Abstract
Acetate supplementation increases brain acetyl-CoA and histone acetylation and reduces lipopolysaccharide (LPS)-induced neuroglial activation and interleukin (IL)-1β expression in vivo. To determine how acetate imparts these properties, we tested the hypothesis that acetate metabolism reduces inflammatory signaling in microglia. To test this, we measured the effect acetate treatment had on cytokine expression, mitogen-activated protein kinase (MAPK) signaling, histone H3 at lysine 9 acetylation, and alterations of nuclear factor-kappa B (NF-κB) in primary and BV-2 cultured microglia. We found that treatment induced H3K9 hyperacetylation and reversed LPS-induced H3K9 hypoacetylation similar to that found in vivo. LPS also increased IL-1β, IL-6 and tumor necrosis factor-alpha (TNF-α) mRNA and protein, while treatment returned the protein to control levels and only partially attenuated IL-6 mRNA. In contrast, treatment increased mRNA levels of transforming-growth factor-β1 (TGF-β1) and both IL-4 mRNA and protein. LPS increased p38 MAPK and JNK phosphorylation at 4 and 2–4 hr respectively, while treatment reduced p38 MAPK and JNK phosphorylation only at 2 hr. In addition, treatment reversed the LPS-induced elevation of NF-κB p65 protein and phosphorylation at serine 468 and induced acetylation at lysine 310. These data suggest that acetate metabolism reduces inflammatory signaling and alters histone and non-histone protein acetylation.
Keywords: Histone, acetylation, neuroinflammation, microglia, cytokine, MAPK
Introduction
Neuroinflammation involves an innate immune response that is advantageous with regard to normal brain physiology. However, uncontrolled neuroinflammation is detrimental and associated with numerous neurological pathologies. The physiological functions of cytokines include regulating cell growth, differentiation, and body temperature regulation (Rothwell & Hopkins 1995, Hopkins & Rothwell 1995). Cytokines also have a pleiotropic function in neuroimmune communication between neuroglia, neurons, and endothelium involving both injury resolution and progression (Suzuki et al. 2009). Excessive pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin-(IL)1β, and IL-6 are implicated in the pathogenesis of numerous neuroinflammatory diseases (Denes et al. 2010, Helmy et al. 2011, Johnston et al. 2011, Merson et al. 2010, Qian et al. 2010). Transforming growth factor-beta1 (TGF-β1), IL-4, and IL-10 collectively share anti-inflammatory features that counteract the pro-inflammatory cytokines and provide control over the neuroinflammatory response. Anti-inflammatory cytokines are generally involved in tissue repair, enhancing neuronal survival, and downregulating the expression of pro-inflammatory cytokines (Vitkovic et al. 2001, Ledeboer et al. 2000).
Acetate supplementation increases brain acetate levels (Mathew et al. 2005) as well as the metabolically active intermediate acetyl-CoA in normal animals (Reisenauer et al. 2011). In a rat model of neuroinflammation, acetate supplementation attenuates lipopolysaccharide (LPS)-induced neuroglial activation and the loss of cholinergic immunoreactivity (Reisenauer et al. 2011). Acetate supplementation is also neuroprotective in a rat model of head trauma (Arun et al. 2010a) and a tremor model of Canavan’s disease (Arun et al. 2010b). To understand the mechanism underlying the neuroprotective and anti-inflammatory effects of acetate supplementation, we examined the effect that acetate metabolism has on histone hyperacetylation, which is associated with anti-inflammatory and neuroprotective responses (Adcock 2007, Langley et al. 2005). A single oral dose of glyceryl triacetate, used to induce acetate supplementation, results in site- and time-specific histone hyperacetylation in the brains of normal animals (Soliman & Rosenberger 2011). In addition, long-term acetate supplementation in a rat model of neuroinflammation induces site-specific brain histone hyperacetylation, reverses LPS-induced hypoacetylation of histone H3 at lysine 9 (H3K9), and suppresses IL-1β expression (Soliman et al. 2012).
Microglia, the primary immune cells in the brain, transform into phagocytic cells upon changes in the structural or functional integrity of the brain and produce a wide range of inflammatory cytokines (Hanisch & Kettenmann 2007, Lehnardt 2010, Ransohoff & Perry 2009, Streit et al. 1999). The BV-2 mouse microglia cell line, immortalized through oncogenes carrying retrovirus, exhibit morphological, functional, and phenotypical properties similar to primary microglia (Blasi et al. 1990, Bocchini et al. 1992). Therefore, BV-2 microglial cells are commonly used as an alternative to primary microglia to study various microglial responses and interactions (Woo et al. 2003, Petrova et al. 1999, Rojanathammanee et al. 2011).
Mitogen-activated protein kinases (MAPK) are key regulators of the biosynthesis of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β and are hence potential therapeutic targets in inflammatory and autoimmune diseases (Kumar et al. 2003, Pearson et al. 2001). MAPK include p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK), whose activities are crucial for normal immune and inflammatory responses. The activation of these kinases is implicated in a number of biological processes including cell differentiation and survival, and the response to stress. Specific lysine acetylation activates MAPK phosphatase-1, which subsequently dephosphorylates and deactivates MAPK signaling (Cao et al. 2008), which provides a link between acetylation and phosphorylation as a regulator of inflammation. Another signaling complex that regulates inflammatory responses and is altered by acetylation is nuclear factor-kappa B (NF-κB) which is most commonly a heterodimer of p65 and p50. In the cytoplasm, NF-κB is associated with inhibitors of kappa B (IκB) which mask the nuclear export motif. Upon stimulation by pro-inflammatory cytokines, B and T cell receptor signaling, and viral and bacterial toxins, NF-κB is released from IκB and translocates to the nucleus where it binds DNA sequences and alters the transcriptional activity of genes involved in inflammatory responses and cell survival (Chen & Ghosh 1999). p65, but not p50, binds transcriptional co-activators p300 and CREB-binding protein (Perkins et al. 1997). The p65 subunit of NF-κB can be modified by acetylation at certain lysine residues with variable functional outcomes (Kiernan et al. 2003, Chen et al. 2001, Huang et al. 2010).
No reports are available that describe a decline in brain acetate levels in response to LPS or other neurological pathologies. Consequently, rather than replenishing endogenous acetate stores, we propose that acetate supplementation acts to increase intracellular levels of acetyl-CoA as an inducer of metabolic and molecular processes that ultimately result in the reduction of inflammatory phenotype. To test this hypothesis we measured the ability of acetate treatment to alter inflammatory signaling in LPS-challenged microglia. We found that acetate treatment effectively reversed the LPS-induced H3K9 hypoacetylation and increases in the pro-inflammatory cytokine protein, but not mRNA levels. Further, treatment upregulated the mRNA levels of the anti-inflammatory cytokine TGF-β1, and both the protein and mRNA levels of IL-4. Because MAPK and NF-κB signaling are also associated with the neuroinflammatory responses, we expanded our study to quantify the effect acetate treatment had on these signaling pathways. In this regard, treatment attenuated p38 and JNK phosphorylation at 2, and not 4, hr, and increased phosphorylated ERK1/2 at 4 hr only in the presence of LPS. In addition, acetate treatment returned LPS-mediated increases in p65 protein levels and phosphorylation at serine 468 to control levels, and induced p65 hyperacetylation at lysine 310. These data suggest that, in LPS-challenged microglia, acetate metabolism can modulate inflammatory signaling and shift cytokine balance towards a more anti-inflammatory state.
Materials and Methods
Reagents
LPS (Escherichia Coli 055:B5) was purchased from Sigma, antibodies against total histone H3, acetylated H3K9, phosphorylated p38 (Thr180/Tyr182), p38, phosphorylated JNK (Thr183/Tyr185, Thr221/Tyr223), phosphorylated ERK1/2 (Th202/Tyr204, Thr185/Tyr187), and ERK1/2 were from Millipore, and anti-JNK and NF-κB p65 antibodies were purchased from Cell Signaling Technology Incorporated. Rabbit polyclonal antibodies to IL-1β, IL-6, TNF-α, TGF-β1, IL-4, IL-10 and acetyl-CoA synthetase were from Abcam and all Western blot supplies and a goat anti-rabbit horse radish peroxidase (HRP)-linked antibody and iScript cDNA synthesis kits were purchased from Bio-Rad Laboratories. Reverse and forward IL-1β, IL-6, TNF-α, IL-4, IL-10, TGF-β1 and β-actin primers from SA Biosciences, FastStart Universal SYBR Green Master from Roche Applied Science from Bio-Rad, TRIzol® reagent from Life Technologies, DMEM–F-12 media from Invitrogen, and buffering reagents and other chemicals from EMD Biosciences.
Primary and BV-2 microglial cell cultures
Primary microglia were derived from C57BL/6 mouse brains as described previously (Rojanathammanee et al. 2011). The BV2 microglia were obtained from Dr. Gary E. Landreth (Cleveland, OH) and maintained until used as described previously (Dhawan et al. 2012). Cells were plated in 6 well-dishes and allowed to replicate till 90% confluence, (1.1 × 106 cells/dish). Prior to stimulating the cells (3 hr), the media was changed to serum-free media. Plates were divided into 4 different groups; a group treated with 12 mM NaCl as a control group, another group treated with 12 mM sodium acetate, a third group treated with both 6.25 ng/ml LPS and 12 mM NaCl, and a fourth group treated with both 6.25 ng/ml LPS and 12 mM sodium acetate (n = 6 per group for BV-2 cells and n = 5 per group for primary microglia). The concentration of acetate used in this study is based on studies to determine the maximal amount of acetate that did not lead to significant cell death over a 24 hr exposure period, compared to cells grown in serum-fee media. After a single oral gavage of GTA (5.8 g/kg), brain acetate levels rise to 8 μM/g tissue at 1 hr, and then decline to 6 and 2 μM/g tissue at 2 and 4 hr, respectively (Mathew et al. 2005). However, the metabolically active molecule in this process is not acetate, but rather acetyl-CoA which reaches a maximum of 5.7 μg/g brain at 30 min and remains constant out to 4 hr in vivo (Reisenauer et al. 2011). The cellular concentration of acetyl-CoA is controlled metabolically by acetyl-CoA synthetases 1 and 2, and not by cellular levels of free acetate (Fujino et al. 2001, Ariyannur et al. 2010). Therefore, our rationale for using the highest tolerable acetate concentration was not to mimic maximal tissue concentrations of acetate but rather to maximize, over a 4 hr-period, cellular levels of acetyl-CoA in an effort to identify metabolic and the inflammatory pathways that are modulated down-stream of the formation of acetyl-CoA. For dose-response studies, plates were divided in 6 different groups treated with LPS in the following concentrations: 25, 12.5, 6.25, 3.125, 1.56, or 0 ng/ml (n = 3). After 4 hr, the media was collected and stored at −20° C, and the cells were lysed in either TRIzol® reagent for quantitative real-time polymerase chain reaction (qrt-PCR) analysis or ice cold RIPA lysis buffer (150 mM sodium chloride, Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 50 mM Tris, pH 8.0) for Western blot analysis and stored at −80° C until used.
Western blot analysis
Gel electrophoresis and protein transfer was performed as described previously (Soliman & Rosenberger 2011, Soliman et al. 2012). The antibody concentrations used were 1:500 for total H3 and acetyl-CoA synthetase, 1:1000 for each of acetylated H3K9, IL-1β, IL-6, TNF-α, TGF-β1, IL-4, IL-10, p38, phosphorylated p38, JNK, phosphorylated JNK, ERK1/2, phosphorylated ERK1/2 and all NF-κB antibodies, and 1:3000 for α-tubulin. All Western blot data are expressed as the ratio of the optical density of the respective protein to the optical density of α-tubulin, except acetylated H3K9 (normalized to total histone H3), and phosphorylated MAPK p38, JNK and ERK1/2 (normalized to total MAPK p38, JNK and ERK1/2, respectively).
Quantitative real-time polymerase chain reaction
mRNA extraction, quantification, and cDNA synthesis and amplification were performed as described previously (Soliman et al. 2012). The expression of IL-1β, IL-6, TNF-α, IL-4, IL-10, and TGF-β1 transcripts amplified was normalized to the expression of β-actin.
Lactate dehydrogenase assay
Cellular release of lactate dehydrogenase (LDH) used to measure cell viability was measured using a commercial nonradioactive assay kit (Clontech Inc.), according to the manufacturer’s guidelines. Absorbance measurements were taken at 490 nm.
Statistical analysis
One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test using GraphPad InStat software (Version 3.06 for Windows, San Diego, CA). Results are expressed as means ± SD with significance set at p ≤ 0.05.
Results
Optimizing the duration of acetate treatment and LPS concentration
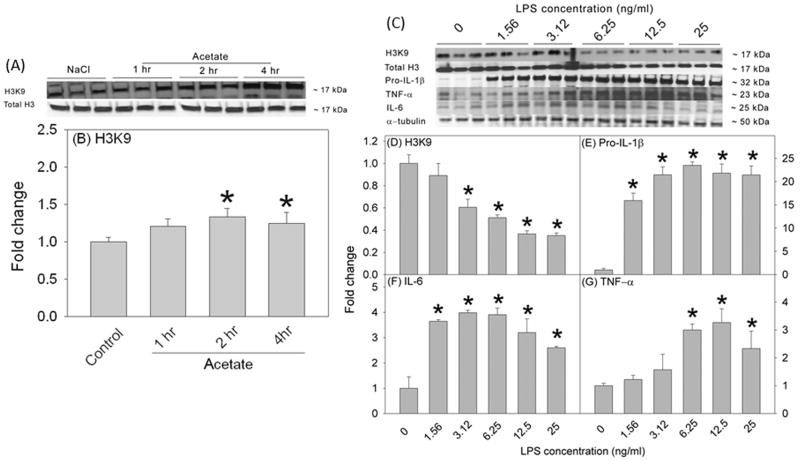
In rat brain, H3K9 acetylation is reduced by 50% in a rat model of neuroinflammation and is returned to control levels with acetate supplementation (Soliman et al. 2012). To determine the duration of acetate treatment required to achieve a similar H3K9 hyperacetylation pattern in vitro, we treated BV-2 microglia with 12 mM sodium acetate for 1, 2 and 4 hr (Figures 1A and B). Cell lysates were used for Western blot analysis to measure acetylated H3K9, total histone H3 (Figure 1A). We found that acetate treatment increased H3K9 acetylation by 2 hr which remained elevated out to 4 hr (Figure 1B). To insure protein expression following treatment, we used 4 hr as the treatment duration for all the experiments with the exception of change in MAPK phosphorylation where additional time points were used. To determine the optimal LPS concentration required to produce the same percentage of H3K9 hypoacetylation found in vivo, we treated BV-2 microglia for 4 hr using a serial dilution of LPS ranging between 0 and 25 ng/ml (Figures 1C and D). Cell lysates were used for Western blot analysis to measure acetylated H3K9, total histone H3 and the pro-inflammatory cytokines pro-IL-1β, IL-6, and TNF-α, which were detected as protein bands corresponding to the molecular weights of 17, 17, 32, 25 and 23, respectively (Figure 1C). LPS reduced H3K9 acetylation and increased the pro-inflammatory cytokine levels in a concentration-dependent manner (Figures 1D–G). Based on these data, we used the LPS concentration 6.25 ng/ml because this concentration resulted in a 50% reduction in H3K9 acetylation, similar to that found in vivo (Soliman et al. 2012), and also increased protein levels of all the pro-inflammatory cytokines measured.
Figure 1.
Time-dependent acetate-induced H3K9 hyperacetylation and dose-response study showing the effects of different LPS concentrations (0–25 ng/ml, 4 hr) on H3K9 acetylation and the expression of pro-inflammatory cytokines in BV-2 microglia. Panels A and C show representative images of the Western blots. Panel B shows the averaged proportion of H3K9 normalized to total H3 (n = 3) after 1, 2 and 4 hr of treatment with 12 mM sodium acetate. Panels D, E, F and G show the optical densities of H3K9 normalized to total H3 and the pro-inflammatory cytokines pro-IL-1β, IL-6, and TNF-α normalized to the loading control α-tubulin (n = 3). The graphs represent the means ± SD where statistical significance (* = compared to LPS 0 ng/ml) was set at p ≤ 0.05, as determined by One Way ANOVA followed by Tukey’s post-hoc test.
Acetate treatment reverses LPS-induced H3K9 hypoacetylation without inducing cytotoxicity in primary microglia
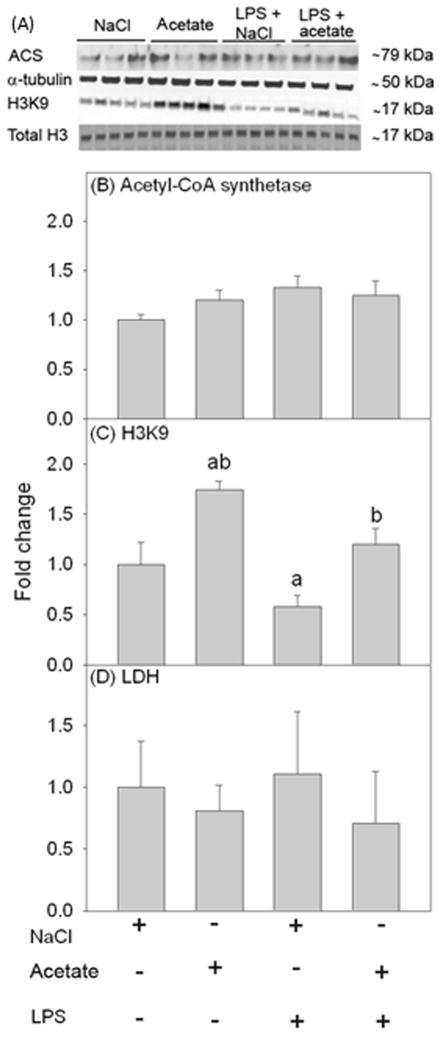
To determine the ability of acetate treatment to reverse LPS-induced H3K9 hypoacetylation in microglia similar to that found in the rat (Soliman et al. 2012), we treated primary mouse microglia with LPS 6.25 ng/ml for 4 hr in the presence and absence of 12 mM sodium acetate, with 12 mM NaCl treatment as control. Using whole cell lysates for Western blot analysis, we found that primary microglia express acetyl-CoA synthetase (ACS) which converts acetate to acetyl-CoA, as protein bands corresponding to the molecular weight of 79 kDa (Figure 2A). The expression level of ACS was not different between groups (Figure 2B). Further, acetate treatment increased H3K9 acetylation by 1.7-fold, whereas LPS reduced H3K9 acetylation by 50% (Figure 2C). Acetate treatment, similar to that found in vivo, effectively increased H3K9 acetylation to control levels in the presence of LPS (Figure 2C). Cell viability assays showed no difference in cell survival between groups (Figure 2D). These data indicate that acetate treatment in vitro reverses LPS-induced H3K9 hypoacetylation in microglia similar to that found in vivo (Soliman et al. 2012).
Figure 2.
Changes in histone acetylation in primary mouse microglial cell culture stimulated for 4 hr with LPS 6.25 ng/ml, and the reversal of these effects upon treatment with 12 mM sodium acetate. Panel A shows representative images of the Western blots. Panels B and C show the optical densities of acetyl-CoA synthetase enzyme normalized to the loading control α-tubulin (n = 3) and H3K9 normalized to total H3 (n = 5), respectively. Panel D shows the quantification of the ratio of secreted LDH in the media to total cellular LDH (n = 5). Bars represent means ± SD where statistical significance (a = compared to NaCl, and b = compared to LPS + NaCl) was set at p ≤ 0.05, as determined by a one way ANOVA followed by Tukey’s post-hoc test.
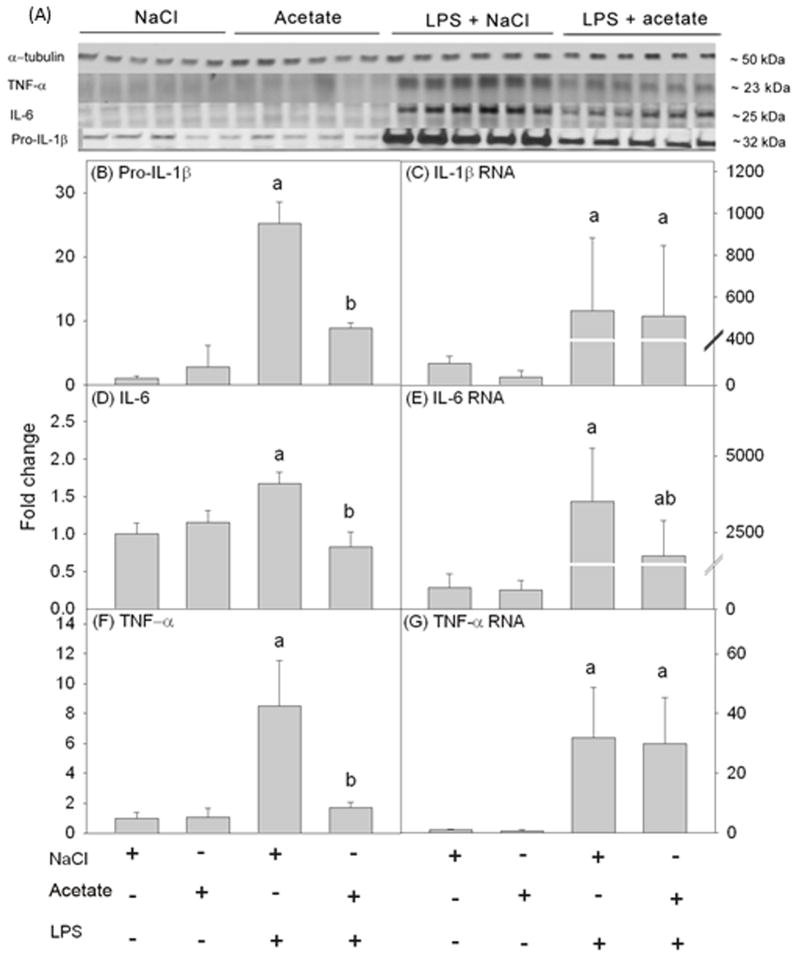
Acetate treatment reverses LPS-induced increases in the pro-inflammatory cytokine proteins, but not mRNA, in primary microglia
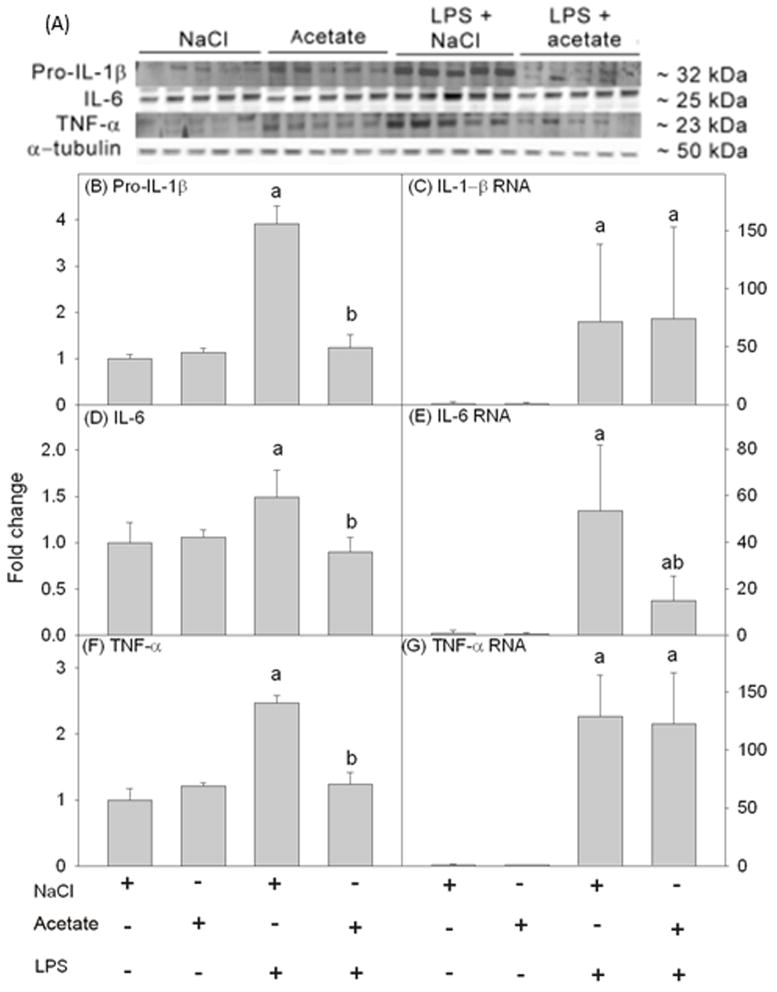
To determine the ability of acetate treatment to reverse pro-inflammatory cytokine production in vitro similar to that found in vivo (Soliman et al. 2012), cell lysates were analyzed using Western blot to probe for IL-1β, IL-6 and TNF-α (Figure 3A). We found that LPS increased pro-IL-1β, IL-6 and TNF-α by about 4, 1.5 and 2.5-fold, respectively which were returned to control levels with acetate treatment (Figures 3B, D and F). In parallel studies, we found that LPS increased the mRNA levels of all the pro-inflammatory cytokines measured but were not altered by acetate treatment (Figures 3C and G) with the exception of IL-6 mRNA which was attenuated 3-fold (Figure 3E). These data demonstrate that this in vitro system reproduces the findings from the animal model, and that acetate treatment decreases pro-inflammatory cytokine levels possibly by disrupting mRNA translation or by increasing protein turnover.
Figure 3.
Changes in the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in primary mouse microglial cell culture stimulated for 4 hr with LPS 6.25 ng/ml with and without 12 mM sodium acetate. Panel A shows representative images of the Western blots. Panels B, D and F show the optical densities of the pro-inflammatory cytokines pro-IL-1β, IL-6 and TNF-α, respectively, normalized to the loading control α-tubulin (n = 5). Panels C, E and G show the changes in the mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α, quantified by qrt-PCR and normalized to β-actin (n = 5). Bars represent means ± SD where statistical significance (a = compared to NaCl, and b = compared to LPS + NaCl) was set at p ≤ 0.05, as determined by a one way ANOVA followed by Tukey’s post-hoc test.
Acetate treatment reverses LPS-induced H3K9 hypoacetylation in BV-2 microglia
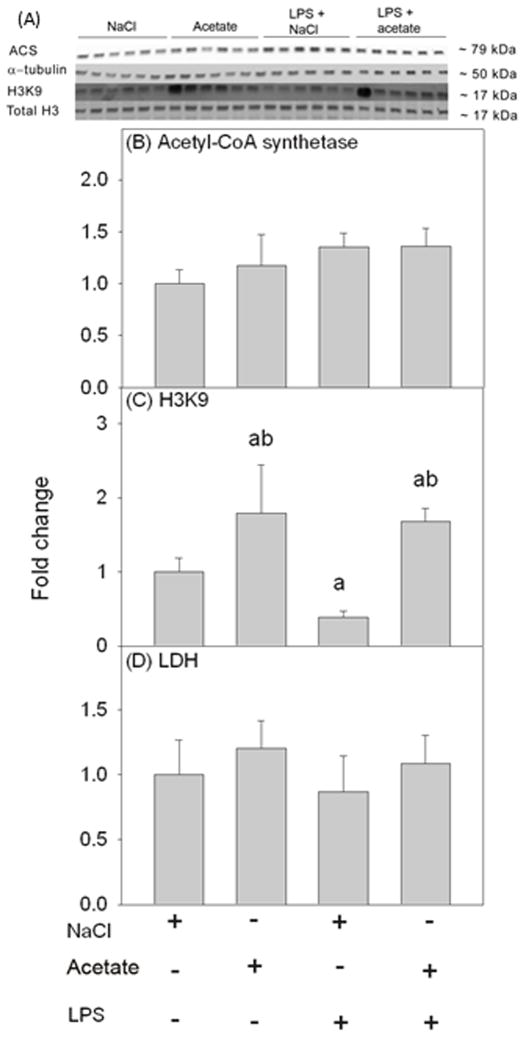
We examined H3K9 acetylation in BV-2 microglia (Figure 4A) under the same experimental conditions used with primary mouse microglia to confirm that both cell types respond similarly. First, we confirmed that BV-2 microglia express ACS; which was not different between groups (Figure 4B). Further, we found that acetate treatment increased H3K9 acetylation by 1.8-fold, and reversed the LPS-induced 50% reduction in H3K9 acetylation (Figure 4C) similar to that found in primary microglia cultures. Further, like the primary microglia cultures, treatment did not alter cell viability (Figure 4D).
Figure 4.
Changes in histone acetylation in BV-2 microglial cell culture stimulated for 4 hr with LPS 6.25 ng/ml, and the reversal of these effects upon treatment with 12 mM sodium acetate. Panel A shows representative images of the Western blots. Panels B and C show the optical densities of acetyl-CoA synthetase enzyme normalized to the loading control α-tubulin and H3K9 normalized to total H3, respectively (n = 6). Panel D shows the quantification of the ratio of secreted LDH in the media to total cellular LDH (n = 6). Bars represent means ± SD where statistical significance (a = compared to NaCl, and b = compared to LPS + NaCl) was set at p ≤ 0.05 (n = 6, per group), as determined by a one way ANOVA followed by Tukey’s post-hoc test.
Acetate treatment reverses the LPS-induced increases in pro-inflammatory cytokine protein, but not mRNA, in BV-2 microglia
We proceeded to determine the effect of acetate treatment and LPS on pro-inflammatory cytokine proteins (Figure 5A) and mRNA levels in BV-2 microglia under the same experimental conditions used with primary microglia to confirm that both cell types respond similarly in this regard. We found that LPS increased pro-IL-1β, IL-6 and TNF-α production by 25-, 1.5-, and 8-fold respectively which were returned to control levels with acetate treatment (Figures 5B, D, and F). In parallel, we found that LPS increased the mRNA levels of the same pro-inflammatory cytokines similar to that found with the primary microglia cultures and were not altered by acetate treatment (Figures 5C and G) with the exception of IL-6 which was attenuated 2-fold (Figure 5E). Therefore, the inflammatory response of BV-2 microglia towards LPS and acetate treatment is similar to primary microglia.
Figure 5.
Changes in the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in BV-2 microglial cell culture stimulated for 4 hr with LPS 6.25 ng/ml with and without 12 mM sodium acetate. Panel A shows representative images of the Western blots. Panels B, D and F show the optical densities of the pro-inflammatory cytokines pro-IL-1β, IL-6 and TNF-α, respectively, normalized to the loading control α-tubulin. Panels C, E and G show the changes in the mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α, quantified by qrt-PCR and normalized to β-actin. Bars represent means ± SD where statistical significance (a = compared to NaCl, and b = compared to LPS + NaCl) was set at p ≤ 0.05 (n = 6, except pro-IL-1β where n = 5), as determined by a one way ANOVA followed by Tukey’s post-hoc test.
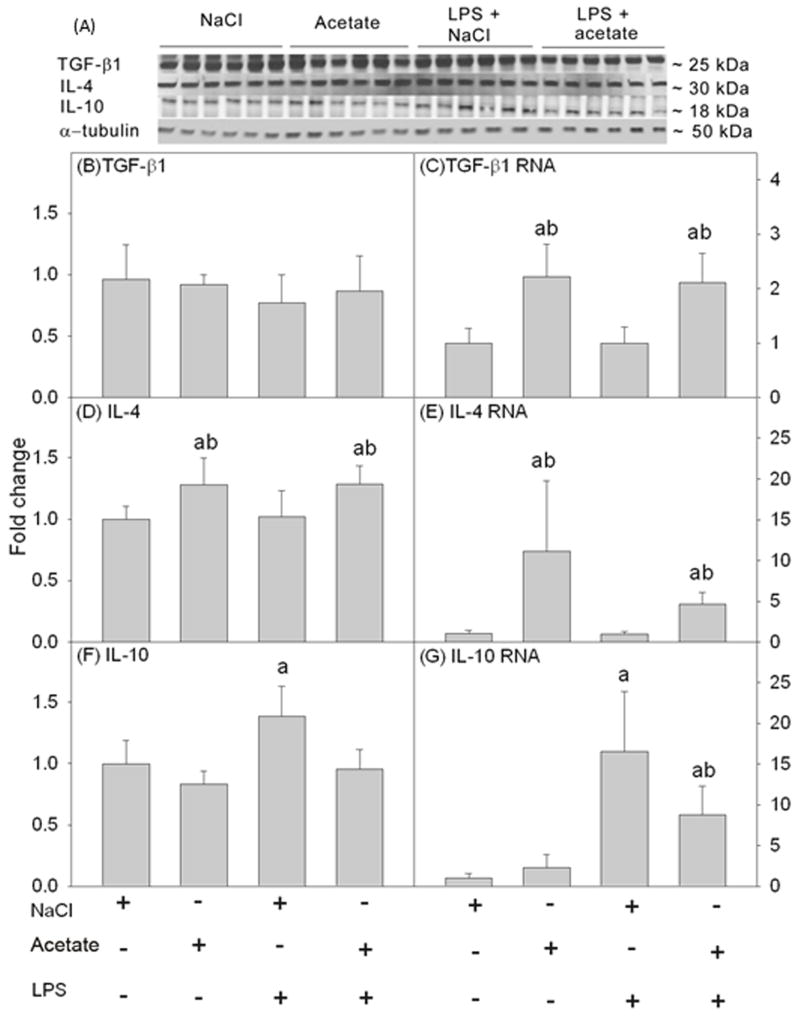
Acetate treatment increases the expression of anti-inflammatory cytokines in BV-2 microglia
Anti-inflammatory cytokines are an integral part of the inflammatory response to minimize the potential of the pro-inflammatory cytokines to produce neuronal damage. We determined the effect of acetate treatment on expression levels of the anti-inflammatory cytokine proteins TGF-β1, IL-4, and IL-10 (Figure 6A). Acetate treatment did not alter the protein levels of TGF-β1 or IL-10 (Figures 6B and F), however IL-4 was increased by 1.3-fold (Figure 6D). In parallel, we found that acetate treatment increased TGF-β1 mRNA by 2-fold (Figure 6C) and IL-4 mRNA by 11- and 4-fold, depending on the group (Figure 6E). Conversely, LPS increased IL-10 protein and mRNA by 1.4- and 16-fold, respectively. Acetate treatment returned IL-10 protein to control levels and attenuated IL-10 mRNA by 8-fold (Figures 6F and G). The possible reasons why increases in mRNA levels are not paralleled by increased protein levels may involve mRNA stability or reflect the short treatment duration. Regardless, these data suggest that acetate treatment modulates pro- and anti-inflammatory cytokine release in BV-2 microglia towards a more anti-inflammatory state.
Figure 6.
Changes in the anti-inflammatory cytokines TGF-β1, IL-4, and IL-10 in BV-2 microglial cell culture stimulated for 4 hr with LPS 6.25 ng/ml with and without treatment with 12 mM sodium acetate. Panel A shows representative images of the Western blots. Panels B, D and F show the optical densities of the anti-inflammatory cytokines TGF-β1, IL-4, and IL-10, respectively, normalized to the loading control α-tubulin (n = 6). Panels C, E and G show the changes in the mRNA levels of the anti-inflammatory cytokines TGF-β1, IL-4, and IL-10, quantified by qrt-PCR and normalized to β-actin (n = 6). Bars represent means ± SD where statistical significance (a = compared to NaCl, and b = compared to LPS + NaCl) was set at p ≤ 0.05, as determined by a one way ANOVA followed by Tukey’s post-hoc test.
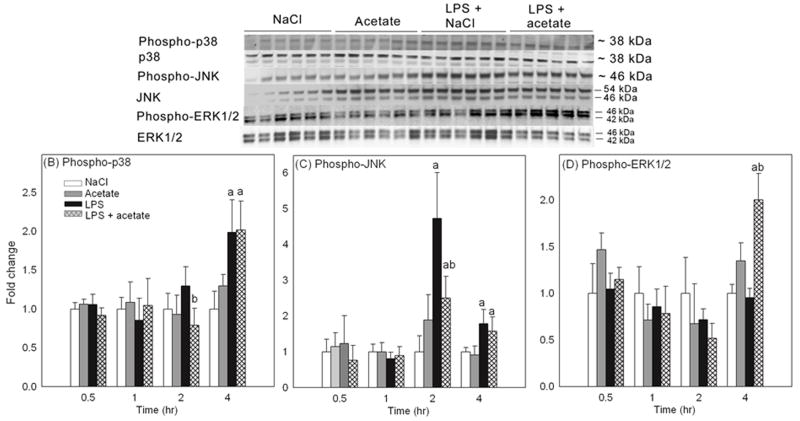
Acetate treatment and LPS alter MAPK phosphorylation in a time-dependent manner in BV-2 microglia
Because MAPK signaling can be inhibited by the acetylation of MAPK phosphatase-1 which induces deacetylation and deactivation of MAPK (Cao et al. 2008), we measured the effects of acetate treatment on LPS-induced MAPK phosphorylation at 0.5, 1, 2, and 4 hr. The rationale for including multiple time points is that other studies reported MAPK activation by LPS at much earlier time points than 4 hr (Schumann et al. 1996, Kraatz et al. 1998). Whole cell lysates were used for Western blot analysis, and phosphorylated p38, p38, phosphorylated JNK, JNK, phosphorylated ERK1/2 and ERK1/2 were detected as protein bands corresponding to the molecular weights of 38, 38, 46, 54 and 46, and 42 and 46 kDa, respectively (Figure 7A). At 0.5 and 1 hr, neither LPS nor acetate treatment had an effect on the levels of phosphorylated MAPK (Figures 7B-D). At 2 hr, acetate treatment reduced the level of phosphorylated p38 as compared to LPS, and LPS increased JNK phosphorylation by 5-fold, which was attenuated 2.5-fold with acetate treatment (Figures 7B and C). At 4 hr, LPS increased phosphorylated p38 and phosphorylated JNK by 2-fold and was not altered upon acetate treatment; however treatment did increase the level of phosphorylated ERK1/2 by 2-fold only in the presence of LPS (Figures 7B–D).
Figure 7.
Changes in the phosphorylation state of MAPK p38, JNK and ERK1/2 in BV-2 microglia stimulated for 0.5, 1, 2 and 4 hr with LPS 6.25 ng/ml with and without 12 mM sodium acetate. Panel A shows representative images of the Western blots from the 4 hr experiment. Panels B, C and D show the optical densities of phosphorylated MAPK p38, JNK and ERK1/2 normalized to the loading controls MAPK p38, JNK and ERK1/2, respectively (n = 6). The data represent the means ± SD where statistical significance (* = compared to NaCl) was set at p ≤ 0.05, as determined by One Way ANOVA followed by Tukey’s post-hoc test.
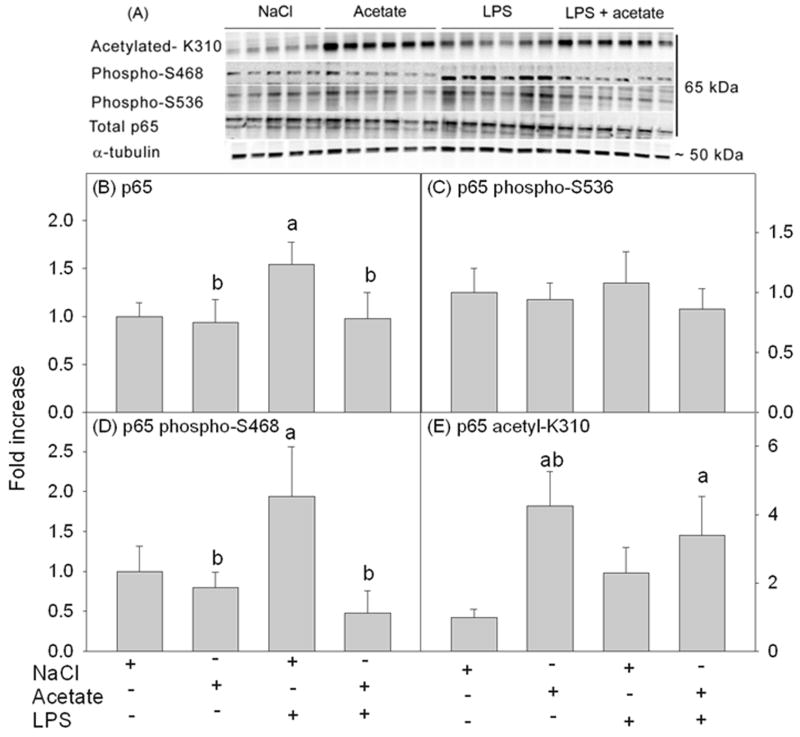
Acetate treatment alters LPS-induced increases in NF-κB p65 protein levels and phosphorylation at serine 468 in BV-2 microglia
Because NF-κB signaling is altered by acetylation of p65 (Kiernan et al. 2003, Chen et al. 2001, Huang et al. 2010) and has a prominent role in the regulation of inflammatory and immune responses, we tested the effect of acetate treatment on LPS-induced changes in p65 protein levels, phosphorylation and acetylation after 4 hr of treatment. Whole cell lysates were used for Western blot analysis, and total p65, phosphorylated p65 at serine 536, phosphorylated p65 at serine 468, and acetylated p65 at lysine 310 were detected as protein bands corresponding to the molecular weight of 65 kDa (Figure 8A). LPS increased the total protein level of p65 by 1.5-fold which returned to control levels with acetate treatment (Figure 8B). While neither acetate treatment nor LPS altered the level of phosphorylated p65 at serine 536, LPS did increase the levels of phosphorylated p65 at serine 468 by 2-fold which was reduced to control levels with acetate treatment (Figures 8C and D). In addition, acetate treatment induced p65 hyperacetylation at lysine 310 by 3.5-fold (Figure 8E). These data suggest that acetate metabolism alters the LPS-induced p65 response in microglia, and that the anti-inflammatory effect of acetate treatment can potentially be attributed to acetylation of non-histone targets.
Figure 8.
Changes in the protein level, phosphorylation and acetylation states of NF-κB p65 in BV-2 microglia cell culture stimulated for 4 hr with LPS 6.25 ng/ml with and without 12 mM sodium acetate. Panel A shows representative images of the Western blots. Panel B shows the optical density of total NF-κB p65 normalized to the loading control α-tubulin. Panels C, D and E show the optical densities of phosphorylated p65 at S536, phosphorylated p65 at serine 468 and acetylated p65 at lysine 310 normalized to total p65, respectively (n = 6). The data represent the means ± SD where statistical significance (a = compared to NaCl and b = compared to LPS) was set at p ≤ 0.05, as determined by One Way ANOVA followed by Tukey’s post-hoc test. Abbreviations are: S536, serine 536; S468; serine 468: and K310, lysine 310.
Discussion
In this study, we demonstrated that acetate treatment reversed the LPS-induced reduction in H3K9 acetylation and decreases pro-inflammatory cytokines in microglia in vitro. Moreover, acetate treatment increased the transcription of the anti-inflammatory cytokines TGF-β1 and IL-4, suggesting that acetate-induced histone modulation may influence more strongly the expression of anti-inflammatory cytokines in this model considering histone hyperacetylation is conventionally linked to increased gene expression. We also demonstrated the time-dependent effects of LPS and acetate treatment on MAPK activation. In addition, acetate treatment reduced LPS-induced increases in total NF-κB p65 protein level, serine 468 phosphorylation, and increased its acetylation at lysine 310. These data suggest that acetate metabolism can modulate cytokine balance in microglia, which correlates to increases in both histone and non-histone protein acetylation.
The differential effect of acetate treatment on mRNA and protein levels suggests that the reduction in pro-inflammatory cytokines may be due to a disruption in mRNA translation rather than gene transcription or pro-inflammatory cytokine turnover. Translation involves the interaction of mRNA with various subsets of proteins which, we speculate, may be regulated by acetylation. For example, nuclear mRNA binds to nuclear proteins that transport mRNA to the cytosol. Some of these proteins repress translation by interfering with the binding of mRNA to ribosomal subunits (Wells 2006). Similarly, the integrity of mRNA is modulated by mRNA stabilizing proteins (Kohn et al. 1996). It is possible that acetylation may alter the expression and/or activity of mRNA-binding and/or stabilizing proteins. Of particular interest is cytosolic polyadenylation element-binding protein (CPEB) expressed both in neuroglia and neurons which prevents the formation of the translation initiation complex and represses translation (Theis et al. 2003, Mendez & Richter 2001). CPEB is regulated by phosphorylation (Atkins et al. 2004) however the effect that acetylation has on its activity remains unknown. Further, the eukaryotic initiation factor 5A (eIF5A), which regulates initiation and elongation, contains a polyamine-lysine conjugated amino acid “hypusine” that is essential to its activity (Zanelli et al. 2006, Gregio et al. 2009, Saini et al. 2009) and is inactivated following acetylation by spermidine/spermine acetyltransferase 1 (Lee et al. 2011). In addition, acetylation by a histone acetyltransferase PCAF leads to eIF5A accumulation in the nucleus that prevents translocation to the cytosol and in turn disrupts translation (Ishfaq et al. 2012). All of which suggests that acetylation may be involved in the regulation of mRNA translation.
Acetate treatment may also reduce pro-inflammatory cytokine levels but not mRNA by increasing protein turnover. A number of histone acetyltransferases possess intrinsic ubiquitin conjugating activity and are associated with ubiquitin transferases in multiprotein complexes that stimulate degradation (Sadoul et al. 2008). Further, acetylation of the translation elongation factor (E2F1) (Galbiati et al. 2005) and the hypoxia-inducible factor 1α (HIF-1) at lysine 532 enhances their ubiquitination and degradation (Jeong et al. 2002). Thus it is plausible that non-histone protein acetylation may alter mRNA translation and the turnover of pro-inflammatory cytokines in activated microglia.
An increase in pro-inflammatory cytokine production is generally considered deleterious based on their involvement in a wide number of neurological and non-neurological disorders. As an example, co-culture of primary rat cortical neurons with LPS-activated microglia results in neuronal death which can be largely blocked using the naturally occurring IL-1 receptor antagonist IL-1ra (Li et al. 2003). Not surprisingly, suppression of pro-inflammatory cytokines is associated with improved behavioral and cognitive endpoints in animal models of neurodegenerative diseases (Hu et al. 2007, Lloyd et al. 2008). On the other hand, IL-4, IL-10, and TGF-β1 share features of anti-inflammatory and neuroprotective actions that can be attributed to downregulating glial production of pro-inflammatory cytokines and/or attenuating their secondary release. IL-4 reduces the production of inflammatory mediators, including inducible nitric oxide (NO) synthase, TNF-α, IL-1β, cyclooxygenase 2, and macrophage chemoattractant protein-1 by activated microglia in vivo and in vitro (Ledeboer et al. 2000, Furlan et al. 2000). In addition, TGF-β has a neuroprotective effect by regulating Bad (pro-apoptotic) and Bcl-2 and Bcl-x1 (anti-apoptotic) proteins (Dhandapani & Brann 2003). Further, anti-inflammatory cytokines reduce the expression levels of the pro-inflammatory cytokines in LPS-stimulated microglial-astroglial co-cultures (Ledeboer et al. 2000). Endogenous and exogenous TGF-β1 and β2 suppress the production of NO but not IL-1β, IL-6 or TNF-α and exogenous IL-4 downregulates NO, IL-6 and TNF-α, but not IL-1β (Ledeboer et al. 2000). Our findings showing that LPS stimulation upregulated IL-10 is not counterintuitive, because stimulation of an inflammatory response can lead to upregulation of both conventional pro-inflammatory and anti-inflammatory mediators as a biological self-checking mechanism. In this regard, IL-10 inhibits the LPS-induced increase of IL-1β and TNF-α (Sawada et al. 1999) and IL-10 release by LPS-stimulated microglia increases simultaneously with TNF-α (Seo et al. 2004). The multiplicity of receptors, signaling cascades, cellular and subcellular targets, and various experimental designs all demonstrate the complexity of how anti-inflammatory cytokines can regulate the transcription and/or translation of the pro-inflammatory cytokines.
Lysine acetylation is a common post-translational modification that occurs on both histones as well as non-histone proteins. Histone acetylation is conventionally linked to an increase in gene expression. Non-histone targets of acetylation include cytoskeletal proteins and transcription and nuclear import factors. Acetylation of these targets have many functional consequences including altering subcellular localization, DNA-binding, transcriptional activity, protein-protein interaction and protein stability (Sadoul et al. 2008, Glozak et al. 2005). MAPK signaling is inducible by pro-inflammatory cytokines and also regulates their transcription and translation. For example, MAPK signaling regulates the production of IL-8 in response to IL-1 and osmotic shock (Shapiro & Dinarello 1995), and regulates the production of IL-6 in response to TNF-α (Beyaert et al. 1996). Animals with genetic deletion of one of the MAPK accessory proteins show diminished IL-6 and TNF-α production in response to LPS stimulation (Kotlyarov et al. 1999). Because a MAPK phosphatase is activated by acetylation which inhibits MAPK signaling, we studied whether acetate treatment alters MAPK phosphorylation (activation) at different time points. We found that the effect of LPS on MAPK phosphorylation was time-dependent, as was the ability of acetate treatment to reduce LPS-induced p38 and JNK phosphorylation. LPS increased phosphorylated p38 at 4 hr and phosphorylated JNK at 2 and 4 hr, whereas acetate treatment reduced phosphorylated p38 and JNK only at 2, but not 4, hr. We did not observe an increase in MAPK activation at 0.5 or 1 hr unlike other studies (Schumann et al. 1996, Kraatz et al. 1998). However, this may be due to our using a lower concentration of LPS or may demonstrate a cell-type specific response. While the therapeutic effect of acetate supplementation is demonstrated in the in vivo studies (Reisenauer et al. 2011) these results further strengthen our understanding of the possible therapeutic mechanism(s) involved in modulating cytokine expression by increasing acetate metabolism. Therefore, because the effect of acetate treatment on the LPS-induced MAPK p38 phosphorylation is transient, the effect of acetate treatment on cytokine release may be due to the convergence of multiple pro- and anti-inflammatory signaling mechanisms.
NF-κB is acetylated on p65 which modulates nuclear translocation, DNA binding, and transcriptional activity (Chen et al. 2001, Chen et al. 2002, Huang et al. 2010). In this study, we found that acetate treatment induced p65 hyperacetylation at lysine 310. This is of interest because p65 interacts with histone deacetylases (HDAC) 1, 2 and 3, but only HDAC3 deacetylates p65 (Kiernan et al. 2003, Chen et al. 2001) which is downregulated with acetate supplementation (Soliman et al. 2012). Therefore, the effect that acetate metabolism has on HDAC3 expression may help to explain the hyperacetylation of p65 at lysine 310 observed in this study. The acetylation of p65 may be associated with anti-inflammatory outcomes as it represses transcriptional activity, reduces binding to κB-DNA, and facilitates its interaction with IκB that increases p65 export to the cytoplasm. Because acetylated p65 accumulates in the cytoplasm suggests that post-activation turn-off of NF-κB-dependent transcription is regulated, at least in part, by acetylation (Kiernan et al. 2003). However, β-amyloid toxicity increases hyperacetylated p65 at lysine 310 in microglia, which is reversed by SIRT1 over-expression and stimulation (Chen et al. 2005). This suggests that changes in the activity and expression of the of the sirtuins and class I HDAC can differentially modulate NF-κB-mediated inflammatory phenotype, possibly as a result of differing inflammatory stimulation or differing intercellular regulation points. Alternately, acetate treatment-induced p65 hyperacetylation in the presence of LPS may be linked to pro-inflammatory signaling that is generally outweighed by the other anti-inflammatory mechanisms. Regardless, the functional consequences of post-translational modification of p65 are diverse and specific to the modification and the residue involved (Huang et al. 2010). Future studies are necessary to determine the impact that acetylation of p65 has on NF-κB functionality in this model.
Since histone acetylation is conventionally associated with enhanced gene expression (Strahl & Allis 2000), we speculate that the increase in H3K9 acetylation may be instrumental in upregulating the transcription of anti-inflammatory cytokines, as found in this study. We chose to focus on H3K9 due to the effect that neuroinflammation and acetate supplementation have on its acetylation-state as opposed to H4K8 and H4K16 which are hyperacetylated during acetate supplementation but not altered by neuroinflammation (Soliman et al. 2012). This is further supported by other reports implicating H3K9 hypoacetylation in neuroinflammation, and microglial activation (Zhang et al. 2008, Silva et al. 2012, Govindarajan et al. 2011). Our data also demonstrate a correlation between acetate treatment-induced inhibition of pro-inflammatory cytokine release and hyperacetylation of H3K9 and p65 at lysine 310. H3K9 can also be modified by methylation where methylated H3K9 is associated with gene repression, contrary to acetylated H3K9 that is associated with active gene expression (Rice & Allis 2001). In this regard, the enrichment of methylated H3K9 at the promoter region of opioid receptors is linked to decreased opioid receptor transcription in mice fed a high fat diet (Vucetic et al. 2011). Similarly, genome-wide mapping demonstrates that an increase H3K9 acetylation corresponds with areas of transcription activity (Shin et al. 2012). H3 methylation is more predominant in areas of enriched acetylated H4, unlike methylated H4 which is more evident in less acetylated chromatin regions (Annunziato et al. 1995). Thus it is possible that H3K9 hyperacetylation may alter the expression and/or activity of effector proteins involved in translation, which may help to explain the decrease in pro-inflammatory cytokines in the absence of a reduction in their mRNA levels.
In conclusion, we describe microglial specific responses to acetate treatment, where modulation of cytokine balance is attributable to a reduction in pro-inflammatory cytokine levels and induction of anti-inflammatory cytokine transcription. All of which correspond to a reversal of LPS-induced changes in the acetylation of histone and non-histone proteins with acetate treatment. To better understand the contribution that histone versus non-histone acetylation has in the control of cytokine balance, it will be necessary to determine the differential impact that an increase in histone acetylation has on pro- and anti-inflammatory cytokine transcription.
Acknowledgments
This publication was made possible by Grant Number 5P20RR017699 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). We thank Drs. Othman Ghribi and Joyce Ohm for their technical support and generous use of their equipment.
Defining Abbreviations
- LPS
lipopolysaccharide
- IL-1β
Interleukin- 1beta
- IL-6
interleukin-6
- H3K9
histone H3 at lysine 9
- TNF-α
tumor necrosis factor-alpha
- TGF-β1
transforming growth factor-beta1
- IL-4
interleukin-4
- IL-10
interleukin-10
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- ERK
extracellular signal-regulated kinase
- NF-κB
nuclear factor-kappa B
- Acetyl-CoA
acetyl coenzyme A
- ACS
acetyl-CoA synthetase
- IL-8
interleukin-8
- NO
nitric oxide
- CPEB
cytoplasmic polyadenylation element binding protein
- HDAC
histone deacetylase
Footnotes
The authors declare no conflict of interests.
References
- Adcock IM. HDAC inhibitors as anti-inflammatory agents. Br J Pharmacol. 2007;150:829–831. doi: 10.1038/sj.bjp.0707166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato AT, Eason MB, Perry CA. Relationship between methylation and acetylation of arginine-rich histones in cycling and arrested HeLa cells. Biochemistry. 1995;34:2916–2924. doi: 10.1021/bi00009a023. [DOI] [PubMed] [Google Scholar]
- Ariyannur PS, Moffett JR, Madhavarao CN, Arun P, Vishnu N, Jacobowitz DM, Hallows WC, Denu JM, Namboodiri AM. Nuclear-cytoplasmic localization of acetyl coenzyme a synthetase-1 in the rat brain. J Comp Neurol. 2010;518:2952–2977. doi: 10.1002/cne.22373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun P, Ariyannur PS, Moffett JR, Xing G, Hamilton K, Grunberg NE, Ives JA, Namboodiri AM. Metabolic acetate therapy for the treatment of traumatic brain injury. J Neurotrauma. 2010a;27:293–298. doi: 10.1089/neu.2009.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun P, Madhavarao CN, Moffett JR, et al. Metabolic acetate therapy improves phenotype in the tremor rat model of Canavan disease. J Inherit Metab Dis. 2010b;33:195–210. doi: 10.1007/s10545-010-9100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Nozaki N, Shigeri Y, Soderling TR. Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2004;24:5193–5201. doi: 10.1523/JNEUROSCI.0854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. Embo J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Bocchini V, Mazzolla R, Barluzzi R, Blasi E, Sick P, Kettenmann H. An immortalized cell line expresses properties of activated microglial cells. J Neurosci Res. 1992;31:616–621. doi: 10.1002/jnr.490310405. [DOI] [PubMed] [Google Scholar]
- Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views. Oncogene. 1999;18:6845–6852. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. Embo J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010;24:708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003;39:13–22. doi: 10.1385/CBB:39:1:13. [DOI] [PubMed] [Google Scholar]
- Dhawan G, Floden AM, Combs CK. Amyloid-beta oligomers stimulate microglia through a tyrosine kinase dependent mechanism. Neurobiol Aging. 2012;33:2247–2261. doi: 10.1016/j.neurobiolaging.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Kondo J, Ishikawa M, Morikawa K, Yamamoto TT. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J Biol Chem. 2001;276:11420–11426. doi: 10.1074/jbc.M008782200. [DOI] [PubMed] [Google Scholar]
- Furlan R, Bergami A, Lang R, Brambilla E, Franciotta D, Martinelli V, Comi G, Panina P, Martino G. Interferon-beta treatment in multiple sclerosis patients decreases the number of circulating T cells producing interferon-gamma and interleukin-4. J Neuroimmunol. 2000;111:86–92. doi: 10.1016/s0165-5728(00)00377-5. [DOI] [PubMed] [Google Scholar]
- Galbiati L, Mendoza-Maldonado R, Gutierrez MI, Giacca M. Regulation of E2F-1 after DNA damage by p300-mediated acetylation and ubiquitination. Cell Cycle. 2005;4:930–939. doi: 10.4161/cc.4.7.1784. [DOI] [PubMed] [Google Scholar]
- Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26:187–197. doi: 10.3233/JAD-2011-110080. [DOI] [PubMed] [Google Scholar]
- Gregio AP, Cano VP, Avaca JS, Valentini SR, Zanelli CF. eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun. 2009;380:785–790. doi: 10.1016/j.bbrc.2009.01.148. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Helmy A, De Simoni MG, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol. 2011;95:352–372. doi: 10.1016/j.pneurobio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- Hu W, Ralay Ranaivo H, Roy SM, Behanna HA, Wing LK, Munoz L, Guo L, Van Eldik LJ, Watterson DM. Development of a novel therapeutic suppressor of brain proinflammatory cytokine up-regulation that attenuates synaptic dysfunction and behavioral deficits. Bioorg Med Chem Lett. 2007;17:414–418. doi: 10.1016/j.bmcl.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NF-kappaB: another layer of regulation for NF-kappaB signaling pathway. Cell Signal. 2010;22:1282–1290. doi: 10.1016/j.cellsig.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishfaq M, Maeta K, Maeda S, Natsume T, Ito A, Yoshida M. Acetylation regulates subcellular localization of eukaryotic translation initiation factor 5A (eIF5A) FEBS Lett. 2012 doi: 10.1016/j.febslet.2012.06.042. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- Johnston H, Boutin H, Allan SM. Assessing the contribution of inflammation in models of Alzheimer’s disease. Biochem Soc Trans. 2011;39:886–890. doi: 10.1042/BST0390886. [DOI] [PubMed] [Google Scholar]
- Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- Kohn DT, Tsai KC, Cansino VV, Neve RL, Perrone-Bizzozero NI. Role of highly conserved pyrimidine-rich sequences in the 3′ untranslated region of the GAP-43 mRNA in mRNA stability and RNA-protein interactions. Brain Res Mol Brain Res. 1996;36:240–250. doi: 10.1016/0169-328x(95)00239-o. [DOI] [PubMed] [Google Scholar]
- Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- Kraatz J, Clair L, Bellingham J, Wahlstrom K, Rodriguez JL, West MA. Lipopolysaccharide pretreatment produces macrophage endotoxin tolerance via a serum-independent pathway. J Trauma. 1998;45:684–691. doi: 10.1097/00005373-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Breve JJ, Poole S, Tilders FJ, Van Dam AM. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30:134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lee SB, Park JH, Folk JE, Deck JA, Pegg AE, Sokabe M, Fraser CS, Park MH. Inactivation of eukaryotic initiation factor 5A (eIF5A) by specific acetylation of its hypusine residue by spermidine/spermine acetyltransferase 1 (SSAT1) Biochem J. 2011;433:205–213. doi: 10.1042/BJ20101322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd E, Somera-Molina K, Van Eldik LJ, Watterson DM, Wainwright MS. Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J Neuroinflammation. 2008;5:28. doi: 10.1186/1742-2094-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Arun P, Madhavarao CN, Moffett JR, Namboodiri MA. Progress toward acetate supplementation therapy for Canavan disease: glyceryl triacetate administration increases acetate, but not N-acetylaspartate, levels in brain. J Pharmacol Exp Ther. 2005;315:297–303. doi: 10.1124/jpet.105.087536. [DOI] [PubMed] [Google Scholar]
- Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Merson TD, Binder MD, Kilpatrick TJ. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. Neuromolecular Med. 2010;12:99–132. doi: 10.1007/s12017-010-8112-z. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Felzien LK, Betts JC, Leung K, Beach DH, Nabel GJ. Regulation of NF-kappaB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Akama KT, Van Eldik LJ. Selective modulation of BV-2 microglial activation by prostaglandin E(2). Differential effects on endotoxin-stimulated cytokine induction. J Biol Chem. 1999;274:28823–28827. doi: 10.1074/jbc.274.40.28823. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Reisenauer CJ, Bhatt DP, Mitteness DJ, Slanczka ER, Gienger HM, Watt JA, Rosenberger TA. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J Neurochem. 2011;117:264–274. doi: 10.1111/j.1471-4159.2011.07198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Current opinion in cell biology. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Rojanathammanee L, Murphy EJ, Combs CK. Expression of mutant alpha-synuclein modulates microglial phenotype in vitro. J Neuroinflammation. 2011;8:44. doi: 10.1186/1742-2094-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie. 2008;90:306–312. doi: 10.1016/j.biochi.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem. 1999;72:1466–1471. doi: 10.1046/j.1471-4159.1999.721466.x. [DOI] [PubMed] [Google Scholar]
- Schumann RR, Pfeil D, Lamping N, Kirschning C, Scherzinger G, Schlag P, Karawajew L, Herrmann F. Lipopolysaccharide induces the rapid tyrosine phosphorylation of the mitogen-activated protein kinases erk-1 and p38 in cultured human vascular endothelial cells requiring the presence of soluble CD14. Blood. 1996;87:2805–2814. [PubMed] [Google Scholar]
- Seo DR, Kim KY, Lee YB. Interleukin-10 expression in lipopolysaccharide-activated microglia is mediated by extracellular ATP in an autocrine fashion. Neuroreport. 2004;15:1157–1161. doi: 10.1097/00001756-200405190-00015. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci U S A. 1995;92:12230–12234. doi: 10.1073/pnas.92.26.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Li RW, Gao Y, Baldwin Rt, Li CJ. Genome-wide ChIP-seq mapping and analysis reveal butyrate-induced acetylation of H3K9 and H3K27 correlated with transcription activity in bovine cells. Functional & integrative genomics. 2012;12:119–130. doi: 10.1007/s10142-012-0263-6. [DOI] [PubMed] [Google Scholar]
- Silva PF, Garcia VA, da Dornelles SA, et al. Memory impairment induced by brain iron overload is accompanied by reduced H3K9 acetylation and ameliorated by sodium butyrate. Neuroscience. 2012;200:42–49. doi: 10.1016/j.neuroscience.2011.10.038. [DOI] [PubMed] [Google Scholar]
- Soliman ML, Rosenberger TA. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem. 2011;352:173–180. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- Soliman ML, Smith MD, Houdek HM, Rosenberger TA. Acetate supplementation modulates brain histone acetylation and decreases interleukin-1beta expression in a rat model of neuroinflammation. J Neuroinflammation. 2012;9:51. doi: 10.1186/1742-2094-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- Theis M, Si K, Kandel ER. Two previously undescribed members of the mouse CPEB family of genes and their inducible expression in the principal cell layers of the hippocampus. Proc Natl Acad Sci U S A. 2003;100:9602–9607. doi: 10.1073/pnas.1133424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovic L, Maeda S, Sternberg E. Anti-inflammatory cytokines: expression and action in the brain. Neuroimmunomodulation. 2001;9:295–312. doi: 10.1159/000059387. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Reyes TM. Chronic high-fat diet drives postnatal epigenetic regulation of mu-opioid receptor in the brain. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:1199–1206. doi: 10.1038/npp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DG. RNA-binding proteins: a lesson in repression. J Neurosci. 2006;26:7135–7138. doi: 10.1523/JNEUROSCI.1795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MS, Jang PG, Park JS, Kim WK, Joh TH, Kim HS. Selective modulation of lipopolysaccharide-stimulated cytokine expression and mitogen-activated protein kinase pathways by dibutyryl-cAMP in BV2 microglial cells. Brain Res Mol Brain Res. 2003;113:86–96. doi: 10.1016/s0169-328x(03)00095-0. [DOI] [PubMed] [Google Scholar]
- Zanelli CF, Maragno AL, Gregio AP, Komili S, Pandolfi JR, Mestriner CA, Lustri WR, Valentini SR. eIF5A binds to translational machinery components and affects translation in yeast. Biochem Biophys Res Commun. 2006;348:1358–1366. doi: 10.1016/j.bbrc.2006.07.195. [DOI] [PubMed] [Google Scholar]
- Zhang B, West EJ, Van KC, Gurkoff GG, Zhou J, Zhang XM, Kozikowski AP, Lyeth BG. HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]