Abstract
Quaking is a prototypical member of the STAR protein family, which plays key roles in posttranscriptional gene regulation by controlling mRNA translation, stability and splicing. QkI-5 has been shown to regulate mRNA expression in the central nervous system, but little is known about its roles in other tissues. STAR proteins function as dimers and bind to bipartite RNA sequences, however, the structural and functional roles of homo- and hetero-dimerization are still unclear. Here, we present the crystal structure of the QkI dimerization domain, which adopts a similar stacked helix-turn-helix arrangement as its homologs GLD-1 and Sam68, but differs by an additional helix inserted in the dimer interface. Variability of the dimer interface residues likely ensures selective homodimerization by preventing association with non-cognate STAR family proteins in the cell. Mutations that inhibit dimerization also significantly impair RNA binding in vitro, alter QkI-5 protein levels, and impair QkI function in a splicing assay in vivo. Together our results indicate that a functional Qua1 homodimerization domain is required for QkI-5 function in mammalian cells.
INTRODUCTION
Quaking (QkI) is a conserved multi-functional protein in vertebrates that belongs to the STAR (signal transduction and activation of RNA) family of RNA-binding proteins. QkI regulates a multitude of cellular functions, both at different developmental times and in various tissues in mice and humans (reviewed in 1). STAR family proteins link signaling pathways to various aspects of posttranscriptional regulation of messenger RNAs (mRNAs). QkI is important for several biological processes including myelination, smooth muscle cell differentiation, vascular and heart development 2; 3; 4. The cellular processes controlled by QkI are reported to include target mRNA stabilization, translation, sub-cellular localization and alternative splicing 5; 6; 7; 8. The consensus RNA binding sequence of QkI is A(C/U)UAA(C/U) and is often accompanied by a so-called half site UAA(C/U) in close proximity 9; 10; 11. The activity of QkI and other STAR family members is regulated via post-translational modifications including phosphorylation 12; 13; 14. However, the molecular mechanisms linking RNA binding and homodimerization to the diverse regulatory roles of QkI are still poorly understood.
Several alternatively spliced isoforms of Quaking protein differ in their C-terminal 8-30 amino acid sequence, with the three major isoforms being QkI-5, QkI-6 and QkI-7. These unique tails determine the localization within the cell and confer specific functions. QkI-6 and QkI-7 are mainly cytoplasmic, while QkI-5 contains a non-canonical nuclear localization signal within its tail 15; 16; 17.
The most extensively studied aspect of QkI function is its crucial role in myelin formation in the central nervous system (CNS), where QkI controls the expression of many genes important for myelination through a complex mechanism involving all pathways mentioned above. Defects in QkI expression and/or splicing in the CNS have been implicated in several psychiatric diseases including schizophrenia and ataxia 18; 19. In quaking-viable (qkv) mice, myelin fails to undergo compaction in the CNS due to a large deletion upstream of the quaking locus 20. The alternative splicing of several myelin-specific genes regulated by QkI has been implicated as an important mechanism in regulation of myelin formation 5; 21; 22. Recently, QkI expression was correlated with alternative splicing of the histone variant MacroH2A1 in several different human tissues and cancer cell lines, linking defects in MacroH2A1 splicing to a variety of cancers, including testicular, lung, bladder, cervical, breast, colon, ovarian and endometrial, and suggesting that QkI may be an important tumor suppressor 23; 24.
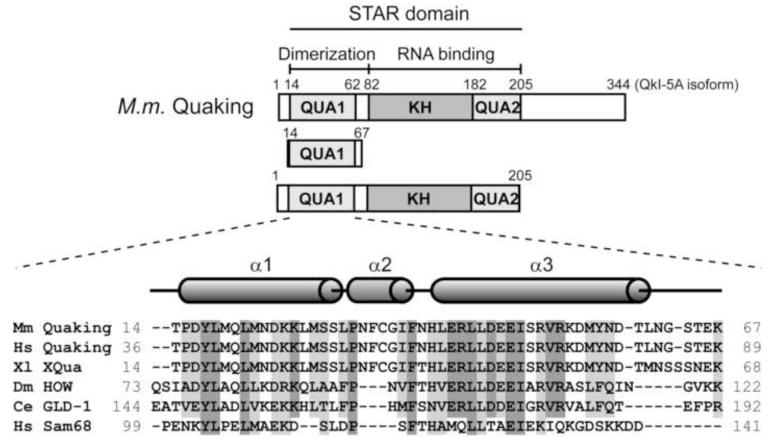
The conserved STAR domain defines the STAR protein family, and consists of a central hnRNP K homology (KH) RNA binding domain flanked by two conserved subdomains referred to as Qua1 and Qua2 (Figure 1) 2. STAR proteins bind RNA via the KH and Qua2 regions, while the Qua1 domain is essential for homodimerization 25; 26; 27. The current model for a STAR protein dimer binding to RNA suggests a bipartite binding mode. One STAR protomer is thought to bind one 6-7 nucleotide consensus sequence, while the second protomer binds to an additional full consensus site or shorter (3-4 nucleotide) half-site spaced by 1 to 20 nucleotides 9; 11; 27; 28. Alternatively, recognition of two distantly spaced consensus sites could organize mRNA structure for regulation by QkI29; 30; 31.
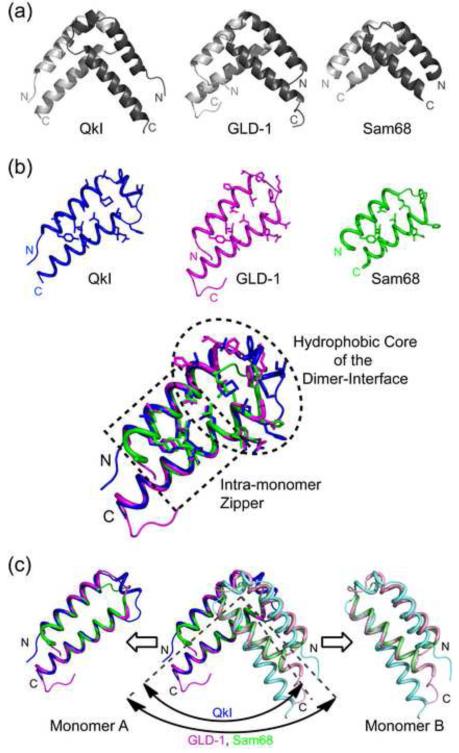
Figure 1. The STAR family of RNA-binding proteins.
Domain structure of QkI and constructs used in this study. The Qua1, KH and Qua2 subdomains are shaded (top). Sequence alignment of the Qua1 domain of representative members of the STAR/GSG protein family (bottom). Identical conserved residues are highlighted in dark grey, similar residues in light grey. The secondary structure of the Quaking Qua1 domain is shown above. Members of the Quaking subfamily feature a 3-residue insertion between the two large helices which form an additional short helix, instead of a simple turn, that is not present in the Qua1 structures of GLD-1 and Sam68.
The structural basis for the recognition of a single RNA consensus site has been described for the splicing factor SF1 32, indicating that the Qua2 domain extends the RNA-binding surface of the KH domain and that this KH-Qua2 tandem domain is essential for sequence-specific RNA recognition. Interestingly, SF1 is the only STAR family member that lacks the Qua1 dimerization domain. The solution structure of Xenopus Quaking KH-Qua2 domain shows that the Qua2 domain does not contact the KH domain in the absence of RNA 33. Qua1 homodimerization domain structures have been solved for the GLD-1 and Sam68 homologs of QkI, revealing a helix-turn-helix fold 30; 31. The hydrophobic dimer interface is located at the top of the hairpin and the protomers are stacked at a 90° angle. Although the full STAR domain is conserved within the family, the sequence identity in the Qua1 region is only 30% between QkI and its close homolog GLD-1. Interestingly, the hydrophobic ‘zipper’ residues mediating the contact between the two helices within each monomer are highly conserved, while the hydrophobic patch at the top of the hairpin that forms the dimer interface in the GLD-1 and Sam68 Qua1 structures contain significant variation throughout the STAR family (Figure 1). Furthermore, the Quaking orthologs feature a 3-residue insertion in this region that is not present in other STAR proteins.
Some studies suggest that STAR protein dimerization is essential for their function, but little is known about how dimerization translates into biological function on a cellular level. In C. elegans GLD-1, the KH-Qua2 domain is sufficient for RNA binding, but dimerization enables the bipartite binding mode, and deletion of the Qua1 domain reduces the RNA binding affinity by one order of magnitude 27. QkI mutants that abolish dimerization 26 cause an embryonic lethal phenotype in mice, suggesting that homodimerization serves other important functions in addition to facilitating RNA binding. While the splicing factor SF1 lacks the Qua1 dimerization domain and functions as a monomer, Sam68 mutants that impair homodimerization reduce its activity in an alternative splicing assay 31, suggesting that proper splicing regulation requires dimerization for at least some STAR family members. The role of QkI dimerization for its function as an alternative splicing regulator is unknown.
Here we present the crystal structure of the QkI Qua1 domain. The helix-turn-helix like fold is expanded by an additional short helix at the top of the hairpin. Based on thermal melting and RNA binding data we show that failure to dimerize impairs RNA binding. Furthermore, a cell-based splicing assay in mouse C2C12 myoblasts demonstrates that QkI-5 function in alternative splicing requires its ability to homodimerize.
RESULTS
Structure of the QkI-Qua1 Homodimer
To select a suitable QkI Qua1 construct for crystallography, 20 C-terminal truncation constructs were cloned based on secondary structure prediction (PredictProtein: https://www.predictprotein.org/) and sequence homology to the GLD-1 and Sam68 Qua1 domains. Residue C35 was changed to Ser for the QkI Qua1 and QkI STAR in vitro constructs used in this study to avoid non-specific aggregation 34. NMR spectra (1H,15N-HSQC, Figure S1) recorded for six constructs, spanning the region from residue 12 or 14 to between 55 and 77, indicated that the majority of the protein is structured and that the structured region is present in all constructs analyzed. The only differences in the spectra between the different C-terminal truncations were observed in the unstructured region (7.8-8.5 ppm) of the spectrum, indicating that the C-terminal region comprising residues 55-77 is in fact unstructured in these constructs.
Native QkI Qua1 (14-67) yielded well diffracting crystals, but molecular replacement using the homologous proteins GLD-1 and Sam68 failed to produce a satisfactory model for phasing. The phases were ultimately solved using selenomethionine (SeMet) labeling for multiwavelength anomalous dispersion (MAD) phasing, and the QkI-Qua1 structure was solved at 2.1 Å resolution with one homodimer in the asymmetric unit. A summary of the data collection and refinement statistics is given in Table 1.
Table 1.
| SeMet | ||||
|---|---|---|---|---|
| Data collection | ||||
|
| ||||
| Space group | P212121 | |||
| Cell dimensions (Å) | a = 33.98, | |||
| b = 36.02, | ||||
| c = 92.81 | ||||
|
| ||||
|
SeMet (peak) |
SeMet (remote) |
SeMet (inflection) |
merged (solve) | |
|
| ||||
| Wavelength (Å) | 0.9791358 | 0.9184018 | 0.979569 | |
| Resolution (Å) | 50 - 2.15 | 50 - 2.10 | 50 - 2.15 | |
| (2.19 - 2.15) | (2.14 – 2.10) | (2.19 - 2.15) | ||
| Total reflections | 50936 ? | 55390 | 51303 | |
| Unique reflections | 11880 (578) | 12905 (659) | 11969 (593) | |
| Rsym (%) | 3.6 (28.8) | 3.4 (33.9) | 3.6 (32.4) | |
| I/σ(I) | 35.1 (5.2) | 40.0 (4.4) | 35.4 (4.4) | |
| Completeness (%) | 99.9 (100) | 99.8 (100) | 99.9 (100) | |
| Redundancy | 4.3 (4.3) | 4.3 (4.2) | 4.3 (4.2) | |
| Wilson B factor | 41.06 | 39.83 | 42.31 | |
|
| ||||
| Refinement | ||||
|
| ||||
| Resolution (Å) | 31.91 – 2.10 | |||
| (2.65 – 2.10) | ||||
| Unique reflections | 7067 (3462) | |||
| Rwork | 21.63 (22.03) | |||
| Rfree | 25.15 (24.66) | |||
| Stereochemistry | ||||
| Res. in favored region (%) | 98.02 | |||
| Res. in allowed region (%) | 1.98 | |||
| Number of atoms | ||||
| Protein | 856 | |||
| Water | 44 | |||
| Ca2+ | 1 | |||
| B factors | ||||
| Protein | 38.84 | |||
| Water | 36.99 | |||
| Ca2+ | 119.53 | |||
| Rmsd | ||||
| Bond lengths (Å) | 0.008 | |||
| Bond angles (°) | 1.102 | |||
| Residues not modeled | ||||
| A | 62 – 67 | |||
| B | −2 – 1 (conformer B), | |||
| 63 – 67 | ||||
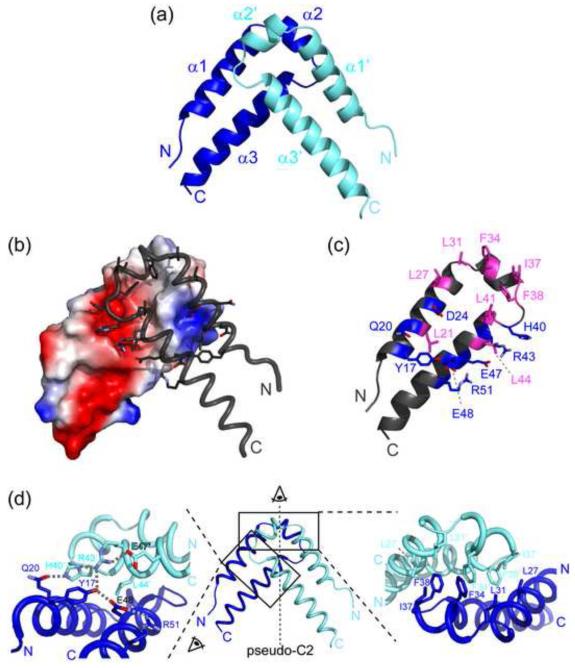
The QkI Qua1 monomer consists of two α-helices arranged in a hairpin-like geometry resembling the helix-turn-helix fold observed for GLD-1 and Sam68 Qua1. The two antiparallel helices are held together by a hydrophobic ‘zipper’ and a hydrogen bond between the conserved residues Y17 and E48. The QkI Qua1 structure contains an additional short helix, including the three residue insertion, in the linker region between the two main helices (Figure 2a). This helix is not present in the homologous GLD-1 and Sam68 Qua1 domain structures and lies perpendicular to the two main helices. The C-terminal residues 55-61 show weak electron density and do not form a regular secondary structure. No electron density was observed for residues 62-67, consistent with the NMR data indicating that the C-terminal tail is likely unstructured and flexible.
Figure 2. Overall structure of the QkI-Qua1 homodimerization subdomain.
(a) Structure of the QkI-Qua1 homodimer. Monomers A and B are colored in dark and light blue, respectively. (b) Dimer interface. Monomer A is shown as electrostatic surface potential, and monomer B as tube with dimer interface residues as sticks. (c) Residues of the QkI-Qua1 homodimer interface. The residues that form the hydrophobic core of the interface, containing the conserved Phe 34 and Phe 38, are highlighted in pink. Residues participating in hydrogen bonds at the edge of the interface are shown in blue. (d) Close-up view on the dimer interface. The prime denotes residues in the other protomer. Side chains of key residues in the dimer interface are shown as sticks and hydrogen bonds are indicated by grey dashed lines.
Structural similarity searches with DALI and PISA 35; 36 confirm that the most closely related structures in the Protein Data Bank (PDB) are the Qua1 dimerization domain structures of the homologous STAR proteins GLD-1 (pdb 3K6T) and Sam68 (pdb 2XA6). The overall hairpin-like structure is conserved in all three Qua1 structures, but QkI Qua1 is unique because of the insertion of an additional helix in the turn region of the hairpin, which is neither present in GLD-1 or Sam68 nor in any other of the many helix-turn-helix structures in the PDB.
The Qua1 dimer is formed by stacking the two monomers at the turn region at an angle of 84°. The dimer interface area covers 815 Å2 per monomer, which corresponds to 19% of the total surface area of the monomer. The hydrophobic dimer interface consists of residues L27, L31, F34, I37, L41 and the highly conserved residues F38 and L44 (Figure 2). Several hydrogen bonding interactions along the periphery towards the open end of the hairpin reinforce the hydrophobic interface. Residue D24 forms conserved hydrogen bonds to the backbone amides of residues N39′ and H40′, while inter-monomer hydrogen bonds between Q20 and H40′, and Y17 to R43′ bridge the dimer interface. These interactions are further facilitated by an intra-monomer hydrogen bonding network between H40 – R43 – E47 and Y17 – E48 – R51, including the highly conserved Y17-E48 monomer ‘clamp’ between the two long helices that has been shown to be important for monomer stability 30.
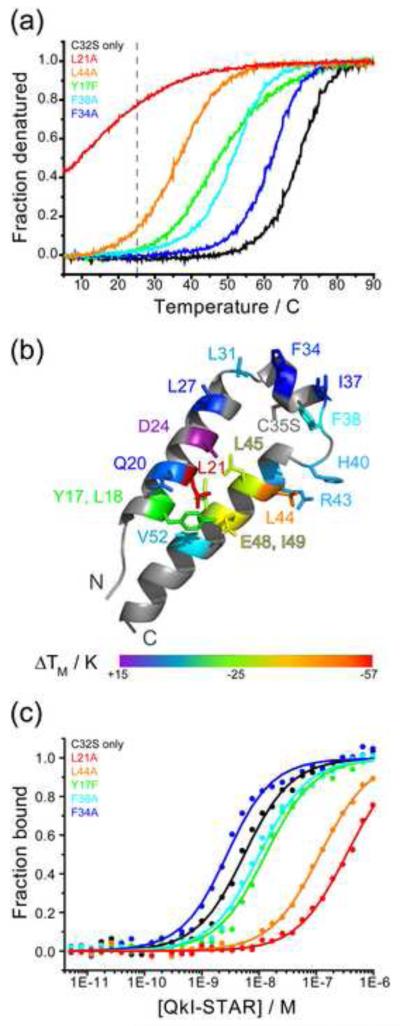
Mutational Analysis of the Dimer Interface and Monomer Zipper
Key dimer interface and zipper residues in QkI Qua1 were mutated to study their contribution to QkI dimerization. The mutation E48G was chosen in addition to E48A because it is biologically relevant, causing an embryonic lethal phenotype in mice. Residue Tyr 17 was mutated to Phe (Y17F) instead of Ala to selectively disrupt the hydrogen bond to residue E48 without disturbing hydrophobic packing of the aromatic side chain. For the GLD-1 Qua1 domain, the zipper, in addition to the dimer interface residues, proved to be crucial for homodimerization by ensuring the structural integrity of the monomer.30 The homodimer stability of the set of QkI Qua1 mutants was determined by thermal melting monitored by CD spectroscopy (Figure 3; Table 2). The curves for most point mutants show a complete melting profile indicating that these constructs are completely folded at low temperatures. Only the QkI Qua1 L21A and E48G point mutants are still significantly unfolded even at temperatures as low as 5°C. All curves show a single transition suggesting that QkI Qua1, like GLD-1 Qua1, forms obligate dimers in which dimer dissociation and unfolding of the monomer secondary structure occur simultaneously and a folded monomeric Qua1 domain likely does not exist. The melting temperatures for the set of mutants, as a measure of dimer stability, vary over a large range from < 15°C to > 85°C. Mutants in the monomer zipper and the Y17-E48 clamp have a generally more detrimental effect on dimer stability than residues in the dimer interface. However, dimer interface mutant L21A confers a large destabilization equaling that of the E48G mutant. Residue L21 plays a dual role in the zipper and dimer interface since it is located at the inner edge of the dimer interface close to the dimer symmetry axis (Figure 3b) and packs against itself (L21′) in the adjacent protomer. Removal of this pair of L21 side chains leads to a large void, while other dimer interface mutants have a more local effect on the hydrophobic packing. Furthermore, L21A’s bottom face participates in the zipper by packing against I49.
Figure 3. Qua1 point mutations that destabilize homodimerization also impair RNA binding.
All mutants are in the C35S background and are therefore compared to QkI-Qua1 C35S. (a) Representative CD melting curves for QkI-Qua1 point mutants. Room temperature, at which the RNA binding experiments (Figure 3c) were performed, is indicated by the gray dashed line. (b) ΔTM mapped onto the monomer structure w. residues probes shown as sticks. (c) Representative RNA binding curves for QkI-STAR constructs with point mutations in the Qua1 domain. The RNA binding experiments were performed at room temperature (indicated by a grey dashed line in Figure 3a), thus only those constructs that are significantly destabilized at this temperature are expected to show a significant impact on the RNA binding affinity. The data was fit to the quadratic binding equation for bimolecular binding. The Hill equation does not apply because the RNA concentration cannot be considered ‘in trace’ (probe concentration << 10·KD) for most QkI STAR constructs.
Table 2.
| Qual domain (residues 14-67) | full STAR domain (residues 1-205) | |||
|---|---|---|---|---|
|
|
||||
| QkI mutant | TM / °C | ΔTM / K | KD / nM | KD / KD(C35S) |
| wt | n.d. | n.d. | 3.4 ± 0.2 | 0.7 |
| C35S only | 69 | 0 | 5.1 ± 0.3 | 1.0 |
|
| ||||
| Zipper | ||||
|
| ||||
| L18A | 49 | − 20 | 11.6 ± 0.6 | 2.3 |
| Q20A | 65 | − 4 | 4.1 ± 0.3 | 0.8 |
| L44A | 37 | − 32 | 118 ± 5 | 23 |
| L45A | 40 | − 29 | 209 ± 9 | 41 |
| I49A | 38 | −31 | 24 ± 1 | 4.7 |
| V52A | 60 | −9 | 3.8 ± 0.2 | 0.7 |
|
| ||||
| Dimer interface and Zipper | ||||
|
| ||||
| Y17F | 45 | − 24 | 12.8 ± 0.7 | 2.5 |
| L21A | < 15 | < −57 | 340 ± 30 | 67 |
| E48A | 40 | − 29 | 6.7 ± 0.4 | 1.3 |
| E48G | < 15 | < −57 | 22 ± 1 | 4.3 |
|
| ||||
| Dimer Interface | ||||
|
| ||||
| D24A | > 85 | > +14 | 4.3 ± 0.2 | 0.8 |
| L27A | 67 | − 2 | 3.5 ± 0.2 | 0.7 |
| L31A | 62 | − 7 | n.d.* | n.d.* |
| F34A | 64 | − 5 | 2.3 ± 0.1 | 0.5 |
| I37A | 67 | − 2 | 4.4 ± 0.3 | 0.9 |
| F38A | 53 | − 16 | 9.8 ± 0.6 | 1.9 |
| H40A | 58 | − 11 | 10.8 ± 0.6 | 2.1 |
| R43A | 57 | − 12 | 9.0 ± 0.5 | 1.8 |
Qkl-STAR L31A could not be expressed.
The lack of the Y17-E48 hydrogen bond is associated with a lethal phenotype for mouse Quaking E48G. Comparing the E48G and E48A mutants however, it becomes apparent that the effect of glycine in this position is much greater than simply removing a hydrogen bond. Considering that glycine residues are known to break α-helices, it is likely that loss of secondary structure in helix α3 in the E48G mutant adds significantly to the dimer destabilization.
To investigate the effect of the point mutations within the Qua1 subdomain in the context of the full QkI STAR domain, the same point mutations were cloned into QkI STAR (1-205) fused to maltose binding protein (MBP). The C35S mutation that prevents aggregation was also introduced into the QkI STAR mutants. This substitution does not affect the RNA binding affinity of QkI STAR (Table 2). The RNA binding affinity of the mutants was measured using a fluorescence polarization (FP) binding assay 29. The MBP-QkI STAR point mutants were titrated to a constant concentration of the previously identified high affinity RNA sequence 5′-UAUUUAAUUUCUUAUCUACUAAUAUCUA -3′ labeled with fluorescein as probe (the high affinity hexamer consensus is highlighted in bold). The KD for both wild type and C35S-only mutant agree well with the published values 29. RNA binding is significantly impaired for only those point mutants that drastically decrease the stability of the QkI Qua1 homodimer, reflected by a melting temperature of the QkI Qua1 construct of TM ≤ 45°C, namely Y17F, L21A, L44A, L45A, E48G, I49A (Table 2). Since the RNA binding assay is performed at room temperature, a drastic effect on RNA binding is only observed for those mutants that are already significantly destabilized at this temperature (Figure 3c). Interestingly, the mutants E48G and L21A which have similar low melting temperatures in the Qua1 construct show quite different RNA binding affinities. Since different length constructs were used in the different assays, it is possible that destabilization caused by any point mutation could be different in the presence of other parts of the protein compared to an isolated domain, especially since the E48G mutation likely combines at least two destabilizing effects: deletion of a stabilizing hydrogen bond and disruption of secondary structure.
All mutant Qua1 and STAR constructs were analyzed on SDS PAGE and size exclusion chromatography (not shown) to ensure intact protein, equal concentrations and homogeneity. All mutants elute in a single peak, with the exception of the D24A mutant in both the Qua1 and STAR construct. The retention time of destabilized mutants slightly increased compared to wild type in accordance with the dimer stability measured by CD spectroscopy, but not as much as it would be expected for a globular monomeric form, which might reflect either a fast equilibrium between monomeric and dimeric forms or an increase of hydrodynamic radius due to unfolding. The D24A mutation causes the appearance of an additional higher molecular weight species for both the Qua1 and STAR domain constructs, indicating that this mutation might cause aggregation, which could explain the surprisingly high melting temperature of the D24A Qua1 construct.
The mutational analysis shows that QkI homodimerization is correlated to its ability to bind RNA, and mutations that disrupt the homodimer also confer a reduced RNA binding affinity. In addition to the QkI Qua1 dimer interface residues themselves, the conserved zipper serves an important function for both homodimer stability and RNA substrate binding. The zipper is well conserved among STAR proteins. It stabilizes the tertiary structure of the α-helical hairpin and ensures the proper display of the dimer interface residues.
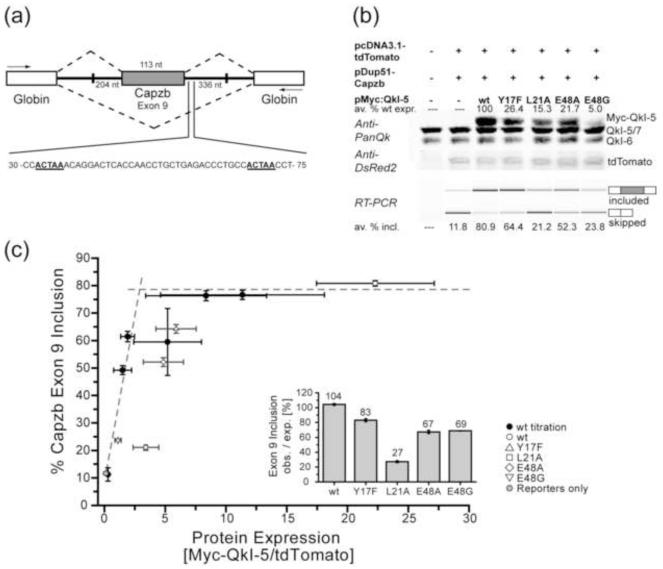
A functional Qua1 dimerization domain is required for robust splicing activation by QkI-5 in muscle cells
Many exons that are activated in muscle development are enriched for a motif similar to the QkI consensus downstream of the activated exon, such as the actin capping protein beta subunit, Capzb, which features two ACUAA sites in the 19-80 region of its Exon 9 downstream intron 37. In mouse C2C12 myoblasts, alternative splicing is regulated during differentiation to myotubes38; 39, and the STAR motif is enriched near exons that change 38 (M. Hall & M. Ares, unpublished), including Capzb exon 9. An in vivo splicing assay using a Capzb Exon 9 minigene construct in C2C12 cells (M. Hall & M. Ares, unpublished) was used to investigate whether a functional Qua1 domain is required for alternative splicing regulation by QkI-5 (Figures 4 and S5). Overexpression of wild type Myc-tagged QkI-5 (wt Myc-QkI-5) increases Capzb Exon 9 inclusion up to 10-fold in a dose-dependent manner, saturating at a maximum Exon 9 inclusion of 77.5%. Four selected mutants (E48G, E48A, Y17F and L21A) that showed significantly impaired homodimerization in the QkI Qua1 thermal melting assay were investigated in the context of full length Myc-QkI-5 using the Capzb splicing assay in C2C12 cells. Protein expression was reduced for all mutants, with the lowest protein levels being observed for the mutants E48G and L21A. Capzb exon 9 inclusion is significantly reduced by 20-74% for all four mutants compared to wt, with the E48G and L21A mutants showing the most drastic effect. However, since the protein levels for the QkI-5 mutants vary significantly, these data alone do not reveal whether the decrease of exon inclusion is caused by a reduced splicing efficiency of the point mutants or simply by the lower protein levels. Varying amounts of wt Myc-QkI-5 expression plasmid were transfected into C2C12 cells along with a constant amount of the Capzb minigene construct. Capzb exon 9 inclusion values were plotted against Myc-QkI-5 expression levels and fit to a simple saturation model, reflecting the per-molecule splicing efficiency. Adding the QkI-5 mutants to the plot allows comparison of their splicing efficiency to wt Myc-QkI-5 (Figures 4c and S5). Points falling on the fitted curve represent a splicing efficiency equal to wild type protein, while points falling under the curve imply a reduced and points above the curve an enhanced splicing efficiency. Furthermore, the splicing efficiency for each mutant was calculated as the ratio of observed exon inclusion to the expected wt value for the given mutant expression level which was obtained from the wild type titration fit. The data points for all 4 mutants fall under the wt titration curve and the splicing efficiency is decreased by 17-73% compared to wt, indicating that the mutations significantly affect the ability of QkI-5 to activate splicing in C2C12 cells.
Figure 4. Dimerization-deficient point mutations in the Qua1 domain reduce QkI-5 splicing activity in vivo.
(a) Schematic presentation of the Capzb Exon9 mini gene organization. White boxes represent the Globin exons, the grey box represents the Capzb exon 9. Numbers by the introns flanking the Capzb Exon 9 indicate the length of intron sequence that was cloned from the Capzb gene. PCR primer positions are indicated as arrows. The 30-75 nt region downstream of the Capzb Exon 9 contains two QkI consensus binding sites, highlighted in bold and underscored, which are essential for QkI-5 splicing activity in C2C12 myoblast cells. (b) Western Blot (top) and RT-PCR analysis of QkI-5 expression levels and minigene splicing patterns from cytoplasmic RNA of C2C12 myoblast cells co-transfected with the Capzb Exon 9 minigene construct (pDup51-Capzb), plasmid for expression of wild type (wt) or mutant QkI-5 protein, and a tdTomato expression vector as transfection control. Each QkI-5 construct was assayed in three independent co-transfection experiments. In the Anti-PanQk blot, the two bottom bands represent endogenous QkI, while the top band represents the overexpressed Myc-QkI-5. The Anti-DSRed blot serves as transfection control. Average values for QkI-5 construct expression relative to wt expression are given above the Western blot. The average percentage of Capzb Exon 9 inclusion for each construct is given below the RT-PCR gel. Plots of these values including standard deviation can be found in Figure S5. (c) Capzb Exon 9 inclusion plotted versus protein expression levels. In order to normalize the splicing efficiencies of the QkI-5 mutants, which show very different protein expression levels, varying amounts of wt Myc-QkI-5 expression vector were transfected in three independent titration experiments. The protein expression level (normalized to tdTomato transfection control) and Capzb minigene splicing efficiency were analyzed as described for the QkI-5 mutants and plotted against each other. Plots of these values including standard deviation can be found in Figure S5. All individual data points were used to fit a simple saturation model (Figure S5). This plot shows the wt titration (black circles) and mutant (open symbols) data averaged over the three replicates. Error bars represent 1/2 standard deviation. The fitted saturation curve is presented as two intersecting dashed lines. Points falling below this curve indicate a lower splicing efficiency per QkI-5 molecule compared to wt, while the area above the curve indicates a higher splicing efficiency. The inset shows the ratio of observed to expected splicing efficiency (PSI, percent splicing included).
DISCUSSION
STAR family members show structural differences in their dimer interface
The STAR domain is highly conserved and defines the family of STAR/GSG proteins in metazoans. The Qua1 subdomains across this family show a high degree of sequence similarity, implying that the structures of the Qua1 homodimers are likely similar, however, the fraction of identical residues between homologs is only 30%. Interestingly, the monomer zipper between the two main α-helices shows high sequence identity, while significant variability is observed within the hydrophobic homodimer interface, including the turn region that links the two main helices.
The Quaking subfamily exhibits a characteristic 3-residue insertion within this turn region that is not present in other paralogs. A homology model of the QkI Qua1 domain using the GLD-1 Qua1 structure 30 as template, consistent with secondary structure prediction from the QkI Qua1 sequence (https://www.predictprotein.org/), yielded a model where the two helices in the helix-turn-helix motif overlap exactly and the longer linker simply results in a further extending turn (Figure S4), prompting the initial attempt using molecular replacement to solve the QkI Qua1 structure.
The structure of the QkI Qua1 subdomain shows that the two main helices forming the helical hairpin fold are in fact conserved in the QkI Qua1 structure. Interestingly, the top of the hairpin fold stands out because it does not simply consist of a larger turn lacking secondary structure as predicted, but forms a short additional α-helix of about 1.5 turns which lies perpendicular to the two main helices (Figures 2 and 5). Formation of this third helix is enabled by the Quaking subfamily-specific three-residue insertion that is not present in the GLD-1 and Sam68 Qua1 structures 30; 31, which likely explains why molecular replacement using the GLD-1 or Sam68 Qua1 structures was not successful.
Figure 5. Comparison of the QkI-Qua1 structure (pdb 4DNN) to the GLD-1 (pdb 3K6T) and Sam68 (pdb 2XA6).
(a) Ribbon presentation. QkI-Qua1 features an additional 3 residues in the turn region that enable to form an additional short helix and expand the surface area of the dimer interface. (b) While the two main helices in each monomer overlay precisely for all three Qua1 domains, the two QkI-Qua1 monomers are stacked at a narrower angle compared to the GLD-1 and Sam68 homodimers. (c) Comparison of dimer interface and zipper residues (shown as sticks) of QkI, GLD-1 and Sam68 Qua1 monomers and overlay of the three structures. While the monomer zipper is highly conserved, more variation is found in the hydrophobic dimer interface residues. All three proteins use stacking of conserved Phe residues as the core of the dimer interface, but the exact position of these residues varies to some degree. The additional helix in the QkI structure broadens the dimer interface significantly and allows for more hydrogen bond interactions across the edge of the interface (see also Figure 2).
The QkI Qua1 dimer interface is enlarged by the inserted helix and covers 815 Å2 per monomer, compared to 700 Å2 per monomer for GLD-1 30 and 624 Å2 per monomer for Sam68 31. The larger interface allows for a more extended hydrogen bonding network at the edge of the dimer interface which, in contrast to GLD-1 and Sam68, also includes the conserved monomer clamp residues Y17 and E48 (Figure 2d). The differences in surface area and number of interactions are also reflected in the melting temperatures of the QkI (69°C), GLD-1 (63°C)30 and Sam68 (<50°C)31 Qua1 constructs. The quaternary dimer structures of GLD-1 and Sam68 Qua1 domains overlay well in the helical segments for both protomers with the main differences in the turn region (backbone RMSD = 1.1 Å)31 and both show a 90° angle between the two protomers. In contrast, the protomers in the QkI Qua1 dimer are stacked at an angle of only 84° (Figure 5c).
Within the QkI Qua1 dimer interface, the conserved F38 residue and F34 are located close to the hairpin turn region and form the center of the hydrophobic dimer interface. F34 stacks face-to-face with its own counterpart F34′ in the second protomer. The F34-F34′ interaction lies over the dimer symmetry axis and requires one side chain to face towards the center of the dimer interface while the other one has to rotate outwards which breaks the symmetry between the two protomers. Each of the F34 residues also engages in a face-to-edge stacking interaction with F38 within the same protomer (Figure 2d). A similar Phe π-sandwich structure is also observed in GLD-1 Qua1, but the position of these side chains within the dimer interface is shifted noticeably. Sam68 with only one Phe (F118) per protomer does not use Phe π-stacking interactions at all but achieves hydrophobic packing with Leu (L114) residues. These differences in the dimer interface between STAR family members might serve to enforce homodimerization as opposed to potential heterodimerization of homologous STAR proteins, for example QkI and Sam68.
Interestingly, the highest degree of sequence identity in the Qua1 subdomain between QkI, GLD-1 and Sam68 as well as other STAR family members is found in the zipper residues that form the contact between the two main helices in the helical hairpin fold. The zipper is important for dimerization because it stabilizes the tertiary structure of the monomer and ensures a proper display of the dimer interface residues. This stabilizing effect was also observed for the homologous GLD-1 Qua1 homodimer 30. The hydrophobic dimer interface residues on the other hand show similarity, in that the hydrophobic properties are conserved, but only the Pro residue (P32 in QkI), ending helix α1 and inducing the (first) turn, and one Phe residue (F38 in QkI) are invariant throughout the STAR family. Overlaying the QkI, GLD-1 and Sam68 Qua1 structures (Figure 5b), it becomes apparent that even the invariant dimer interface residues vary in their spatial arrangement resulting in noticeable variations in the details of interface surface shape, even though the general placement of the surface ridges and grooves as well as the electrostatic surface potential seem similar (Figure S3).
Dimer stability and its effect on RNA binding in vitro
The dimeric STAR proteins bind to bipartite RNA sequences where each monomer is believed to bind to one of the two RNA half-sites 9; 10; 11. Homodimerization is essential for enabling this bipartite binding mode. Close proximity of the half sites, and thus high local concentration, explains the tighter binding of a STAR dimer to a full site in vitro compared to binding of a dimerization-deficient monomeric STAR mutant. For GLD-1, the KH-Qua2 subdomain alone is sufficient to bind RNA, but the absence of the Qua1 domain reduces the RNA binding affinity about one order of magnitude 9. This loss in binding affinity can be explained by the fact that each monomeric GLD-1 protein can only bind to one hexamer consensus site, while the dimer binds a bipartite target site that contains at least one high-affinity consensus element 9; 29. Deletion of the QkI Qua1 domain did not abrogate RNA binding despite the lack of dimerization 26. However, this experiment was of qualitative nature and did not allow to quantitate any changes in QkI RNA binding affinity upon Qua1 deletion.
Mutational analysis of the QkI dimer interface and zipper residues shows that, in addition to the dimer interface residues, the hydrophobic zipper that stabilizes the helix-turn-helix fold of the monomer is essential for homodimerization. Zipper mutations have, on average, a larger effect on the stability of the dimer than mutations within the dimer interface itself as they ensure proper tertiary structure and thus present the dimer interface residues in the correct orientation. This effect was also observed for the GLD-1 Qua1 domain 30 and is consistent with the fact that the zipper residues are more highly conserved within the STAR protein family than the dimer interface residues. Mutational analysis of the Qua1 subdomain within the QkI STAR domain context reveals that RNA binding is indeed significantly reduced in those QkI Qua1 mutants that impair dimerization, whether at the dimer interface or in the zipper, linking QkI dimer stability to its biological functions.
What is the role of homodimerization in QkI function in vivo?
To test Qk function in vivo, we monitored its role as a direct splicing factor in muscle cells (Megan P. Hall et al. manuscript in preparation) using a tranfected splicing reporter carrying Capzb Exon 9, which contains two QkI consensus sites in its downstream intron. Exon 9 inclusion is promoted by co-transfection of wild type Myc-tagged QkI-5 and requires the presence of the QkI binding sequences near exon 9 in the reporter (Hall & Ares unpublished), indicating that QkI-5 binding directly to the intron is required to achieve exon inclusion.
The four QkI mutations (Y17F, L21A, E48A and E48G) tested in the in vivo splicing assay significantly impact the stability, and thus ability to homodimerize, of the QkI Qua1 domain and the RNA binding affinity of the QkI STAR domain in vitro. Their significantly reduced splicing activity observed in C2C12 myoblast cells correlates well with the in vitro data. Interestingly, the reduction of exon inclusion for the mutants is caused by both a diminished splicing efficiency and lower protein expression levels. The observed lower protein levels suggest that the dimerization-deficient mutants with a likely unfolded Qua1 domain are more prone to protein degradation in C2C12 cells. The impaired splicing efficiencies (Figure 4) for the mutants correlate well with their in vitro RNA binding affinities (Table 2). The L21A mutation confers the lowest RNA binding affinity and splicing efficiency, while both mutants E48A and E48G are being equally impaired in both functions and the least destabilizing Y17F mutant confers the least drastic, albeit still significant, loss of function. Overall, the splicing activity of the QkI point mutants correlates well with their ability to homodimerize, indicating that QkI homodimerization is required for QkI splicing activity in vivo.
Homodimerization seems to be important for STAR protein biological function beyond a simple increase in RNA binding affinity. Mutants that fail to dimerize show a loss-of-function phenotype, e.g. QkI E48G causes embryonic lethality26. It is possible that in vivo STAR protein dimers bind to two distant sites looping and arranging the RNA to facilitate binding of other RNA-binding proteins of the spliceosome or transcription regulation complexes 40. The RNA binding and splicing activity of QkI and other STAR proteins is regulated by phosphorylation 12; 13; 14 and it has been proposed that phosphorylation might also be used to disrupt the homodimer and switch to a monomeric, inactive form of the protein 31. Several of the tyrosine residues implicated in phosphorylation are conserved throughout the STAR protein family, suggesting that regulation of dimeric vs. monomeric forms by phosphorylation might be used as a general switch. It is also possible that other proteins recognize and bind to a STAR protein dimer but not a monomer, or vice versa.
QkI exists in multiple alternatively spliced Quaking isoforms, the most prominent ones being QkI-5, QkI-6 and QkI-7. All isoforms are identical in the 1-311 region including the full STAR domain. The C-terminal 8-30 residues differ between isoforms and convey specific functions. QkI-5 (312-341) contains a nuclear localization signal. The 14 C-terminal residues (312-325) of the predominantly cytoplasmic QkI-7 carry an apoptosis-inducing signal. QkI-6 uniquely features an 8 residue tail (residues 312-319) and is found both in the nucleus and the cytoplasm. Heterodimerization between isoforms can occur 16 but it is not well understood under what circumstances these isoforms can heterodimerize in cells, and how this affects QkI function. When cotransfected and overexpressed in HeLa cells, QkI-5 forms heterodimers with QkI-6 and QkI-7 as well as self-homodimers. Furthermore, QkI-5 was able to shuttle QkI-7 into the nucleus and suppress the induction of apoptosis by QkI-7. These results are a first indication that heterodimerization between different isoforms might be used to control protein localization and that the balance in the QkI isoforms is critical for the normal function of the QkI proteins and cell viability.
In addition to multiple Quaking isoforms, there are other STAR proteins like Sam68 present in both the cytoplasm and the nucleus. Considering that the topologies of the Qua1 dimer interfaces in the STAR family are similar but not identical it could be possible that STAR proteins form inter-paralog heterodimers. QkI has been shown to interact with co-transfected GLD-1 but not Sam68 in an immunoprecipitation assay in HeLa cells 25. The structural differences between STAR family members within the dimer interface likely serve to specify and select dimerization partners. STAR protein heterodimers would be expected to form with a lower affinity, if at all, and thus might be controlled by protein concentrations in the cell, which would add another level of functional regulation. To shed light on these questions, further studies are needed addressing the role of STAR protein dimerization in vivo, both between isoforms and different family members, and how dimerization affects interaction with RNA and other regulatory proteins. In summary, we have determined the structure of the QkI homodimerization domain, and correlated the formation of dimers to RNA binding in vitro, and to activation of splicing in cells, thus providing insights into how STAR proteins function in regulation of gene expression.
MATERIALS AND METHODS
Protein expression and purification
MBP-tagged pMAL-QkI-STAR(1-205) was expressed in E. coli strain JM109 (New England Biolabs) and purified via affinity chromatography using amylose resin (New England Biolabs), followed by a HiTrap Q anion exchange column (GE Healthcare), as described previously 9, but omiting the cation exchange chromatography step. Purified protein was stored at −80°C. Cys 35 was changed to Ser for consistency with the Qua1 constructs, and point mutants were generated in the C35S background using Quikchange (Agilent).
QkI Qua1 C35S constructs were cloned into pET22b(+) (Novagen), which results in a C-terminal hexahistidine (His) tag, or a pET22b(+) derived expression vector, introducing an N-terminal His tag and a Thrombin cleavage site (MHHHHHLVPRGS). For a complete list of constructs cloned and tested for expression see Table S1. All QkI-Qua1 constructs, including point mutants, were expressed in E. coli BL21-Gold (DE3) cells (Stratagene).
The N-terminally His-tagged QkI Qua1 (14-67) was used for all structural studies and dimer stability experiments. Single point mutations in the QkI-Qua1 and QkI-STAR context were introduced in the C35S background using Quikchange (Agilent). Unlabeled QkI-Qua1 protein was expressed in LB media with 100 mg/l ampicillin for three hours at 37°C with 1 mM IPTG, added at a cell density of OD600 = 0.6 – 0.8. Six constructs were 15N-labeled for NMR spectroscopy was expressed for 6h at 37°C in M9 minimal media containing (15NH4)2SO4 as the sole nitrogen source, unlabeled glucose and supplemental trace metals and vitamins. Selenomethionine (SeMet) labeled QkI-Qua1 was expressed for 6 hours at 37°C in M9 minimal media containing NH4Cl as nitrogen source, unlabeled glucose, supplemental trace metals and vitamins, and supplemented with the amino acids selenomethionine (SeMet), Lys, Thr, Phe, Leu, Ile, Val and Thiamine.
Unlabeled and 15N-labeled QkI Qua1 samples for thermal melting experiments were purified in 20 mM sodium phosphate (pH 7.5) buffer, while the SeMet sample for crystallography was purified in 20 mM Tris/HCl (pH 7.5) buffer. Cells were lysed by sonication in buffers containing 15 mM imidazole and Complete EDTA-free protease inhibitor cocktail (Roche). Soluble protein was purified in two steps by Ni-affinity chromatography followed by anion exchange chromatography. The lysate was passed over a 5 mL HisTrap HP column (GE Healthcare), washed with 25 ml of purification buffer with 15 mM imidazole, followed by 15 ml of purification buffer with 20 mM imidazole and finally eluted in a single step with 25 ml of purification buffer with 200 mM imidazole. Pure unlabeled protein fractions were dialyzed against 20 mM sodium phosphate buffer (pH 7.5) at 4°C over night. For SeMet-labeled protein fractions, thrombin (5 U/mg of protein) was added, and the protein was dialyzed against 20 mM Tris/HCl pH 7.5 with 0.02% NaN3 at room temperature for 24 h. Complete Thrombin cleavage was confirmed by SDS-tricine PAGE. The protein was further purified by anion exchange chromatography on a 5 ml HiTrap Q HP column (GE Healthcare), where SeMet-labeled QkI-Qua1 eluted with a 120 ml gradient from 0 to 1 M NaCl, while unlabeled, still His-tagged, QkI-Qua1 constructs were found in the flow through fraction. Unlabeled samples for thermal melting experiments were dialyzed against 20 mM sodium phosphate (pH 7.5), 0.02% NaN3, while SeMet-labeled protein for crystallization was dialyzed against 20 mM Tris/HCl (pH 7.5), 0.02% NaN3. All protein samples were concentrated in Centriprep concentrators (Millipore, 3 kD cutoff) and purified protein was stored at 4°C. Protein concentration was determined by measuring the UV absorption at 280 nm. All protein samples (50 uM for QkI Qua1 and 30 uM for QkI STAR) were run on an SDS-PAGE gel to ensure sample integrity and equal concentrations in the assays.
NMR Spectroscopy
The following constructs were labeled with 15N to study by NMR spectroscopy: QkI Qua1 (14-77)-C-His6, QkI Qua1 (14-77)-N-His6, QkI Qua1 (12-77)-N-His6, QkI Qua1 (14-63)-C-His6, QkI Qua1 (12-60)-C-His6, QkI Qua1 (14-55)-C-His6. NMR samples were prepared in 20 mM sodium phosphate (pH 7.5), 0.02% NaN3 containing 10% D2O / 90% H2O with a final protein concentration of 0.5 mM. NMR spectra were recorded at 35°C on a Bruker 750 MHz Avance spectrometer with 5 mm TXI/HCN triple resonance probes.
Analytical Size Exclusion Chromatography
All QkI Qua1 and STAR mutants were subjected to analytical size exclusion chromatography at room temperature to ensure sample integrity and multimerization status. QkI Qua1 proteins were loaded onto a Superdex 75 10/300 gel filtration column (GE Healthcare) and eluted in 20 mM sodium phosphate buffer (pH 7.5). QkI STAR proteins were loaded onto a Superdex 200 10/300 gel filtration column (GE Healthcare) and eluted in 50 mM Tris/HCl (pH 7.9), 20 mM NaCl, 2 mM DTT. Gel filtration standard (Biorad) with Thyroglobulin (bovine, 670,000 Da), γ-globulin (bovine, 158,000 Da), Ovalbumin (chicken, 44,000 Da), Myoglobin (horse, 17,000 Da) and Vitamin B12 (1,350 Da) was used to calibrate molecular weight.
Protein Crystallography
Protein Crystallization
SeMet-QkI-Qua1(14-67) was crystallized by vapor diffusion in 24-well sitting drop plates, 2μl drop volume, at 22°C. Crystals of SeMet-QkI-Qua1 were obtained by mixing 1 μl of protein (5 mg/ml) in 20 mM Tris/HCl (pH 7.5), 0.02% NaN3 with 1 μl of 0.1 M sodium cacodylate (pH 6.5), 0.2 M calcium acetate and 29% (v/v) PEG 600. Crystals were flash-frozen in liquid nitrogen without additional cryo-protectant.
Data Collection and Processing
Diffraction data were collected at the Stanford Synchrotron Radiation Laboratory (SSRL) BL 11-1 processed with HKL200041. Molecular replacement (MR) was attempted using the PHENIX42 AutoSol. Using the Sam68 Qua1 (pdb 2XA6) monomer or dimer, no solution was obtained. Using GLD-1 Qua1 monomer or dimer, solutions in space group P212121 were found. However, the electron density maps were poorly defined, especially in the turn region. The log likelyhood gains (LLG) of LLG = 34 for GLD-1 Qua1 dimer and LLG = 79 for GLD-1 Qua1 monomer as model were both lower than the acceptable value of 100 (http://www.phenix-online.org/documentation/), indicating a poor quality of these solutions. The PHENIX package42 was also used for MAD phasing of SeMet-QkI-Qua1. Initial model building after density modification was carried out in PHENIX AutoBuild, followed by alternating rounds of refinement with PHENIX Refine and manual model building and evaluation in Coot43. In later rounds of refinement, TLS refinement using each monomer as an independent TLS group was performed in addition to coordinate and atomic B-factor refinement. Adding NCS restrains during refinement did not improve the statistic and was therefore not used. Iterative rebuilding and refinement converged on a final model with R = 21.6% and Rfree = 25.2% and good stereochemistry (98% / 2% of residues in the most favored / additionally allowed region). For complete crystallographic statistics see Table 1.
Thermal Melting Experiments by CD Spectroscopy
Thermal melting experiments were performed using a Jasco J-815 CD spectrometer with Jasco PFD-425S/15 temperature unit and a 1 mm quartz cuvette. The samples consist of 50 μM protein in 20 mM sodium phosphate (pH 7.5). Melting curves were recorded between 5°C and 90°C in 0.2°C-steps with a heating rate of 1°C/min monitoring the signal at 222 nm. The raw CD data was normalized to fraction denatured and corrected for the slopes for folded and unfolded signal according to:
with Fu = fraction unfolded, T = temperature in °C; mdeg = CD signal in millidegrees; min = minimum CD signal (at 5°C); max = maximum CD signal (at 90°C); Tmin = 5°C; Tmax = 90°C; sf = slope for folded state; su = slope for unfolded state.
TM was determined as the inflection point of the normalized melting curves.
Samples were run on an SDS-PAGE gel after the melting experiment to ensure sample integrity and equal concentrations in the assay.
Quantitative RNA Binding Assay using Fluorescence Polarization
Quantitative analysis of QkI STAR/RNA interaction was performed using a fluorescence polarization (FP) assay as described previously 9. A constant concentration of 1 nM fluorescein (Fluo)-labeled RNA (5′-Fluo-AUUUAAUUUCUUAUCUACUAAUAUCUA-3′) was equilibrated with varying concentrations of QkI-STAR in a total volume of 100 μL in 96-well opaque Fluotrak 200 μL plates (Greiner) for 3 hours at room temperature. The blank-corrected polarization (mP) was measured using an EnVision 2104 Multilabel Reader (Perkin Elmer). Each experiment was performed in triplicate. The apparent equilibrium dissociation constant KD was determined by fitting the FP data from all repeats globally using the quadratic binding equation for a bimolecular complex, keeping the RNA concentration R0 constant:
with FB = RNA fraction bound; mP = polarization value in mP units; mPmax = maximum mP value at saturation; mPmin = minimum mP value in the absence of protein; P0 = protein concentration; R0 = labeled RNA concentration; KD = apparent equilibrium dissociation constant.
The Hill equation does not apply because the RNA concentration cannot be considered ‘in trace’ (probe concentration << 10·KD) for most QkI STAR constructs. The data points shown in Figure 3 are the average of the normalized FB values.
QkI Splicing Assay
Plasmid Construction
The pDup51 vector was used as a splicing reporter as described 44. Exon 9 of Capzb, including 204 nt 5′ of the exon and 336 nt 3′ of the exon, were PCR-amplified and cloned into the ApaI and BglII sites in pDup51. The pMyc:Qk5 vector was a kind gift from Sean Ryder. Mutations were made by site directed mutagenesis and verified by sequencing. The pcDNA3.1-tdTomato vector was a kind gift from Rohinton Kamakaka.
Cell Culture and Transfections
Mouse C2C12 myoblasts (ATCC) were cultured in 1x Dulbecco’s Modified Eagle Media (Gibco/Invitrogen) supplemented with 10% Fetal Bovine Serum (Gibco/Invitrogen) under sub-confluent conditions as recommended by the distributor. Cells were co-transfected in 6-well plates with 1 μg pcDNA3.1-tdTomato, 0.5 μg pDup51-CapzbEx9, and either 2 μg of mutant and wild-type pMyc:Qk5 (for mutant splicing assays) or varying amounts of wild-type pMyc:Qk5 (for Qk5 titrations). Transfections were performed using Lipofectamine2000 (Invitrogen) according to the manufacturer’s instructions. Cells were harvested and RNA was extracted using TriZOL Reagent (Invitrogen), while protein was extracted by lysis in Laemmli Buffer.
RT-PCR and BioAnalyzer Analysis
Reverse transcription was carried out on 2 μg total RNA using Superscript II (Invitrogen) according to the manufacturer’s instructions. Equal volumes of resulting cDNA were used as PCR template for each sample. Reactions were carried out for 30 cycles with primers directed toward the flanking Dup51 reporter exons, and visualized by ethidium bromide staining of agarose gel analysis. To measure percent inclusion for splicing, we purified PCR products then separated samples on the Agilent Bioanalyzer. This reported the size and concentration of both skipped and Capzb exon 9 included PCR products.
Western Blotting
Equal volumes of cell lysate were loaded onto SDS-Polyacrylamide gels and electrophoresed. Gels were transferred to pure nitrocellulose then blocked for one hour at room temperature. Primary antibodies directed toward Pan-Quaking (Antibodies Incorporated) and DsRed2 (Clontech) were used at respective dilutions of 1:2000 and 1:500 and incubated with membranes overnight at 4°C. IRDye 800CW conjugated goat anti-mouse IgG2b (Li-Cor) and IRDye 680LT conjugated goat anti-mouse IgG1 secondary (Li-Cor) antibodies were both diluted to 1:20,000 and simultaneously used to probe the membrane for 60 min at room temperature. The resulting signals were visualized on a Li-Cor Odyssey Infrared Imager. Bands were quantified by corresponding infrared counts in each signal wavelength, and ectopic Myc-tagged Qk5 expression was normalized to the expression of the tdTomato transfection control.
Calculation of normalized splicing efficiency
Wild type titration data were plotted as Capzb exon 9 inclusion versus protein expression and fitted to a simple saturation model keeping the y intercept fixed to the value determined from the average of the reporter only data:
Mutant splicing efficiency relative to wild type was calculated as the ratio of the averaged exon inclusion for the mutants divided by the expected exon inclusion for wt protein at the given mutant protein level according to the fit above.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ian Wilson for the use of X-ray equipment; Kurt Wüthrich for the use of CD spectrometer; Steven Brown for the use of the fluorescence plate reader. X-ray data were collected at SSRL, a national user facility operated by Stanford University on behalf of the U.S. Dept. of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the DOE, BES, and by the NIH, NCRR, Biomedical Technology Program, and the NIGMS. This work was supported by the National Institutes of Health, grant GM053320 (J.R.W.), a Training Grant 5T32GM008646-14 (WSF), and a postdoctoral fellowship by the DFG (C.B).
Abbreviations
- QkI
Quaking protein
- STAR
signal transducer and activator of RNA
- CNS
central nervous system
- qkv
quaking viable mutation in mice
- KH
hnRNP K homology domain
- SF1
splicing factor 1
- GLD-1
germline development defective-1
- Sam68
Src-associated during mitosis, 68 kDa-protein
- NMR
nuclear magnetic resonance
- HSQC
heteronuclear single-quantum coherence
- MBP
maltose binding protein
- FP
fluorescence polarization
- KD
dissociation constant
- TM
melting temperature
- RMSD
root mean square deviation
- wt
wild type
Footnotes
ACCESSION NUMBERS
Coordinates and structure factors for QkI Qua1 (14-67) C35S have been deposited in the Protein Data Bank with the accession code 4DNN.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Artzt K, Wu JI. STAR trek: An introduction to STAR family proteins and review of quaking (QKI) Adv Exp Med Biol. 2010;693:1–24. [PubMed] [Google Scholar]
- 2.Vernet C, Artzt K. STAR, a gene family involved in signal transduction and activation of RNA. Trends Genet. 1997;13:479–84. doi: 10.1016/s0168-9525(97)01269-9. [DOI] [PubMed] [Google Scholar]
- 3.Lobbardi R, Lambert G, Zhao J, Geisler R, Kim HR, Rosa FM. Fine-tuning of Hh signaling by the RNA-binding protein Quaking to control muscle development. Development. 2011;138:1783–94. doi: 10.1242/dev.059121. [DOI] [PubMed] [Google Scholar]
- 4.Noveroske JK, Lai L, Gaussin V, Northrop JL, Nakamura H, Hirschi KK, Justice MJ. Quaking is essential for blood vessel development. Genesis. 2002;32:218–30. doi: 10.1002/gene.10060. [DOI] [PubMed] [Google Scholar]
- 5.Wu JI, Reed RB, Grabowski PJ, Artzt K. Function of quaking in myelination: regulation of alternative splicing. Proc Natl Acad Sci U S A. 2002;99:4233–8. doi: 10.1073/pnas.072090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saccomanno L, Loushin C, Jan E, Punkay E, Artzt K, Goodwin EB. The STAR protein QKI-6 is a translational repressor. Proc Natl Acad Sci U S A. 1999;96:12605–10. doi: 10.1073/pnas.96.22.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larocque D, Galarneau A, Liu HN, Scott M, Almazan G, Richard S. Protection of p27(Kip1) mRNA by quaking RNA binding proteins promotes oligodendrocyte differentiation. Nat Neurosci. 2005;8:27–33. doi: 10.1038/nn1359. [DOI] [PubMed] [Google Scholar]
- 8.Larocque D, Pilotte J, Chen T, Cloutier F, Massie B, Pedraza L, Couture R, Lasko P, Almazan G, Richard S. Nuclear retention of MBP mRNAs in the quaking viable mice. Neuron. 2002;36:815–29. doi: 10.1016/s0896-6273(02)01055-3. [DOI] [PubMed] [Google Scholar]
- 9.Ryder SP, Williamson JR. Specificity of the STAR/GSG domain protein Qk1: implications for the regulation of myelination. RNA. 2004;10:1449–58. doi: 10.1261/rna.7780504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galarneau A, Richard S. Target RNA motif and target mRNAs of the Quaking STAR protein. Nat Struct Mol Biol. 2005;12:691–8. doi: 10.1038/nsmb963. [DOI] [PubMed] [Google Scholar]
- 11.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr., Jungkamp AC, Munschauer M, Ulrich A, Wardle GS, Dewell S, Zavolan M, Tuschl T. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–41. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Lu Z, Ku L, Chen Y, Wang H, Feng Y. Tyrosine phosphorylation of QKI mediates developmental signals to regulate mRNA metabolism. EMBO J. 2003;22:1801–10. doi: 10.1093/emboj/cdg171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matter N, Herrlich P, Konig H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420:691–5. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 14.Nir R, Grossman R, Paroush Z, Volk T. Phosphorylation of the Drosophila melanogaster RNA-Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity. PLoS Genet. 2012;8:e1002632. doi: 10.1371/journal.pgen.1002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebersole TA, Chen Q, Justice MJ, Artzt K. The quaking gene product necessary in embryogenesis and myelination combines features of RNA binding and signal transduction proteins. Nat Genet. 1996;12:260–5. doi: 10.1038/ng0396-260. [DOI] [PubMed] [Google Scholar]
- 16.Pilotte J, Larocque D, Richard S. Nuclear translocation controlled by alternatively spliced isoforms inactivates the QUAKING apoptotic inducer. Genes Dev. 2001;15:845–58. doi: 10.1101/gad.860301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Zhou L, Tonissen K, Tee R, Artzt K. The quaking I-5 protein (QKI-5) has a novel nuclear localization signal and shuttles between the nucleus and the cytoplasm. J Biol Chem. 1999;274:29202–10. doi: 10.1074/jbc.274.41.29202. [DOI] [PubMed] [Google Scholar]
- 18.Haroutunian V, Katsel P, Dracheva S, Davis KL. The human homolog of the QKI gene affected in the severe dysmyelination “quaking” mouse phenotype: downregulated in multiple brain regions in schizophrenia. Am J Psychiatry. 2006;163:1834–7. doi: 10.1176/ajp.2006.163.10.1834. [DOI] [PubMed] [Google Scholar]
- 19.Noveroske JK, Hardy R, Dapper JD, Vogel H, Justice MJ. A new ENU-induced allele of mouse quaking causes severe CNS dysmyelination. Mamm Genome. 2005;16:672–82. doi: 10.1007/s00335-005-0035-x. [DOI] [PubMed] [Google Scholar]
- 20.Ebersole T, Rho O, Artzt K. The proximal end of mouse chromosome 17: new molecular markers identify a deletion associated with quakingviable. Genetics. 1992;131:183–90. doi: 10.1093/genetics/131.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Mandler MD, Yi H, Feng Y. Quaking I controls a unique cytoplasmic pathway that regulates alternative splicing of myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 2010;107:19061–6. doi: 10.1073/pnas.1007487107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zearfoss NR, Clingman CC, Farley BM, McCoig LM, Ryder SP. Quaking regulates Hnrnpa1 expression through its 3′ UTR in oligodendrocyte precursor cells. PLoS Genet. 2011;7:e1001269. doi: 10.1371/journal.pgen.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novikov L, Park JW, Chen H, Klerman H, Jalloh AS, Gamble MJ. QKI-mediated alternative splicing of the histone variant MacroH2A1 regulates cancer cell proliferation. Mol Cell Biol. 2011;31:4244–55. doi: 10.1128/MCB.05244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G, Fu H, Zhang J, Lu X, Yu F, Jin L, Bai L, Huang B, Shen L, Feng Y, Yao L, Lu Z. RNA-binding protein quaking, a critical regulator of colon epithelial differentiation and a suppressor of colon cancer. Gastroenterology. 2010;138:231–40. e1–5. doi: 10.1053/j.gastro.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, Damaj BB, Herrera C, Lasko P, Richard S. Self-association of the single-KH-domain family members Sam68, GRP33, GLD-1, and Qk1: role of the KH domain. Mol Cell Biol. 1997;17:5707–18. doi: 10.1128/mcb.17.10.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T, Richard S. Structure-function analysis of Qk1: a lethal point mutation in mouse quaking prevents homodimerization. Mol Cell Biol. 1998;18:4863–71. doi: 10.1128/mcb.18.8.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryder SP, Frater LA, Abramovitz DL, Goodwin EB, Williamson JR. RNA target specificity of the STAR/GSG domain post-transcriptional regulatory protein GLD-1. Nat Struct Mol Biol. 2004;11:20–8. doi: 10.1038/nsmb706. [DOI] [PubMed] [Google Scholar]
- 28.Galarneau A, Richard S. The STAR RNA binding proteins GLD-1, QKI, SAM68 and SLM-2 bind bipartite RNA motifs. BMC Mol Biol. 2009;10:47. doi: 10.1186/1471-2199-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmel AB, Wu J, Lehmann-Blount KA, Williamson JR. High-affinity consensus binding of target RNAs by the STAR/GSG proteins GLD-1, STAR-2 and Quaking. BMC Mol Biol. 2010;11:48. doi: 10.1186/1471-2199-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beuck C, Szymczyna BR, Kerkow DE, Carmel AB, Columbus L, Stanfield RL, Williamson JR. Structure of the GLD-1 homodimerization domain: insights into STAR protein-mediated translational regulation. Structure. 2010;18:377–89. doi: 10.1016/j.str.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer NH, Tripsianes K, Vincendeau M, Madl T, Kateb F, Lehmann-erner R, Sattler M. Structural basis for homodimerization of the Src-associated during mitosis, 68-kDa protein (Sam68) Qua1 domain. J Biol Chem. 2010;285:28893–901. doi: 10.1074/jbc.M110.126185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Luyten I, Bottomley MJ, Messias AC, Houngninou-Molango S, Sprangers R, Zanier K, Kramer A, Sattler M. Structural basis for recognition of the intron branch site RNA by splicing factor 1. Science. 2001;294:1098–102. doi: 10.1126/science.1064719. [DOI] [PubMed] [Google Scholar]
- 33.Maguire ML, Guler-Gane G, Nietlispach D, Raine AR, Zorn AM, Standart N, Broadhurst RW. Solution structure and backbone dynamics of the KH-QUA2 region of the Xenopus STAR/GSG quaking protein. J Mol Biol. 2005;348:265–79. doi: 10.1016/j.jmb.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 34.Ali M, Broadhurst RW. Structural Studies of Quaking Protein Qua1 domain. XXIII. ICMRBS; San Diego: 2008. p. 193. [Google Scholar]
- 35.Holm L, Kaariainen S, Rosenstrom P, Schenkel A. Searching protein structure databases with DaliLite v.3. Bioinformatics. 2008;24:2780–1. doi: 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–97. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Sugnet CW, Srinivasan K, Clark TA, O’Brien G, Cline MS, Wang H, Williams A, Kulp D, Blume JE, Haussler D, Ares M., Jr Unusual intron conservation near tissue-regulated exons found by splicing microarrays. PLoS Comput Biol. 2006;2:e4. doi: 10.1371/journal.pcbi.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bland CS, Wang ET, Vu A, David MP, Castle JC, Johnson JM, Burge CB, Cooper TA. Global regulation of alternative splicing during myogenic differentiation. Nucleic Acids Res. 2010;38:7651–64. doi: 10.1093/nar/gkq614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jungkamp AC, Stoeckius M, Mecenas D, Grun D, Mastrobuoni G, Kempa S, Rajewsky N. In vivo and transcriptome-wide identification of RNA binding protein target sites. Mol Cell. 2011;44:828–40. doi: 10.1016/j.molcel.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography. 1997;276(Pt A):307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 42.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–54. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 43.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 44.Dominski Z, Kole R. Selection of splice sites in pre-mRNAs with short internal exons. Mol Cell Biol. 1991;11:6075–83. doi: 10.1128/mcb.11.12.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.