Abstract
Background
There is debate as to whether chronic irritability (operationalized as severe mood dysregulation, SMD) is a developmental form of bipolar disorder (BD). Although structural brain abnormalities in bipolar disorder (BD) have been demonstrated, no study compares neuroanatomy among SMD, BD, and healthy volunteers (HV) either cross-sectionally or over time. Furthermore, the developmental trajectories of structural abnormalities in BD or SMD are unknown. This study provides such data in BD, SMD, and HV.
Methods
An optimized, modulated voxel-based morphometry (VBM) analysis was conducted on structural MRI scans from 201 children (78 SMD, 55 BD, and 68 HV). Additionally, 92 children (31 SMD, 34 BD, and 27 HV) were re-scanned after two years (mean interval 1.99 ± 0.94 years), to compare time-related changes among the three groups.
Results
Cross-sectionally, the groups differed in gray matter (GM) volume in pre-supplementary motor area (pre-SMA), dorsolateral prefrontal cortex (DLPFC), insula, and globus pallidus. The cortical differences were driven mainly by increased GM volume in HV compared to BD and SMD. In globus pallidus, there was increased GM in BD compared to HV and SMD. Longitudinally, group-by-time interactions were evident in two clusters in the superior/inferior parietal lobule (R SPL/IPL) and in the precuneus. In both clusters, the interactions were driven by an abnormal increase in volume in BD.
Conclusions
Cross-sectionally, both BD and SMD are associated with structural abnormalities in frontal cortex, insula, and basal ganglia. While some of these deficits overlap (insula and DLPFC), others differentiate SMD and BD (pre-SMA and globus pallidus). Abnormal developmental trajectories in lateral parietal cortex and precuneus are present in, and unique to, BD. Because of the high proportion of co-occurring ADHD in the SMD subjects, we could not separate effects of ADHD from those of SMD, and future research including a non-irritable ADHD group must address this issue.
It remains unclear whether BD presents in youth, as in adults, with only episodic mood symptoms or if chronic, severe non-episodic irritability is a developmental phenotype of BD. To study this question, Leibenluft et al. defined a new syndrome, severe mood dysregulation (SMD), to codify youth with chronic, non-episodic irritability. Although outcome and family history data (Brotman et al., 2007; Stringaris et al., 2010) differentiate SMD from `classic,' episodic BD, the underlying pathophysiology of these disorders is unclear. Functional magnetic resonance imaging (fMRI) studies comparing children with SMD and BD suggest that these two groups have both overlapping and distinct neural dysfunction (Adleman et al., 2011; Brotman et al., 2010; Deveney et al., 2012). However, no study compares neuroanatomy in children with SMD to those with BD and healthy volunteers (HV).
In pediatric BD, research demonstrates structural abnormalities in many brain regions. Most studies report decreased cortical gray matter (GM) in BD vs. HV in a range of association cortex areas (Dickstein et al., 2005; Frazier et al., 2005; James et al., 2011; Wilke, Kowatch, DelBello, Mills, & Holland, 2004), and increased basal ganglia volumes in pediatric BD (DelBello, Zimmerman, Mills, Getz, & Strakowski, 2004; Liu et al., 2011; Wilke, et al., 2004). A recent meta-analysis in children and adults with BD reported that decreased prefrontal cortex and increased globus pallidus volumes were two of the most consistent findings (Arnone et al., 2009).
Although there have been no structural MRI (sMRI) studies of SMD, the syndrome combines hyperarousal symptoms seen in attention-deficit/hyperactivity disorder (ADHD) with severe irritability (Leibenluft, 2011). In the ADHD neuroanatomical literature, the most replicated abnormality is decreased basal ganglia GM (Nakao, Radua, Rubia, & Mataix-Cols, 2011). However, since data suggest that SMD and non-irritable ADHD differ in neural activity (Brotman, et al., 2010), ADHD may differ from SMD neuroanatomically, and a study of children with SMD is warranted.
While an emerging cross-sectional literature demonstrates structural brain abnormalities in adult and pediatric BD (Arnone, et al., 2009), the developmental trajectory of these abnormalities is unclear, and the trajectory is completely unstudied in SMD. Most previous longitudinal sMRI studies in pediatric BD include small samples, averaging around 10 patients (Blumberg et al., 2005; Gogtay et al., 2007; Kalmar et al., 2009). The most recent, and largest, study combined 58 late-adolescent and adult patients and reported greater GM volume increases over time in BD vs. HV in several regions, including superior temporal gyrus, basal ganglia, and medial temporal structures (Lisy et al., 2011).
Given the limited literature in pediatric BD and the absence of research in SMD, we conducted a study comparing brain volume both cross-sectionally and longitudinally over two years in SMD, BD and HV. As previous fMRI and behavioral findings suggest that BD and SMD have both overlapping and distinct dysfunction, we hypothesized that we would find regions in which both SMD and BD differed from HV, and regions where the two patient groups differed from each other as well as HV. We expected group differences in association cortices and in basal ganglia, as such abnormalities have been reported in both BD and ADHD. However, given the lack of structural studies in SMD, we did not have specific hypotheses about the directionality of these findings. To explore both whole-brain and regional effects, as well as between- and within-group effects, we employed optimized voxel-based morphometry (VBM), a semi-automated method used to measure volume differences.
Methods
Subjects
Data from 201 subjects (78 SMD, 55 BD, 68 HV) were included. All research was approved by the NIH Institutional Review Board, and informed consent (parent/guardian) and assent (minor) were acquired before participation. Full recruitment and diagnostic methods are described previously (Brotman, et al., 2010). None of the data in this study have been published previously.
BD subjects met full DSM-IV criteria for BD, representing the BD “narrow phenotype” (Leibenluft, Charney, Towbin, Bhangoo, & Pine, 2003). SMD inclusion criteria were: (1) abnormal mood (anger or sadness), present at least half the day most days; (2) hyperarousal (at least three of: insomnia, agitation, distractibility, racing thoughts or flight of ideas, pressured speech, intrusiveness); (3) markedly increased reactivity to negative emotional stimuli manifest verbally or behaviorally at least three times a week; and (4) severe impairment in at least one setting (home, school or peers) and at least mild impairment in a second setting. To qualify for SMD, symptom onset must be before age 12 and symptoms must be currently present for at least 12 months without symptom-free periods >2 months (Leibenluft, et al., 2003). HV inclusion criteria were: negative psychiatric history for Axis I disorders in the proband and for mood disorders in first-degree relatives; normal physical and neurologic examinations; and lack of current, regular medication use.
IQ>70, as ascertained by the Wechsler Abbreviated Scale of Intelligence (WASI), was required for all subjects. Within 48 hours of scanning, BD and SMD subjects were rated by clinicians using the Childhood Global Assessment Scale (CGAS) assessing the past six months and the Childhood Depression Rating Scale Revised (CDRS-R). Clinicians also administered the Young Mania Rating Scale (YMRS) to BD patients. Subjects were contacted every 6 months to record mood episodes and medication changes.
All 201 subjects were included in the cross-sectional analysis. A subset of 92 subjects (31 SMD, 34 BD, and 27 HV) were scanned twice, an average of 1.99 ± 0.94 years apart. (See Tables 1 and 2, and Supplemental tables S1 and S2 for detailed demographic information).
Table 1.
| Cross-sectional Subjects | Healthy Volunteers (n=68) | Bipolar Disorder (n=55) | Severe Mood Dysregulation (n=78) |
|---|---|---|---|
|
| |||
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Sex (male) | 36 (52.9%) | 30 (54.5%) | 53 (67.9%) |
| Age (y)*** | 13.9 ± 2.3 | 14.2 ± 2.6 | 12.7 ± 2.4 |
| IQ† | 111 ± 14 | 110 ± 15 | 107 ± 14 |
| YMRS† | --- | 9.31 ± 7.3 | --- |
| CDRS† | --- | 28.6 ± 8.5 | 27.9 ± 6.7 |
| CGAS† | --- | 49.7 ± 12.0 | 46.8 ± 7.4 |
| TBV^ | 1142 ± 82 | 1103 ± 102 | 1123 ± 101 |
Note: YMRS = Young Mania Rating Scale; CDRS = Childhood Depression Rating Scale; CGAS = Childhood Global Assessment Scale; TBV = total brain volume.
p ≤ 0.001
p≤ 0.1
Data unavailable: IQ: 1 HV, 1 BD, 1 SMD; YMRS: 3 BD; CDRS: 5 BD, 2 SMD; CGAS: 5 BD, 6 SMD.
Table 2.
| Longitudinal Subjects | Healthy Volunteers (n=27) | Bipolar Disorder (n=34) | Severe Mood Dysregulation (n=31) |
|---|---|---|---|
|
| |||
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Sex (male)* | 9 (33.3%) | 16 (47.1%) | 22 (71%) |
| Age, Scan 1 (y) | 12.9 ± 2.0 | 13.7 ± 2.5 | 12.9 ± 2.4 |
| Age, Scan 2 (y) | 15.0 ± 2.0 | 15.6 ± 2.5 | 15.0 ± 2.6 |
| Time between scans | 2.1 ± 1.0 | 1.8 ± 0.9 | 2.1 ± 1.0 |
| IQ | 110 ± 16 | 111 ± 15 | 110 ± 12 |
| YMRS, Scan 1 | --- | 10.0 ± 7.7 | --- |
| YMRS, Scan 2† | --- | 7.88 ± 5.6 | --- |
| CDRS, Scan 1† | --- | 28.0 ± 8.8 | 29.3 ± 8.0 |
| CDRS, Scan 2†^ | --- | 30.0 ± 9.5 | 25.8 ± 6.3 |
| CGAS, Scan 1 | --- | 49.5 ± 11.4 | 46.8 ± 8.3 |
| CGAS, Scan 2†^ | --- | 46.3 ± 11.8 | 51.5 ± 10.3 |
| TBV, Scan 1 | 1131 +77 | 1093 ± 105 | 1130 ± 103 |
| TBV, Scan 2 | 1125 ± 74 | 1097 ± 110 | 1127 ± 105 |
| N (%) | N (%) | N (%) | |
|
| |||
| Lithium exposure between scans^ | --- | 17 (50.0) | 9 (29.0) |
| Valproate exposure between scans | --- | 11 (32.4) | 5 (16.1) |
Note: YMRS = Young Mania Rating Scale; CDRS = Childhood Depression Rating Scale; CGAS = Childhood Global Assessment Scale; TBV = total brain volume.
p ≤ 0.05
p≤ 0.1
Data unavailable: YMRS, Scan2:1 BD; CDRS, Scan1: 2 SMD; CDRS, Scan2:5 BD, 6 SMD; CGAS, Scan2: 2 BD, 1 SMD.
MR imaging protocol
Morphometric images were acquired on a 1.5-T GE scanner using an axial 3-dimensional fast spoiled gradient recalled echo in the steady state with a minimum full echo time, 20° flip angle, 224×224 acquisition matrix, 1 excitation, 22cm field of view, and 300ms inversion recovery preparation pulse used for T1-weighting with bandwidth of 15.63 Hz. 124 contiguous 1.2mm axial sections were collected with a voxel size of 0.859×0.859×1.2mm.
VBM protocol & statistical analysis
Before processing, scans were checked visually for artifacts and reoriented manually to a common origin point (anterior commissure) to facilitate automated registration. Processing, including the optimized protocol (Good et al., 2001), used the VBM2 utility (Gaser, Volz, Kiebel, Riehemann, & Sauer, 1999), version 1.08, run in SPM2 and Matlab7.
Cross-Sectional Analysis
A custom template and priors (Good et al., 2001) were created using the scans from all 55 BD and, to balance groups, 55 randomly-selected HV and 55 randomly-selected SMD (total=165 images). See Supplemental Information for further discussion on the rationale for using a custom template. Processing was conducted with the cross-sectional analysis data options in VBM2. Images were segmented using the custom template and priors and default settings. A modulation step was used, allowing for analysis of absolute GM (volume) differences (Ashburner & Friston, 2000). Images were smoothed in SPM2 at the individual level (8mm FWHM kernel).
The VBM2 cross-sectional data analysis option was used to run a whole-brain F-test to examine volume differences across the three groups. Groups differed in the age at scan and also tended to differ in total brain volume (TBV, i.e., sum of GM and white matter volumes); as a result, age and TBV were included as nuisance covariates. A GM threshold of 0.1 was used. Significant clusters were identified using a voxel-wise height threshold of p<0.001 uncorrected, with a cluster extent≥200 voxels, a threshold used in previous VBM studies of pediatric BD (Adler et al., 2007; Adler, Levine, DelBello, & Strakowski, 2005; Lisy, et al., 2011). In addition, correction for non-stationarity of smoothness was employed. Because we used a custom template, peak coordinates are reported in study-specific space. Location of clusters was determined visually. A VBM value representing the average probable volume of GM per voxel was calculated for each subject in each suprathreshold cluster. Post-hoc tests were run on these values using PASW Statistics 18.0.1 for Windows. All post-hoc tests were Bonferroni-corrected.
Longitudinal Analysis
Custom template and priors were created using the scans from both time points for all 27 HV and, to balance groups, 27 randomly-selected BD and 27 randomly-selected SMD subjects (total=162 images, 2 time points each from 81 subjects). All processes were run using the longitudinal analysis data options in VBM2. Images were segmented using the custom template and priors and the same settings as the cross-sectional analysis, with 50mm FWHM smoothing for additional bias correction between time points. Because the VBM2 toolbox does not allow for a modulation step in longitudinal analyses, the utility's modulation step from cross-sectional analyses was added to the longitudinal analysis processing protocol. Images were smoothed in SPM2 at the individual level (8mm kernel).
The VBM2 longitudinal data analysis option was employed to run a whole-brain F-test comparing group (SMD, BD, HV) and scan time (first vs. second scan), and their interaction. Because groups differed in sex distribution, sex was included as a nuisance covariate. The same thresholding and value extraction was employed as in the cross-sectional analysis. Again, Bonferroni-corrected post-hoc tests were run in PASW on extracted data.
Results
Cross-Sectional Analysis
Age at scan differed between groups (p=0.001); the SMD group was younger than the BD (p<0.01) and HV (p=0.02) groups. There was a trend difference in TBV (p=0.09), driven by a trend for larger TBV in HV than BD (p=0.08). Sex composition and IQ did not differ between groups.
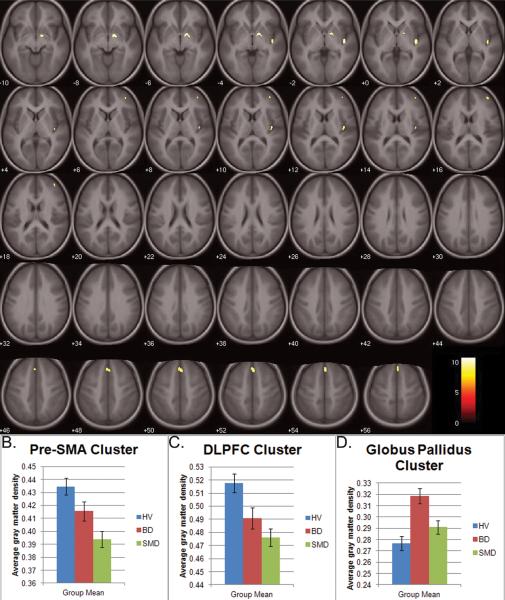
After covarying for age and TBV, there were four GM clusters in which volume differed among SMD, BD, and HV: bilateral pre-supplementary motor area (pre-SMA, BA 6/8, peak: 4,26,53), right globus pallidus (peak: 16,−2,−7), right insula (peak: 43,−13,−1), and right dorsolateral prefrontal cortex (DLPFC, BA 9/46, peak: 41,52,16) (Figure 1A). Post-hoc analyses indicated that between-group differences in the cortical clusters were driven mainly by increased GM volume in HV compared to both BD and SMD, while the globus pallidus differences were driven by increased GM volume in BD compared to both HV and SMD.
Figure 1.
Specifically, in the pre-SMA cluster, SMD had less GM volume (Figure 1B) than HV (p<0.001) and BD at a trend level (p=0.08). In the right insula, HV volume was larger than both BD and SMD (both p<0.001), but BD and SMD did not differ. Similarly, in the right DLPFC cluster, HV volume was larger than BD (p=0.04) and SMD (p<0.001); patient groups did not differ (Figure 1C).
However, in the right globus pallidus cluster (Figure 1D), the BD group had larger volume than HV (p<0.001) and SMD (p<0.01), who did not differ from each other.
Longitudinal Analysis
Sex distribution differed between groups (χ2 p=0.01). Age at each scan, time between scans, IQ, and TBV at each time point did not differ between groups (all p's>0.21).
Two clusters revealed group-by-time interactions, after covarying for sex (Figure 2). These were in the right superior/inferior parietal lobule (R SPL/IPL, BA 7/40, peak: 36,−48,54) and bilateral precuneus (BA 7, peak: 5,−68,40). Post-hoc decompositions revealed that the interaction in R SPL/IPL was driven by an increase in volume in the BD group over time (p=0.01) that was opposite to the decrease over time evident in both HV and SMD (both p<0.001) (Figure 2A). The interaction in the precuneus cluster also was driven by an increase in volume over time in the BD group (p<0.001), with no change in the other two groups (Figure 2B). In this cluster, at time 1, BD had significantly less GM than HV (p=0.02).
Figure 2.
Discussion
This is the first study comparing brain structure and development in SMD, BD, and HV. There are two major sets of findings. First, cross-sectional analyses demonstrated between-group differences in pre-SMA, insula, DLPFC, and globus pallidus. Differences in the three cortical regions were driven by larger GM volumes in HV as compared to the patient populations; in the DLPFC and insula, both patient groups differed from HVs, and in the pre-SMA, SMD differed from HV. Globus pallidus differences, however, were driven by increased volume in BD vs. SMD and HV. Second, longitudinal analyses showed group-by-time interactions in the right SPL/IPL and bilateral precuneus. These reflected an abnormal increase in volume over time in children with BD, as compared to HV and SMD.
Perhaps the most interesting cross-sectional finding is increased globus pallidus volume in BD vs. both SMD and HV. Post-hoc analyses indicate that this finding was not driven by atypical antipsychotic treatment (see Supplemental Information). Increased basal ganglia volume has been reported in pediatric BD using both VBM (Wilke, et al., 2004) and manual tracing (Liu, et al., 2011), and increased globus pallidus volume was the most robust finding in a meta-analysis of 72 pediatric and adult BD sMRI studies (Arnone, et al., 2009). Comorbidity with ADHD is frequent in both our patient samples, and abnormalities in the basal ganglia have been reported in children with ADHD; however, the most consistent findings have been reduced basal ganglia GM in ADHD compared to HV (Nakao, et al., 2011). Consistent with this, Liu and colleagues (2011) studied BD with or without comorbid ADHD and found increased caudate, putamen and globus pallidus volumes in BD, but decreased caudate and putamen volumes in ADHD.
In understanding why globus pallidus abnormalities may be present in BD but not SMD, it is notable that associations between functional responsivity in the right globus pallidus and type and severity of mood state have been reported (Caligiuri et al., 2006; Caligiuri et al., 2003). In addition, deep brain stimulation in globus pallidus has been shown to cause recurrent manic episodes in patients with Parkinson's disease (Miyawaki, Perlmutter, Troster, Videen, & Koller, 2000), although some suggest this may be a “fiber of passage” effect. Therefore, the fact that the globus pallidus abnormality is present in BD only may reflect the fact that only BD patients experience extremely severe and aberrant mood states, including manic episodes. In this sense, our finding of volumetric specificity in this region is consistent with other data showing differences in longitudinal course between SMD and BD i.e., in youth, BD, but not SMD, is associated with manic episodes on follow-up (Stringaris, et al., 2010).
While the cross-sectional data showed differences between BD and SMD in globus pallidus volume, both groups showed cortical abnormalities in comparison to HV. This is consistent with prior research reporting diffusely decreased cortical GM in BD adolescents (Frazier, et al., 2005; Lisy, et al., 2011; Wilke, et al., 2004). Recent meta-analyses report decreased prefrontal (Arnone, et al., 2009) and insular (Ellison-Wright & Bullmore, 2010) cortices in BD children and adults. Indeed, decreased GM density in the right prefrontal cortex and insula, both evident here, may be associated with genetic risk for BD (van der Schot et al., 2010).
Aron and colleagues (2007) posit that pre-SMA, DLPFC, and striatal regions comprise a network mediating motor and cognitive inhibition. These functions are impaired in SMD (Leibenluft, 2011) and BD, regardless of mood state (Martinez-Aran et al., 2004), and in unaffected BD relatives (Bora, Yucel, & Pantelis, 2009). Furthermore, fMRI studies using response inhibition tasks report abnormal activation in DLPFC, pre-SMA, and basal ganglia in BD and SMD (Deveney, et al., 2012; Roth et al., 2006; Singh et al., 2010). Regarding the insula, both SMD and BD are characterized by impaired decision-making. Insula activation has been associated with the use of somatic markers in decision-making (Bechara, 2001), and there is an association between right insula activation and risk-taking (Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003).
While cortical volume abnormalities have been reported in BD, this is the first study examining such abnormalities in SMD. However, as noted above, comparison to studies in ADHD may be informative, since SMD and ADHD share hyperarousal symptoms. sMRI studies of pediatric ADHD report decreased GM, although more diffusely than we found here in a three-group comparison (Batty et al., 2010; Carmona et al., 2005). Indeed, the decreased pre-SMA volume that we observed only in SMD has been reported in ADHD (Mahone et al., 2011). Although there is evidence that brain function may differ between SMD and non-irritable ADHD (Brotman et al, 2010), here we cannot isolate deficits secondary to ADHD in the SMD group, since approximately 70% of SMD had co-occurring ADHD and there is no non-irritable ADHD group. We were, however, able to compare BD with or without ADHD to SMD with ADHD, and to HV (see Supplemental Information), and these post-hoc exploratory analyses suggest that our between-group findings are not driven by co-occurring ADHD. However, future direct comparisons of SMD to non-irritable ADHD are required to definitively separate the effects of SMD and ADHD on brain morphology.
Longitudinally, between-group differences were limited to parietal regions, and were driven by abnormal trajectories in BD only. The group-by-time interaction in the R SPL/IPL was driven by a volume increase in the BD group over time that was directly opposite to the significant decrease in the HV and SMD groups over time. This parietal region is part of a top-down attentional control system (Hopfinger, Buonocore, & Mangun, 2000; Vandenberghe & Gillebert, 2009), and it is activated during tasks with emotional distracters (Luo et al., 2007). The precuneus interaction was also driven by GM increase in the BD group only. The precuneus mediates attention shifting, episodic memory retrieval, and theory of mind, and is part of the default mode network (Cavanna & Trimble, 2006). Studies in BD have reported deficits in several of these processes (Bora, et al., 2009; Schenkel, Marlow-O'Connor, Moss, Sweeney, & Pavuluri, 2008) and decreased reduction in default mode network in response to increasing task demands (Costafreda et al., 2011). The abnormal increases in BD in these parietal attentional regions may reflect deficient pruning, leading to less efficient function. The most recent longitudinal study examining adults and adolescents (Lisy, et al., 2011) also reported widespread GM increases in BD compared to HV, although mostly limited to frontal and subcortical regions.
Although data suggest that lithium and valproate may have neurotrophic effects (Manji, Moore, & Chen, 2000), patient exposure to lithium or valproate between scans did not appear to account for our findings (see Supplemental Information for more details). This is consistent with another longitudinal study that found no inter-scan changes in GM volume in BD adolescents and adults receiving lithium (Lisy, et al., 2011).
The biggest limitation of the study is the differences in age between the groups, especially in the cross-sectional analysis. In addition, we were unable to examine pubertal effects directly; given the potential role of pubertal development on brain structure, this is an important limitation when considering the results of this study. Patients were medicated with a variety of regimens, so we are unable to clarify the effects of specific medications on brain development. It would be ethically untenable to study developmental trajectories in medication-free children with BD, as that would require keeping patients off medications for years. Another limitation is the high rate of comorbid illnesses. While this is characteristic of these populations, it limits our ability to establish the specificity of the findings to the groups of interest, especially in the context of differentiating the effects of SMD from those of ADHD. Therefore, it is important that future studies include a comparison sample of non-irritable youth with ADHD. The study is also limited by small sample sizes, although our samples are larger than most other longitudinal sMRI studies in similar populations. Future studies should include larger samples and more scans to more clearly delineate developmental trajectories in these three groups.
Conclusions
The present study provides important preliminary data regarding brain development in SMD and BD. Similar to previous fMRI findings (Adleman, et al., 2011; Brotman, et al., 2010; Deveney, et al., 2012), SMD and BD have both shared and distinct deficits in GM volume cross-sectionally. However, abnormal development in the BD group alone drove the findings in the longitudinal analysis. It is noteworthy that children with SMD, in contrast to those with BD, displayed relatively normal structural development, although this finding should be replicated in larger samples. Our findings give further support to existing data indicating that SMD is not a developmental phenotype of BD, but a distinct disease process with a different etiology.
Supplementary Material
Key Points
Researchers debate whether chronic severe irritability (operationalized as severe mood dysregulation, SMD) is a developmental phenotype of bipolar disorder (BD), but no data compare the neuroanatomy of these groups to each other and to healthy volunteers (HV).
Development of structural abnormalities in BD is poorly understood, and there is no such information in SMD.
Using voxel-based morphometry, we compared children with SMD, BD, and HV, cross-sectionally and longitudinally.
Cross-sectionally, both BD and SMD had decreased gray matter volume compared to HV in cortical regions that mediate cognitive and motor control. Children with BD had abnormally increased volumes in the globus pallidus.
Longitudinally, BD exhibited abnormal developmental trajectories in parietal regions.
Acknowledgements
This research was supported by the Intramural Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH).
Footnotes
The authors have declared that they have no competing or potential conflicts of interest.
References
- Adleman NE, Kayser R, Dickstein D, Blair RJ, Pine D, Leibenluft E. Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(11):1173–1185. e1172. doi: 10.1016/j.jaac.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biological Psychiatry. 2007;61(6):776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Adler CM, Levine AD, DelBello MP, Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biological Psychiatry. 2005;58(2):151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. The British Journal of Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. The Journal of Neuroscience. 2007;27(44):11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, et al. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):229–238. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Seminars in Clinical Neuropsychiatry. 2001;6(3):205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disorders. 2005;7(6):570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113(1–2):1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Kassem L, Reising MM, Guyer AE, Dickstein DP, Rich BA, et al. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. American Journal of Psychiatry. 2007;164(8):1238–1241. doi: 10.1176/appi.ajp.2007.06101619. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Brown GG, Meloy MJ, Eberson S, Niculescu AB, Lohr JB. Striatopallidal regulation of affect in bipolar disorder. Journal of Affective Disorders. 2006;91(2–3):235–242. doi: 10.1016/j.jad.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Brown GG, Meloy MJ, Eberson SC, Kindermann SS, Frank LR, et al. An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Research. 2003;123(3):171–182. doi: 10.1016/s0925-4927(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, et al. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neuroscience Letters. 2005;389(2):88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Picchioni M, Toulopoulou T, McDonald C, Kravariti E, et al. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorders. 2004;6(1):43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Deveney CM, Connolly ME, Jenkins SE, Kim P, Fromm SJ, Pine DS, et al. Neural recruitment during failed motor inhibition differentiates youths with bipolar disorder and severe mood dysregulation. Biological Psychology. 2012;89(1):148–155. doi: 10.1016/j.biopsycho.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Archives of General Psychiatry. 2005;62(7):734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia Research. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Makris N, Giuliano AS, Herbert MR, Seidman L, et al. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disorders. 2005;7(6):555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H. Detecting structural changes in whole brain based on nonlinear deformations-application to schizophrenia research. NeuroImage. 1999;10(2):107–113. doi: 10.1006/nimg.1999.0458. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. Journal of Child Psychology and Psychiatry. 2007;48(9):852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- James A, Hough M, James S, Burge L, Winmill L, Nijhawan S, et al. Structural brain and neuropsychometric changes associated with pediatric bipolar disorder with psychosis. Bipolar Disorders. 2011;13(1):16–27. doi: 10.1111/j.1399-5618.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Spencer L, Edmiston E, Lacadie CM, Martin A, et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. Journal of the International Neuropsychological Society. 2009;15(3):476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. American Journal of Psychiatry. 2011;168(2):129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. American Journal of Psychiatry. 2003;160(3):430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Lisy ME, Jarvis KB, Delbello MP, Mills NP, Weber WA, Fleck D, et al. Progressive neurostructural changes in adolescent and adult patients with bipolar disorder. Bipolar Disorders. 2011;13(4):396–405. doi: 10.1111/j.1399-5618.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- Liu IY, Howe M, Garrett A, Karchemskiy A, Kelley R, Alegria D, et al. Striatal volumes in pediatric bipolar patients with and without comorbid ADHD. Psychiatry Research. 2011;194(1):14–20. doi: 10.1016/j.pscychresns.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Mitchell D, Jones M, Mondillo K, Vythilingam M, Blair RJ. Common regions of dorsal anterior cingulate and prefrontal-parietal cortices provide attentional control of distracters varying in emotionality and visibility. Neuroimage. 2007;38(3):631–639. doi: 10.1016/j.neuroimage.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Ranta ME, Crocetti D, O'Brien J, Kaufmann WE, Denckla MB, et al. Comprehensive Examination of Frontal Regions in Boys and Girls With Attention-Deficit/Hyperactivity Disorder. Journal of the International Neuropsychological Society. 2011:1–11. doi: 10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biological Psychiatry. 2000;48(8):740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161(2):262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Miyawaki E, Perlmutter JS, Troster AI, Videen TO, Koller WC. The behavioral complications of pallidal stimulation: a case report. Brain and Cognition. 2000;42(3):417–434. doi: 10.1006/brcg.1999.1113. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry. 2011;168(11):1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Roth RM, Koven NS, Randolph JJ, Flashman LA, Pixley HS, Ricketts SM, et al. Functional magnetic resonance imaging of executive control in bipolar disorder. Neuroreport. 2006;17(11):1085–1089. doi: 10.1097/01.wnr.0000227979.06013.57. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, Marlow-O'Connor M, Moss M, Sweeney JA, Pavuluri MN. Theory of mind and social inference in children and adolescents with bipolar disorder. Psychological Medicine. 2008;38(6):791–800. doi: 10.1017/S0033291707002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, et al. Neural correlates of response inhibition in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20(1):15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Baroni A, Haimm C, Brotman M, Lowe CH, Myers F, et al. Pediatric bipolar disorder versus severe mood dysregulation: risk for manic episodes on follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(4):397–405. [PMC free article] [PubMed] [Google Scholar]
- van der Schot AC, Vonk R, Brouwer RM, van Baal GC, Brans RG, van Haren NE, et al. Genetic and environmental influences on focal brain density in bipolar disorder. Brain. 2010;133(10):3080–3092. doi: 10.1093/brain/awq236. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gillebert CR. Parcellation of parietal cortex: convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behavioural Brain Research. 2009;199(2):171–182. doi: 10.1016/j.bbr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Research. 2004;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.