4 to enhance the activity of HSC/HPC adhesion receptors during homing and engraftment, thereby allowing circulating cells to undergo trans-endothelial migration into the BM extravascular space [15, 16]. Inactivation of SDF-1 by endogenous peptidases can impair the functional role of HSC/HPC homing and engraftment in cells.
CD26, also known as dipeptidyl peptidase IV (DPPIV), is a membrane-bound extracellular peptidase that cleaves dipeptides from the N-terminus of polypeptide chains after an N-terminal X-Pro or X-Ala motif [17]. The catalytically active soluble form of CD26 is also present in plasma [18]. In vitro, CD26 cleaves SDF-1 into a truncated analog, SDF-1(3–68), and is a likely in vivo target of CD26 [19]. The cleavage of SDF-1 by CD26 can be blocked by the CD26 inhibitor, Diprotin A (Ile-Pro-Ile) [20]. Treatment with Diprotin A can be used to improve the functional response of cells to CXCL12 by reducing inactivation of SDF-1 [21].
We have previously shown that inhibition of cell surface CD26 by Diprotin A treatment increases the chemotactic response of human CD34+ cord blood (CB) cells to SDF-1 in vitro [22]. In addition, we have shown that long-term engraftment of human CD34+ CB cells in non-obese diabetic/severe combined immunodeficient/beta 2 microglobulin null (NOD/SCID/β2mnull) mice increases in response to treatment of donor CD34+ cells with CD26 inhibitors. However, treatment of CD34+ cells from G-CSF mobPB with CD26 inhibitors have reported to have no effect on SDF-1 induced migration [23]. The effect of CD26 inhibition on human mobPB mononuclear cells (MNC) is unknown. We therefore employed in vitro methods to determine the effects of inhibiting CD26 activity on MNC from CB, BM, and mobPB.
Materials and Methods
Isolation of Primary Cells
Human umbilical cord blood (CB) was obtained from Labor and Delivery and bone marrow (BM) was obtained from the Hematology Outpatient Clinic at Rush University Medical Center (RUMC) with institutional review board (IRB) approval. Human G-CSF mobilized peripheral blood (mobPB) was purchased from AllCells, LLC (Emeryville, CA, USA). Mononuclear cells (MNC) were isolated by density gradient centrifugation over Ficoll-Paque Plus (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and then adhered for 90 minutes on a tissue culture plate in Iscove’s Modified Dulbecco’s Medium (IMDM) + 10% Fetal Bovine Serum (FBS) at 37°C, 5% CO2, 100% humidity to obtain non-adherent MNC.
CD26 Expression by Flow Cytometry
CD26/DPPIV expression on CD45+ cells was measured by multivariate flow cytometry. Non-adherent MNC were stained with anti-CD26 [FITC] (Invitrogen, Carlsbad, CA, USA) and anti-CD45 [Alexa Fluor 700] (Invitrogen, Carlsbad, CA, USA) using a previously described staining protocol [24]. No less than 100,000 events were collected by an LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). Analysis was done with BD FACSDiva Software and presented as percent (%) positive cells as compared to isotype control and Mean Fluorescent Intensity (MFI).
CD26 Expression by Western Blot Analysis
Total protein was extracted from non-adherent MNC (CB, BM, mobPB) using M-PER (Pierce, Rockford, IL, USA)As a positive control, total protein was extracted from Karpas 299, a human T-cell lymphoma cell line (DSMZ- the German Resource Center for Biological Material, Germany) and Jurkat, an acute T-cell leukemia cell line as a negative control (American Tissue Culture Collection (ATCC), Manassas, VA) [25, 26]. Additional human cell lines tested for CD26 expression were: AML-193 (monocytic leukemia cell line), CCRF-CEM (acute lymphocytic leukemia (ALL) cell line), GA-10 (Burkitt’s lymphoma cell line), HL-60 (promyelocytic leukemia cell line), HS5 (bone marrow stromal cell line), MOLT-4 (acute lymphoblastic leukemia cell line), and THP-1 (acute monocytic leukemia cell line). Overall protein concentration was determined by Micro BCA protein assay (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. One microgram (except Karpas 299, which had 0.5 μg) of each protein sample was loaded onto a 4–12% Bis-Tris gel and ran with NuPAGE MOPS SDS Running Buffer. Protein was then transferred onto a polyvinylidene difluoride (PVDF) membrane (0.45 μm pore; Invitrogen, Carlsbad, CA, USA). Membranes were blocked with 3% Bovine Serum Albumin Fraction V (BSA-V) + PBS-T (0.15% Tween) and then stained with an anti-hCD26/DPPIV goat polyclonal IgG primary antibody (200 ng/mL; R&D Systems, Minneapolis, MN, USA). Membranes were washed with PBS-T and stained with donkey anti-goat IgG-HRP secondary antibody (80 ng/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in 5% milk + 2% BSA-V + PBS-T and developed with ECL Plus (GE Healthcare, Little Chalfont, Buckinghamshire, UK). To verify equal loading of protein, blots were re-probed with β-Actin (C4) mouse monoclonal IgG (40 ng/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and secondary antibody goat anti-mouse IgG-HRP (20 ng/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
CD26 (DPPIV) Activity Assay
A working stock concentration of Diprotin A (Sigma-Aldrich, St. Louis, MO, USA) of 100mM was created using Dulbecco’s Phosphate Buffered Saline (DPBS). Non-adherent MNC were then re-suspended in 0.2% BSA-V + DPBS to a concentration of 1×106 cells/mL and either treated with Diprotin A at a final concentration of 5 mM or untreated (DPBS control) for 15 minutes at 37°C. 5×104 cells were loaded per well on a 96-well tissue culture plate. Each sample (untreated or Diprotin A treated) was performed in 3–8 replicates. CD26 peptidase activity was measured using the chromogenic substrate Gly-Pro-p-nitoanilide (Gly-Pro-pNA, Sigma-Aldrich, St. Louis, MO, USA) [24]. Using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) and Soft Max Pro software, CD26 activity was calculated from the linear portion of the curve generated by monitoring production of pNA per minute for one hour at 405 nm [22]. Activity was expressed as picomoles pNA/minute (U) per 50,000 cells. Data was normalized by subtracting BSA-V + Gly-Pro-pNA substrate control at each time point. Data was analyzed using the unpaired two-tailed Student’s t-Test assuming equal variance and presented as mean ± SEM.
Cell Migration Assay
Chemotaxis assays were performed on non-adherent MNC (Figure 1). Cells were diluted to a final concentration of 1×106 cells/mL, and treated with 5 mM Diprotin A or DPBS. 2×105 cells were then loaded per well on a 24-well 5μm Transwell plate (Corning Inc, Corning, NY, USA). Each sample (untreated or Diprotin A treated) was performed in 2–9 replicates at each dose of SDF-1. Cells were exposed to a dilution range of 0–400 ng/mL SDF-1α (Stem Cell Technologies, Vancouver, BC, Canada) in 1 mL of 0.2% BSA-V + DPBS with 5 mM Diprotin A for 2 hours at 37°C, 5% CO2, 100% humidity. Percent (%) migration was determined after removing Transwell membranes and counting the number of cells in the lower chamber with a hemacytometer. Data was normalized by subtracting percent migration of untreated cells without SDF-1α. Data was analyzed using the unpaired two-tailed Student’s T-test assuming equal variance and presented as mean ± SEM.
Figure 1. Chemotaxis and Adhesion Assays.
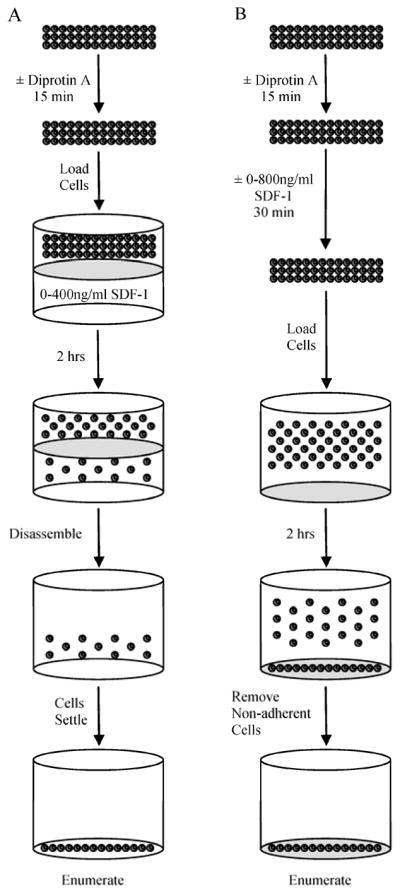
(A) Chemotaxis Assays were performed following treatment of cells with Diprotin A for 15 min. Cells were then loaded onto a Transwell plate with a 5μm membrane containing 0–400 ng/mL SDF-1 in the lower well. After 2 hrs the plate was dissembled, the remaining cells settled, and cell number in the lower well was enumerated. (B) Cell Adhesion Assays were performed following treatment of cells with Diprotin A for 15 min. Cells were then incubated with 0–800 ng/mL SDF-1 for 30 min and then loaded onto fibronectin coated plates. After incubation for an additional 30 minutes, non-adherent cells were removed and the number of adherent cells was enumerated.
Cell Adhesion Assay
Cell adhesion assays were performed on non-adherent MNC (Figure 1). Cells were diluted to a final concentration of 5×104 cells/mL with DPBS + 0.2% BSA-V. MNC were treated with 5 mM Diprotin A or DPBS and incubated for 15 minutes at 37°C, 5% CO2, 100% humidity. Each sample (untreated or Diprotin A treated) was performed in 6 replicates at each dose of SDF-1. Cells were treated with SDF-1α (Stem Cell Technologies, Vancouver, BC, Canada) of concentrations ranging from 0–800 ng/mL and incubated at 37°C, 5% CO2, 100% humidity for 30 minutes. 5×103 cells per well were loaded onto prefabricated human fibronectin 96-well plates containing 1 μg fibronectin (R&D Systems, Minneapolis, MN, USA) and centrifuged at 400 g for 2 minutes. Plates were incubated at 37°C, 5% CO2, 100% humidity for 30 minutes. Non-adherent cells were washed off with DPBS + 0.2% BSA-V. Percent (%) adherence was determined by counting remaining cells at 20X and comparing to a standard curve of cells. Data was normalized by subtracting percent adherence of untreated cells without SDF-1α. Results were presented as mean ± SEM and analyzed using the unpaired two-tailed Student’s t-Test assuming equal variance.
Results
CD26/DPPIV Expression
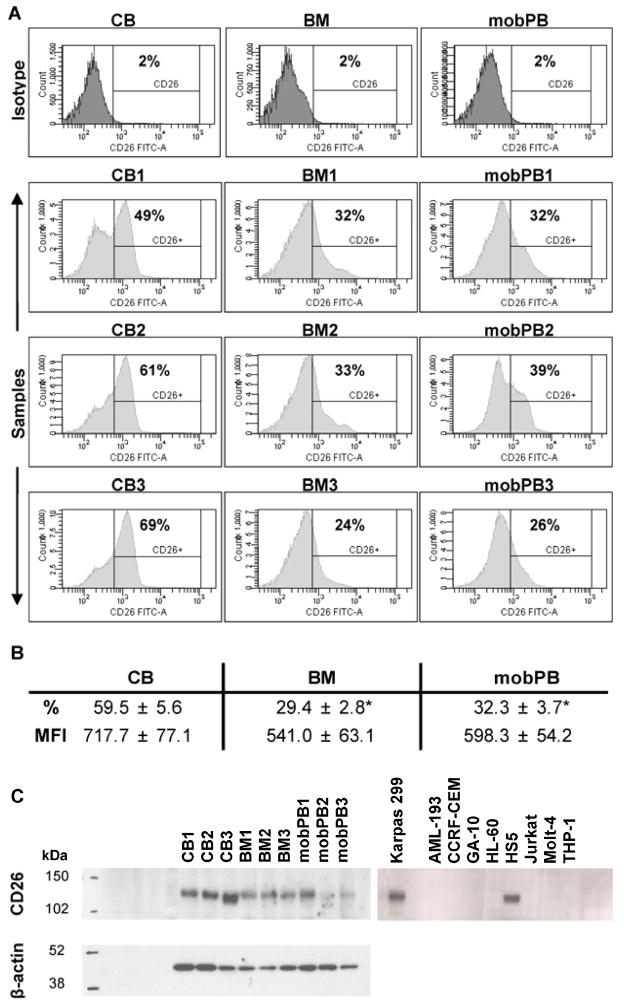
CD26 cell surface expression of non-adherent CB (n=3), BM (n=3), and mobPB (n=3) MNC was quantitated by multivariate flow cytometry (Figure 2A). Variation in CD26 expression was determined between the different cell types. The average CD26 expression of CD45+ cells on CB, BM, and mobPB was 59.5±5.6, 29.4±2.8%, and 32.3±3.7%, and of CD45+ cells (Figure 2B). CB had a much greater percent of CD26+ cells than either BM or mobPB (P<0.05). There was no significant difference between CD26 expression on BM and mobPB cells. The average normalized Mean Fluorescent Intensity (MFI) of CB, BM, and mobPB was 717.7±77.1, 541.0±63.1, and 598.3±54.2 respectively. Although CB trended to a higher MFI, there was no significant difference from BM or mobPB. CB had considerable variation as seen with the larger SEM. In addition, expression of CD26 was also confirmed by Western blot using the same cell sources (Figure 2C). CD26 was detected in all cell sources, but mobPB MNC had qualitatively less CD26 expression. Of the cell lines tested, only Karpas 299 (positive control) and HS5 cell lines were positive for CD26 by western blot. The band for CD26 was located at 110 kDa for the positive control (Karpas 299), HS5 and all primary MNC sources.
Figure 2. CD26 expression on non-adherent MNC by flow cytometry and Western blot analysis.
(A) Flow cytometry was performed on non-adherent CB, BM, and G-CSF mobPB MNC using fluorochrome-conjugated monoclonal antibodies. Representative isotype and sample cell count vs. CD26-FITC histograms are shown. (B) Average CD26 expression and MFI are shown. *P≤0.05 as compared to CB MNC. (C) CD26 expression in corresponding non-adherent MNC and cell lines by Western blot. The representative molecular weight markers (kDa) are shown to the left of each blot. When probed with anti-hCD26/DPPIV goat polyclonal IgG, CD26 (110 kDa) bands appeared at various intensities for CB, BM, and G-CSF mobPB MNC. In the cell lines, Karpas 299 was positive for CD26 and Jurkat was negative, as previously reported. In addition, HS5 cell line was positive for CD26, and all others tested were negative. Both blots were re-probed with anti-β-actin (42 kDa) to ensure equal loading.
CD26 Activity
The amount of pNA produced per minute (CD26 activity) due to cleavage of the Gly-Pro-pNA substrate was assessed for all three cell types (Figure 3). CD26 activity on non-adherent cells was 14.7±0.5, 14.6±0.6, and 10.4±0.6 pmol pNA/minute (U) per 5×104 cells in untreated CB (n=4), BM (n=3), and mobPB (n=3), respectively. Untreated CB and BM MNC had significantly greater CD26 activity than untreated mobPB MNC (P<0.05). When treated with Diprotin A, CD26 activity decreased to 5.3±0.5, 8.4±0.4, and 5.3±0.5 pmol pNA/minute (U) per 5×104 cells in CB, BM, and mobPB respectively.
Figure 3. CD26 activity of non-adherent MNC.
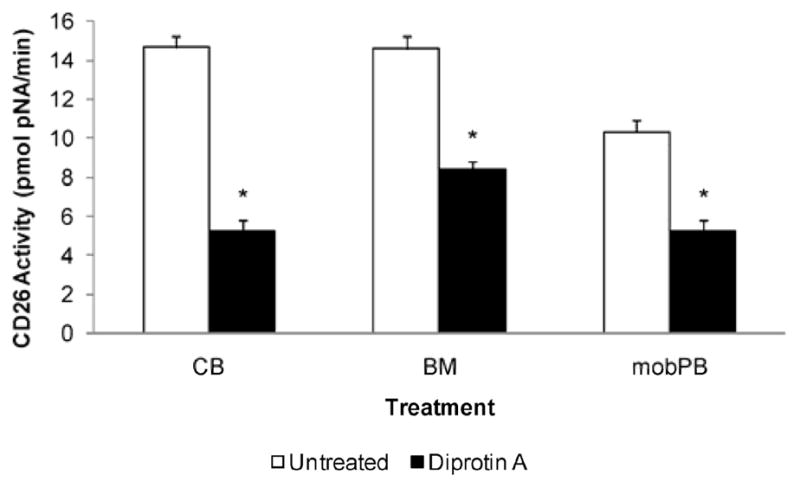
The CD26 activity was assessed in untreated or CD26 inhibitor (5 mM Diprotin A) treated CB (n=4), BM (n=3), and G-CSF mobPB (n=3) MNC. CD26 activity was displayed as pmol pNA/min per 5 × 104 cells. *P≤0.05 as compared to untreated cells.
Treatment with 5mM Diprotin A results in a 3-fold reduction in CD26 activity in CB and a 2-fold reduction in CD26 activity in BM or mobPB (P<0.05).
CD26 and Cell Migration
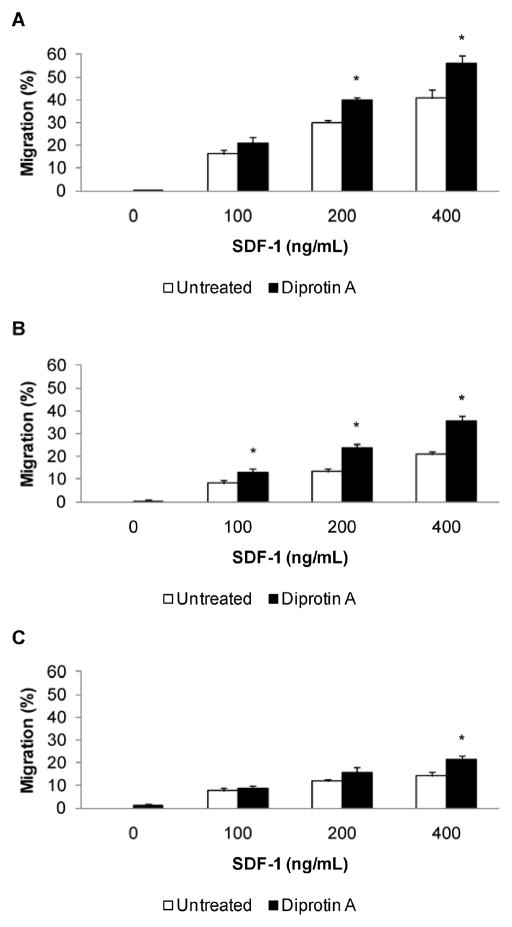
To assess the effect of CD26 inhibition, migration of non-adherent MNC was evaluated by chemotaxis assay (Figure 4). CB, BM, and mobPB cells displayed a dose-dependent response to SDF-1α. Mobilized PB had the lowest chemotactic response of the three sources at all doses of SDF-1α (P<0.05). The absolute percent migration of CB was greater than either BM or mobPB at 200–400 ng/mL SDF-1α (P<0.05). CD26 inhibitor (5 mM Diprotin A) treatment resulted in a significant increase in percent migration for CB, BM, or mobPB. The concentration of SDF-1α used dictated the response to Diprotin A. At 200 ng/mL or greater of SDF-1α, Diprotin A treatment of CB cells resulted in a significant increase in migration (n=3, P<0.05). At 100 ng/mL or greater of SDF-1α, BM cells treated with Diprotin A had an increased migration (n=4, P<0.05). Migration of mobPB cells following CD26 inhibitor treatment was only increased significantly at 400 ng/mL SDF-1α (n=3, P<0.05). This data suggests that the effect of Diprotin A on mobPB is limited to higher doses of SDF-1α, whereas CD26 inhibitor treatment affects CB and BM over a wider range of SDF-1α concentrations. At 400 ng/mL SDF-1α, treatment of CB with Diprotin A resulted in the greatest overall percent migration (56.1±3.2%). Although treatment of mobPB with Diprotin A yieldeds the lowest overall percent migration at 400 ng/mL SDF-1α, CD26 inhibitor treatment resulted in a 50% increase.
Figure 4. Increased migration of CD26 inhibited non-adherent MNC.
SDF-1 induced chemotaxis was assessed in untreated or CD26 inhibitor (5 mM Diprotin A) treated (A) CB (n=3), (B) BM (n=4), and (C) G-CSF mobPB (n=3) MNC respectively. *P≤0.05 as compared to untreated cells.
CD26 and Cell Adhesion
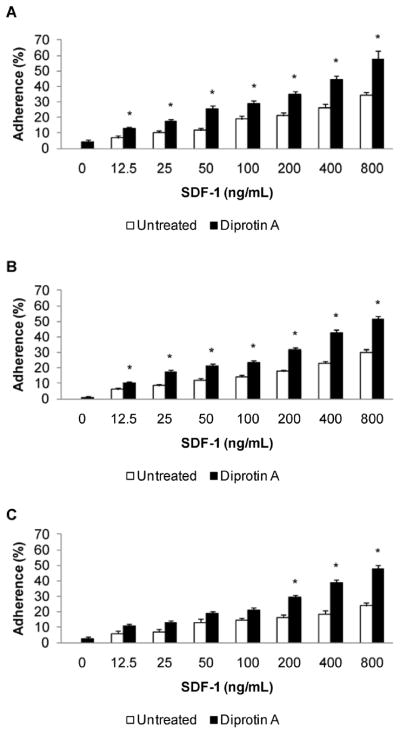
Adhesion of non-adherent MNC in response to SDF-1α was assessed using a static adhesion assay (Figure 5). Cells exhibited a dose-dependent response to SDF-1α. Adhesion of untreated cells was similar regardless of cell source, however, noticeable differences between CB, BM, and mobPB adhesion arise at 800 ng/mL SDF-1α. At this chemokine dose, mobPB MNC have the lowest adhesion in response to SDF-1α (P<0.05). The absolute percent adhesion of CB was greater than BM at 800 ng/mL SDF-1α although this difference was not significant. CD26 inhibition due to 5mM Diprotin A treatment resulted in a significant increase in percent adhesion for CB, BM, and mobPB. The increase in percent adhesion varied depending upon the concentration of SDF-1α from the three sources. At 12.5 ng/mL or greater of SDF-1α, Diprotin A treatment of CB (n=4) or BM (n=3) cells resulted in a significant increase in adhesion (P<0.05). At 200 ng/mL or greater of SDF-1α, mobPB cells treated with Diprotin A had increased adhesion (n=3, P<0.05). This data suggests that the effect of Diprotin A on mobPB is limited to SDF-1α concentrations greater than or equal to 200 ng/mL. Diprotin A treatment of CB resulted in the greatest overall percent adhesion (34.9±1.8%) at 200 ng/mL SDF-1α. Diprotin A treated mobPB had the lowest overall percent adhesion at 200 ng/mL SDF-1α, but treatment with the CD26 inhibitor resulted in a 78% increase. Regardless, adhesion of CB, BM, and mobPB treated with CD26 inhibitor showed no significant differences compared to each other.
Figure 5. Increased adhesion of CD26 inhibited non-adherent MNC.
SDF-1 induced adhesion was assessed in untreated or CD26 inhibitor (5 mM Diprotin A) treated (A) CB (n=4), (B) BM (n=3), and (C) G-CSF mobPB (n=3) MNC respectively. *P≤0.05 as compared to untreated cells.
Discussion
Prior studies have investigated CD34+ cells from various cell sources to examine methods to enhance trafficking during HSCT [22, 23, 27]. Our previous studies have documented that inhibition of CD26/DPPIV activity on donor CD34+ cells from CB can improve transplant efficiency into mice [22]. Other studies have shown that Diprotin A treatment of CD34+ cells from G-CSF mobPB does not result in a statistically significant increase in in vitro migration in response to SDF-1 or in vivo engraftment in immune-deficient mice [23]. A common theme of these prior studies is examination of isolated CD34+ cells. However, CD26 is known to be expressed in B, T, natural killer (NK), epithelial, endothelial, and fibroblast cells [23]. The abundance of CD26 expressed on non-CD34+ hematopoietic cells in the donor graft, T-cells in particular, suggest that that non-CD34+ accessory cells contained within the un-fractionated donor graft may be relevant for the kinetics of engraftment. Additionally, investigation of MNC is clinically relevant because HSCT patients do not typically receive isolated CD34+ cells. In the present study, we specifically determined the effects of CD26 inhibition on MNC; comparing CB, BM, and G-CSF mobPB.
We show that non-adherent MNC from CB, BM, and mobPB express CD26 and exhibit measureable CD26 activity. Although CB has the greatest CD26 expression, CD26 activity is similar to that of BM. Mobilized PB have low CD26 expression similar to BM but also have the lowest CD26 activity. Therefore, our data suggest that CD26 expression does not necessarily correlate directly to the level of peptidase activity in MNC. Depending on the cell source, CD26 on the cell surface may not be enzymatically active at all times although it is expressed. CD26 activity on CB, BM, or mobPB cells can be suppressed by CD26 inhibitor (Diprotin A) treatment but is not entirely suppressed. The concentration of CD26 inhibitor may not be high enough to suppress all activity. More likely, activity may not be solely due to CD26 and may extend to target other related family members. This data suggests that all three cell sources (CB, BM, or mobPB) exhibit peptidase activity that can be suppressed using Diprotin A, indicating that this activity can be attributed to CD26.
Untreated MNC migratory response to SDF-1 is greatest in CB and least in G-CSF mobPB, but adhesion in response to SDF-1 is similar between each cell source. Inhibition of endogenous CD26 with Diprotin A enhances the functional response of CB, BM, and mobPB to SDF-1. All cells treated with CD26 inhibitor have increased migration in response to SDF-1 at 400 ng/mL. However, Diprotin A treated CB and BM exhibit increased migratory responses to lower concentrations of SDF-1 whereas mobPB does not. In addition, all cells treated with Diprotin A have increased adhesion in response to SDF-1 at 200 ng/mL. Diprotin A treated CB and BM have increased adhesion in response to lower doses of SDF-1 whereas mobPB does not. Therefore, functional responses of mobPB treated with CD26 inhibitor occur at higher doses of SDF-1. The differences in SDF-1 induced migration and adhesion of mobPB as compared to CB or BM may be due to specific regulatory differences within the SDF-1/CXCR4 signaling pathway. Furthermore, the absolute percent migration of cells treated with CD26 inhibitor is greatest in CB and least in mobPB. Although adhesion of all cell types increases in the presence of CD26 inhibitor at higher doses of SDF-1, the absolute percent adhesion is not different. In contrast to what has been described for CD34+ cells, this data suggests that endogenous CD26/DPPIV activity acts to regulate the response of MNC from all three sources (CB, BM, and G-CSF mobPB) to SDF-1.
It remains to be determined whether differences in the response of AMD3100 versus G-CSF mobPB cells to SDF-1 can be attributed to differences in CD26 expression and activity. Other studies have shown in mice that CD26 expression on G-CSF mobilized c-kit+ cells is reduced as compared to c-kit+ cells treated with the CXCR4 antagonist, AMD3100 [28]. It has also been reported that human CB CD34+ cells express little to no CD26 on the cell surface [23]. The mechanism by which G-CSF leads to HSC/HPC mobilization is believed to be involved in the disruption of the SDF-1/CXCR4 axis [29]. Previous studies in CD26 deficient mice have shown that CD26 is required for normal G-CSF mobilization [24], but AMD3100 induced mobilization is not altered in the absence of CD26 [30]. Therefore, the use of a CD26 inhibitor on un-fractionated cells mobilized with G-CSF, AMD3100, or G-CSF+AMD3100 has clinical relevance for potential use in cell therapy and may improve engraftment.
Acknowledgments
The authors thank Sefer Gezer, M.D., and Amy Rizman, P.A., for collecting research bone marrow specimens and the Labor and Delivery unit for collecting research cord blood at Rush University Medical Center. The authors thank Elizabeth R. Barraza for assistance with editing. This study was supported by research funding from the Leukemia & Lymphoma Society (6044-08) to K.W.C. In addition, K.W.C was supported during this time period by the American Association for Cancer Research (07-10-19-CHRI), the National Blood Foundation/American Association of Blood Banks (031824), the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases award (DK074892), the Rubschlager Foundation, and the Coleman Foundation (5008).
Footnotes
Authorship
Conception and design: Kent W. Christopherson II
Collection and assembly of data: Robin R. Frank, Sucheta Jagan, Laura A. Paganessi
Data analysis: Robin R. Frank, Laura A. Paganessi, Kent W. Christopherson II
Interpretation and manuscript writing: Robin R. Frank, Laura A. Paganessi, Kent W. Christopherson II
Final approval of manuscript: Robin R. Frank, Laura A. Paganessi, Kent W. Christopherson II, Henry Fung, Stephanie A. Gregory
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95:3702–3709. [PubMed] [Google Scholar]
- 2.Fruehauf S, Haas R, Conradt C, et al. Peripheral blood progenitor cell (PBPC) counts during steady-state hematopoiesis allow to estimate the yield of mobilized PBPC after filgrastim (R-metHuG-CSF)-supported cytotoxic chemotherapy. Blood. 1995;85:2619–2626. [PubMed] [Google Scholar]
- 3.Duhrsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074–2081. [PubMed] [Google Scholar]
- 4.Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte-macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;1:1194–1198. doi: 10.1016/s0140-6736(88)92012-0. [DOI] [PubMed] [Google Scholar]
- 5.Molineux G, Migdalska A, Szmitkowski M, Zsebo K, Dexter TM. The effects on hematopoiesis of recombinant stem cell factor (ligand for c-kit) administered in vivo to mice either alone or in combination with granulocyte colony-stimulating factor. Blood. 1991;78:961–966. [PubMed] [Google Scholar]
- 6.Brasel K, McKenna HJ, Morrissey PJ, et al. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- 7.Orazi A, Cattoretti G, Schiro R, et al. Recombinant human interleukin-3 and recombinant human granulocyte-macrophage colony-stimulating factor administered in vivo after high-dose cyclophosphamide cancer chemotherapy: effect on hematopoiesis and microenvironment in human bone marrow. Blood. 1992;79:2610–2619. [PubMed] [Google Scholar]
- 8.Bensinger W, Appelbaum F, Rowley S, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–2555. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 9.Flomenberg N, Devine SM, Dipersio JF, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 10.Harris DT. Cord blood transplantation: implications for graft vs. host disease and graft vs. leukemia. Blood Cells. 1994;20:560–564. discussion 564–565. [PubMed] [Google Scholar]
- 11.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 12.Xu R, Reems JA. Umbilical cord blood progeny cells that retain a CD34+ phenotype after ex vivo expansion have less engraftment potential than unexpanded CD34+ cells. Transfusion. 2001;41:213–218. doi: 10.1046/j.1537-2995.2001.41020213.x. [DOI] [PubMed] [Google Scholar]
- 13.Oppenheim JJ. Overview of chemokines. Adv Exp Med Biol. 1993;351:183–186. doi: 10.1007/978-1-4615-2952-1_19. [DOI] [PubMed] [Google Scholar]
- 14.Christopherson K, 2nd, Hromas R. Chemokine regulation of normal and pathologic immune responses. Stem Cells. 2001;19:388–396. doi: 10.1634/stemcells.19-5-388. [DOI] [PubMed] [Google Scholar]
- 15.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 16.Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 17.Bongers J, Lambros T, Ahmad M, Heimer EP. Kinetics of dipeptidyl peptidase IV proteolysis of growth hormone-releasing factor and analogs. Biochim Biophys Acta. 1992;1122:147–153. doi: 10.1016/0167-4838(92)90317-7. [DOI] [PubMed] [Google Scholar]
- 18.Durinx C, Lambeir AM, Bosmans E, et al. Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur J Biochem. 2000;267:5608–5613. doi: 10.1046/j.1432-1327.2000.01634.x. [DOI] [PubMed] [Google Scholar]
- 19.Lambeir AM, Proost P, Durinx C, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol. 2003;10:19–25. doi: 10.1038/nsb882. [DOI] [PubMed] [Google Scholar]
- 21.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 22.Christopherson KW, 2nd, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage- human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem cells and development. 2007;16:355–360. doi: 10.1089/scd.2007.9996. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Choi U, Liu PC, Whiting-Theobald NL, Linton GF, Malech HL. Diprotin A infusion into nonobese diabetic/severe combined immunodeficiency mice markedly enhances engraftment of human mobilized CD34+ peripheral blood cells. Stem cells and development. 2007;16:361–370. doi: 10.1089/scd.2007.9997. [DOI] [PubMed] [Google Scholar]
- 24.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 25.Aytac U, Claret FX, Ho L, et al. Expression of CD26 and its associated dipeptidyl peptidase IV enzyme activity enhances sensitivity to doxorubicin-induced cell cycle arrest at the G(2)/M checkpoint. Cancer Res. 2001;61:7204–7210. [PubMed] [Google Scholar]
- 26.Yamada K, Hayashi M, Du W, et al. Localization of CD26/DPPIV in nucleus and its nuclear translocation enhanced by anti-CD26 monoclonal antibody with anti-tumor effect. Cancer Cell Int. 2009;9:17. doi: 10.1186/1475-2867-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonig H, Chudziak D, Priestley G, Papayannopoulou T. Insights into the biology of mobilized hematopoietic stem/progenitor cells through innovative treatment schedules of the CXCR4 antagonist AMD3100. Exp Hematol. 2009;37:402–415. e401. doi: 10.1016/j.exphem.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rettig MP, Ramirez P, Nervi B, DiPersio JF. CXCR4 and mobilization of hematopoietic precursors. Methods Enzymol. 2009;460:57–90. doi: 10.1016/S0076-6879(09)05203-3. [DOI] [PubMed] [Google Scholar]
- 30.Paganessi LA, Walker AL, Tan LL, et al. Effective mobilization of hematopoietic progenitor cells in G-CSF mobilization defective CD26−/− mice through AMD3100-induced disruption of the CXCL12-CXCR4 axis. Exp Hematol. 2011;39:384–390. doi: 10.1016/j.exphem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]