Abstract
Purpose
Acute kidney injury (AKI) is a common source of morbidity after trauma. We sought to determine novel risk factors for AKI, by Acute Kidney Injury Network (AKIN) criteria, in critically ill trauma patients.
Materials and Methods
Prospective cohort study of 400 patients admitted to the ICU of a level one trauma center, followed for development of AKI over five days.
Results
AKI developed in 147/400 (36.8%) patients. In multivariable regression analysis, independent risk factors for AKI included African American race (OR 1.86; 95% CI 1.08,3.18; p=0.024), body mass index ≥30 (OR 4.72 versus normal BMI, 95% CI 2.59, 8.61, p<0.001), diabetes mellitus (OR 3.26; 95% CI 1.30,8.20; p=0.012), abdominal Abbreviated Injury Scale score ≥4 (OR 3.78; 95% CI 1.79,7.96; p<0.001), and unmatched packed red blood cells administered during resuscitation (OR 1.13 per unit; 95% CI 1.04,1.23; p=0.004). AKIN stages 1, 2, and 3 were associated with hospital mortality rates of 9.8%, 13.7%, and 30.4%, respectively, compared with 3.8% for those without AKI (p<0.001).
Conclusions
AKI in critically ill trauma patients is associated with substantial mortality. The findings of African American race, obesity, and blood product administration as independent risk factors for AKI deserve further study to elucidate underlying mechanisms.
Keywords: acute kidney injury, trauma, critical illness, race, obesity, transfusion, epidemiology, risk factors
Introduction
Injury results in over 170,000 deaths annually in the United States, and is the leading cause of death among those aged 1 to 44 years [1]. Acute kidney injury (AKI) is common and associated with substantial mortality in critically ill populations in general [2], and in severely injured patients in particular [3–5]. Risk factors for AKI in trauma, however, have been incompletely studied.
Most prior studies of trauma-associated AKI used non-consensus definitions of AKI [6–9]. The establishment of the Risk, Injury, Failure, Loss, End-stage (RIFLE) criteria and their subsequent modification by the Acute Kidney Injury Network (AKIN) provides a consensus definition for use in epidemiologic studies of AKI [10,11]. This definition has been used in a variety of populations to identify risk factors in an effort to enhance both pathophysiologic understanding of and stratification of risk for AKI [2,12].
Our primary goal in this study was to identify novel risk factors for AKI in trauma patients, including both blunt and penetrating injury mechanisms, and doing so using all components of the AKI consensus criteria (creatinine, urine output, and need for renal replacement therapy). We utilized a cohort of prospectively enrolled critically ill trauma patients with detailed baseline data, followed for the first five days after ICU admission. As the AKIN modifications of RIFLE have not been used to define AKI in prior trauma studies, we further sought to determine the association of AKIN-defined AKI and stage with mortality after severe injury.
Materials and Methods
Study population
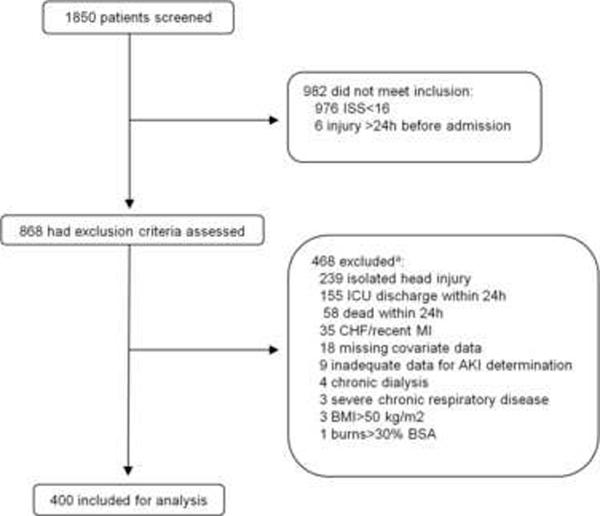
We screened all trauma patients admitted through the emergency department to the surgical ICU of our urban, university hospital from October 2005 to July 2009 for enrollment in a prospective cohort study originally designed to study the development of acute lung injury following trauma [13]. We included patients 14 years of age or older who had an Injury Severity Score (ISS) ≥16. Key exclusion criteria were isolated severe head injury and death or discharge from the ICU within 24 hours of presentation to the ED. For our analysis of AKI, we excluded patients on chronic dialysis. Complete data on inclusions and exclusions are shown in Figure 1. The Institutional Review Board of the University of Pennsylvania approved this study with a waiver of informed consent given its observational nature.
Fig. 1. Screening and enrollment.
aSome subjects excluded for more than one reason.
Risk factor selection
Variables previously reported to be associated with AKI in other populations and those with a plausible association with AKI were included in the risk factor analysis. Baseline data, including demographics, medical history, trauma mechanism and severity, and transfusions were collected prospectively on each patient by review of the medical record. Race was investigator-determined. Key risk factors analyzed are displayed in Table 1.
Table 1.
Subject demographics and clinical characteristics (n=400).
| AKI (n=147) n(%) or mean(range) | No AKI (n=253) n(%) or mean(range) | Odds ratio (95% CI) for AKI | p | |
|---|---|---|---|---|
|
| ||||
| Age, years | 42 (16–91) | 38 (15–96) | 1.13 (1.01,1.26) per 10 years | 0.032 |
| Male sex | 111 (76) | 186 (74) | 1.11 (0.70,1.77) | 0.660 |
| Race | 0.035e | |||
| Caucasian | 61 (42) | 127 (50) | reference | |
| African American | 85 (58) | 116 (46) | 1.53 (1.01,2.31) | 0.046f |
| Asian | 1 (1) | 9 (4) | 0.23 (0.03,1.87) | 0.169f |
| American Indian | 0 (0) | 1 (<1) | - | - |
| Hispanic ethnicity | 2 (1) | 6 (2) | 0.57 (0.11,2.85) | 0.716 |
| BMI category (kg/m2) | <0.001e | |||
| Underweight (<18.5) | 0 (0) | 9 (4) | - | - |
| Normal (18.5–24.9) | 35 (24) | 114 (45) | reference | - |
| Overweight (25.0–29.9) | 44 (30) | 86 (34) | 1.67 (0.99,2.82) | 0.056f |
| Obese (≥30.0) | 67 (46) | 42 (17) | 5.20 (3.03,8.92) | <0.001f |
| ASA or NSAID | 8 (5) | 23 (9) | 0.58 (0.26,1.30) | 0.188 |
| Smoking | 0.752e | |||
| Never | 76 (52) | 125 (49) | reference | |
| Current | 57 (39) | 108 (43) | 0.87 (0.57,1.33) | 0.518f |
| Former | 9 (6) | 15 (6) | 0.99 (0.41,2.37) | 0.976f |
| Unknown | 5 (3) | 5 (2) | 1.64 (0.46,5.87) | 0.443f |
| Chronic alcohol use | 25 (17) | 55 (22) | 0.72 (0.43,1.21) | 0.219 |
| Hypertension | 33 (22) | 34 (13) | 1.86 (1.10,3.16) | 0.020 |
| Diabetes mellitus | 15 (10) | 9 (4) | 3.08 (1.34,7.08) | 0.007 |
| Trauma mechanism (blunt) | 97 (66) | 188 (74) | 0.67 (0.43,1.04) | 0.076 |
| ISS | 24.3 (16–45) | 24.3 (16–48) | 0.99 (0.85,1.16) per 5 points | 0.946 |
| AIS score | ||||
| Head | 1.5 (0–4) | 2.0 (0–4) | 0.84 (0.75,0.95) | 0.005 |
| Thorax | 2.1 (0–5) | 2.1 (0–5) | 1.00 (0.88,1.13) | 0.978 |
| Abdomen | 2.0 (0–5) | 1.4 (0–5) | 1.27 (1.11,1.45) | <0.001 |
| Extremities | 1.7 (0–4) | 1.6 (0–4) | 1.07 (0.90,1.27) | 0.442 |
| External | 0.4 (0–2) | 0.5 (0–2) | 0.84 (0.58,1.20) | 0.329 |
| APACHE III | 73 (39–152) | 63 (32–139) | 1.31 (1.17,1.47) per 10 points | <0.001 |
| APACHE III, nonrenala | 55 (29–118) | 53 (27–127) | 1.10 (0.97,1.24) per 10 points | 0.128 |
| Low SBP, mm Hgb | 96.5 (37–192) | 99.8 (40–172) | 0.95 (0.87,1.03) per 10 mm Hg | 0.203 |
| Hemoglobin, mg/dLc | 12.5 (5.6–17.4) | 12.9 (3.3–17.2) | 0.92 (0.83,1.02) | 0.098 |
| OR prior to ICU | 93 (63) | 126 (50) | 1.74 (1.15,2.63) | 0.009 |
| Receipt of albumin | 3 (2) | 4 (2) | 1.30 (0.32,5.26) | 0.711 |
| Receipt of IV contrastd | 95 (65) | 202 (80) | 0.46 (0.29,0.73) | 0.001 |
| Crystalloid, litersd | 4.4 (0–14.5) | 3.7 (0–12.2) | 1.08 (1.01,1.16) | 0.025 |
| Blood products | ||||
| FFP, unitsd | 2.9 (0–20) | 1.2 (0–27) | 1.14 (1.07,1.22) | <0.001 |
| PRBC, unitsd | 4.3 (0–23) | 2.3 (0–36) | 1.09 (1.04,1.14) | <0.001 |
| Cross-matched | 2.0 (0–16) | 1.2 (0–35) | 1.06 (1.00,1.12) | 0.055 |
| Unmatched | 2.4 (0–18) | 1.1 (0–13) | 1.17 (1.08,1.26) | <0.001 |
| Platelets, unitsd | 0.4 (0–4) | 0.2 (0–3) | 1.72 (1.24,2.39) | 0.001 |
Definition of abbreviations: ASA or NSAID= aspirin or non-steroidal anti-inflammatory drug use; BMI= body mass index; ISS= Injury Severity Score; AIS= Abbreviated Injury Scale; APACHE III= Acute Physiology And Chronic Health Evaluation III; FFP= fresh frozen plasma; PRBC= packed red blood cells; SBP= systolic blood pressure.
APACHE III score with removal of contributions from creatinine, BUN, and urine output.
Out-of-hospital or in the emergency department.
In the emergency department.
During resuscitation prior to ICU admission.
χ2 test comparing 4 categories.
Wald test for OR compared with reference.
Outcome definitions
Presence and stage of AKI within the first five days were defined according to AKIN criteria [11] (Table 2). For AKI by creatinine increase, successive 2-day time windows were tracked (e.g., days 0–2, days 1–3), using the first measured creatinine of each window as the baseline value. Since a significant sub-population of trauma patients has no prior interaction with the medical system, available pre-hospitalization baseline creatinine values from the medical record were not used in order to prevent ascertainment bias. As only 24-hour urine output data were consistently available, a modification was made in the urine output definition such that transient (6- or 12-hour) reductions in urine output were not captured (Table 2 caption). AKI was considered to have occurred on the first day that any criterion was met, though full staging continued through day 5 or to ICU discharge, whichever came first. Day 0 was defined as the calendar day of emergency department presentation, and thus its length varied depending on time of presentation. We determined vital status at the time of discharge from the hospital for all patients.
Table 2.
Acute kidney injury definition.
| Creatinine (over 48 hours) & renal replacement therapy criteria | Modified urine output criteriaa | |
|---|---|---|
|
| ||
| AKI | 1) Acute increase in serum creatinine of ≥0.3 mg/dL or to ≥150% of baseline, OR | <0.5mL/kg/h for 24 hours |
| 2) Creatinine ≥4.0 mg/dL with an acute increase of ≥0.5 mg/dL, OR | ||
| 3) Need for renal replacement therapy | ||
| Stage 1 | Acute increase in serum creatinine of ≥0.3mg/dL or to 150% to 200% of baseline | Not applicablea |
| Stage 2 | Acute increase in serum creatinine to >200% to 300% of baseline | <0.5mL/kg/h and ≥0.3mL/kg/h for 24 hours |
| Stage 3 | 1) Acute increase in serum creatinine to >300% of baseline, OR | <0.3mL/kg/h for 24 hours |
| 2) Creatinine ≥4.0 mg/dL with an acute increase of ≥0.5 mg/dL, OR | ||
| 3) Need for renal replacement therapy | ||
Cr, RRT, and stage 3 urine output criteria are taken from the Acute Kidney Injury Network (AKIN) consensus criteria (17).
Given the lack of 6- or 12-hour urine output data, urine output criteria for both AKI and stage 2 are modified to use 24-hour data (AKIN criteria are <0.5mL/kg/h for 6 hours (AKI) and <0.5mg/kg/h for 12 hours (stage 2)). Urine output criterion for stage 1 is not used in this definition (AKIN criterion is <0.5 mL/kg/h for 6 hours).
Prior studies of organ failure in trauma patients have suggested a biphasic pattern, with most early organ failure occurring within the first 2–3 days, and late organ failure peaking a week or later after injury [14]. Hence, our 5-day study time frame was designed to capture most cases of early AKI, and to reduce the confounding effects of time-varying interventions (e.g., nephrotoxic medications) and late complications (e.g., sepsis) which would likely have had a greater impact in cases of AKI occurring later in the hospital course.
Statistical analysis
Associations of baseline characteristics with AKI were tested using Pearson's chi-square test, Fisher's exact test, Student's unpaired t-test, or Wilcoxon rank-sum test, as appropriate. Because of potential pathophysiologic differences between blunt and penetrating trauma, unadjusted analyses stratified by injury mechanism were also performed to determine if there were differences in effect across injury type.
All variables with an unadjusted association with AKI and a p-value <0.20 as well as all potential confounders were considered for inclusion in the multivariable logistic regression model. Confounders were included in the final model if they had a significant impact on the unadjusted association of candidate exposure variables (as defined by a change in odds ratio ≥15%) [15]. A trauma resuscitation protocol at our institution outlining a recommended ratio of blood product transfusion (6 units PRBC:6 units FFP:1 unit platelets) made unfeasible a determination of the effects of these blood products independent of each other. Therefore, only packed red blood cells (PRBC) were used in the final model. Standardized risks were determined for each variable in the model using post-estimation marginal analysis [16]. This method allows an estimation of the absolute risk of AKI in the presence or absence of a single characteristic (e.g., race), holding the other covariates in the model at the average values of the cohort.
To further explore the time-dependent effects of blood transfusion on AKI, a discrete time model was constructed [17]. The exposure variable for each day was the cumulative number of PRBC units transfused from admission through the previous day. Only those at risk for AKI (patients still in the ICU who had not yet developed AKI) were included in each day's analysis.
For mortality analyses, the unadjusted associations of AKI and AKIN stage with death at discharge were evaluated using Pearson's chi-square test. Associations of baseline variables with mortality were also determined to identify possible confounders (defined as in the primary multivariable model). Multivariable logistic regression was then used to adjust the effect of AKIN stage on death for these confounders. All statistical analyses were done using Stata/IC 11.1 (StataCorp LP, College Station, TX 77845).
Results
Four hundred subjects were included in the final analysis (Figure 1). Within the first five ICU days, 147 subjects (36.8%, 95% CI 32.0,41.7) developed AKI: 14.8% (95% CI 11.4,18.6) by creatinine only, 13.3% (95% CI 10.1,17.0) by urine output only, and 8.8% (95% CI 6.2,12.0) by both criteria. Among those with AKI, 53.1% (95% CI 44.7,61.3) met criteria on day 0 or 1. AKIN stages 1, 2, and 3 developed in 34.7% (95% CI 27.0,43.0), 49.7% (95% CI 41.3,58.0), and 15.7% (95% CI 10.2,22.5), respectively. Nine subjects required acute dialysis by day 5.
A comparison of baseline characteristics between those who did and did not develop AKI is shown in Table 1. The following characteristics were noted in less than 1% of subjects: cirrhosis, HIV/AIDS, leukemia or multiple myeloma, lymphoma, immunosuppression, and solid tumor.
In the multivariable model, African American race, BMI, diabetes mellitus, Abbreviated Injury Scale (AIS) score for abdomen ≥4, and unmatched PRBC units administered during resuscitation were independently associated with subsequent AKI (Table 3). The standardized risk differences show the adjusted increase in AKI risk associated with each characteristic. For example, African American race imparts a 12% absolute increase in AKI risk (43% v. 32% for non-African Americans), holding all other model covariates at the average cohort values. The standardized risk difference for AKI associated with Type I diabetes (0.19, 95% CI −0.14,0.51, p=0.268; n=7) was not substantially different from that associated with Type II diabetes (0.27, 95% CI 0.06,0.47, p=0.010; n=20). Serum creatinine at presentation was not significantly different in subjects with and without diabetes (1.19 mg/dL v. 1.14 mg/dL, respectively, p=0.560) but was higher in African Americans than non-African Americans (1.26 mg/dL v. 1.04 mg/dL, respectively, p<0.001).
Table 3.
Multivariable model for AKI risk (n=391a).
| Standardizedc AKI risk (95% CI) | Standardizedc risk difference (95% CI) | Adjustedd odds ratio (95% CI) for AKI | pe | |
|---|---|---|---|---|
|
| ||||
| Race | ||||
| African American | 0.43 (0.36,0.50) | 0.11 (0.01,0.22) | 1.83 (1.04,3.24) | 0.036 |
| Non-African American | 0.32 (0.25,0.38) | |||
| Diabetes mellitus | ||||
| Present | 0.58 (0.39,0.77) | 0.22 (0.03,0.41) | 3.01 (1.15,7.91) | 0.025 |
| Absent | 0.36 (0.32,0.41) | |||
| AIS score abdomen | ||||
| ≥4 | 0.63 (0.49,0.78) | 0.30 (0.15,0.46) | 4.45 (2.01,9.85) | <0.001 |
| ≤3 | 0.33 (0.28,0.38) | |||
| BMI category (kg/m2) | ||||
| Underweight (<18.5)a | - | - | - | |
| Normal (18.5–24.9) | 0.26 (0.19,0.32) | Ref | Ref | - |
| Overweight (25.0–29.9) | 0.35 (0.27,0.42) | 0.09 (–0.01,0.19) | 1.65 (0.93,2.92) | 0.086 |
| Obese (≥30.0) | 0.57 (0.48,0.67) | 0.32 (0.20,0.43) | 4.72 (2.59,8.61) | <0.001 |
|
| ||||
| Unmatched PRBC, unitsb | 1.13 (1.03,1.24) per 1 unit | 0.007 | ||
| 0 | 0.34 (0.29,0.39) | - | ||
| 3 | 0.41 (0.36,0.46) | - | ||
| 6 | 0.49 0.39,0.58) | - | ||
| 9 | 0.56 (0.42,0.71) | - | ||
All independently associated variables shown in table. Categorical variables are above, continuous variables below, the dashed line.
No underweight subjects developed AKI, resulting in their exclusion from the multivariable model when adjusting for BMI category.
Total amount during resuscitation prior to ICU admission.
Using post-estimation marginal standardization (see methods).
Associations also adjusted for age, hypertension, trauma mechanism, AIS score for head, need for surgery prior to ICU admission, and liters of crystalloid and cross-matched PRBCs administered during resuscitation.
Wald test for odds ratio by multivariable logistic regression.
The unadjusted association of intravenous (IV) contrast during resuscitation with decreased risk of subsequent AKI (Table 1) was most likely not a true biological protective effect. Rather, it suggests that contrast administration identified a sub-population at lower risk for AKI—subjects without evident renal injury on admission. Serum creatinine at presentation was likely a factor in the clinical decision to administer contrast, as only 53% of those with creatinine >1.4 mg/dL received contrast compared with 80% with creatinine ≤1.4 mg/dL, Though inclusion of IV contrast in the multivariable model did not alter the significance of the independent associations noted above, the variable was excluded from the final model since it did not appear to represent the known potential nephrotoxic effects of contrast.
We did not have data on the presence of non-dialysis dependent chronic kidney disease (CKD). We therefore performed a sensitivity analysis including 326 subjects less likely to have CKD, defined as an admission serum creatinine ≤1.4 mg/dL, in an alternate multivariable model. Compared with the primary model, this model showed minimal changes in the standardized risk difference point estimates for all variables except age, which had an attenuated association with AKI (Supplemental Table 1). There is an inherent association of the units defining BMI (kg/m2) with those of AKI defined by urine output (mL/kg/24h). Given the possibility of a resultant spurious BMI-AKI association, a second alternate model excluding BMI as a covariate was constructed. It was similar to the primary model with the exception that the association of age with AKI became statistically significant (Supplemental Table 2). Of note, the association of obesity (BMI≥30) with creatinine-defined AKI was significant (risk ratio 1.83, p<0.001) though less than that of AKI defined by urine output (risk ratio 3.17, p<0.001).
Stratifying the main analyses by mechanism of injury, most risk factors had similar associations with AKI (Supplemental Table 3). A comparison of baseline characteristics by race showed African American subjects to be younger (mean age 34 v. 45 for non-African Americans, p<0.001), less likely to have diabetes (3% v. 9%, p<0.001) and blunt trauma (46% v. 97%, p<0.001), and to have received more FFP, PRBC, and platelet transfusions during resuscitation (Supplemental Table 4).
FFP and platelet transfusions were independently associated with AKI in alternate models in which each was included as the representative blood product (OR 1.12 per unit FFP, 95% CI 1.04,1.20, p=0.002; OR 1.49 per unit platelets, 95% CI 1.04,2.14, p=0.028). The analysis of PRBC transfusion by day (Supplemental Table 5) demonstrated a statistically significant association between cumulative PRBC and AKI only on day 1, though similar odds ratio point estimates were seen on days 2 and 3.
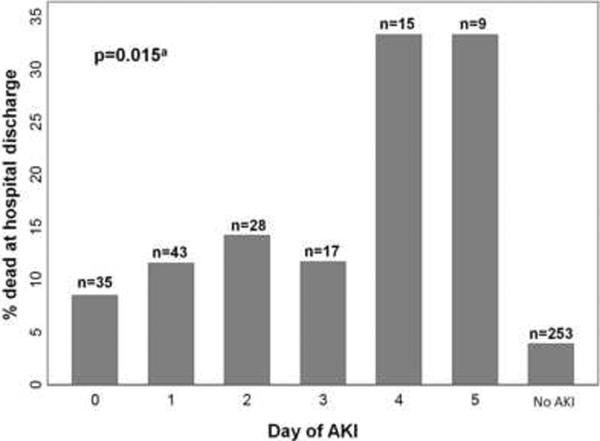
Eight percent (32/400) of subjects died during hospitalization. In unadjusted analysis, AKI was associated with a substantial increase in hospital mortality (14.9% v. 3.8% for non-AKI, p<0.0001). This association was significant in both African Americans (RR 5.46, 95% CI 1.19,25.06, p=0.013) and non-African Americans (RR 3.87, 95% CI 1.71,8.74, p<0.001). Subjects meeting both creatinine and urine output criteria had higher mortality than those meeting creatinine or urine output criteria alone (31.4%, 11.9%, and 7.6%, respectively). Later onset of AKI conferred a higher mortality risk (Figure 2). Among subjects not developing AKI, those who remained in the ICU through day 5 had the highest risk of death (10/146, 6.9%), but this was still lower than the risk for subjects developing AKI on any study day (range 8.6–33.3%). All of the 90 subjects who were discharged from the ICU prior to day 5 without meeting AKI criteria were alive at hospital discharge. Of subjects who died by day 5, the majority (6/8) met criteria for AKI.
Fig. 2. Risk of death by day of AKI diagnosis.
Numbers at the top of each bar represent the total number of subjects who developed AKI on each day (or, for “No AKI,” the number who never developed AKI). aNon-parametric test of trend for those with AKI.
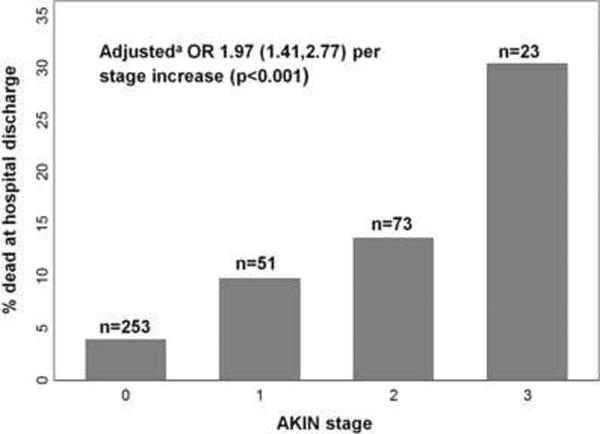
The risk of death increased with higher AKIN stage (Figure 3). Several baseline characteristics were associated with mortality. Adjusting for these variables, however, did not significantly change the association of AKIN stage with mortality. Given this finding and the limited number of deaths, only age and non-renal APACHE III score [18] were included in the multivariable model to avoid overfitting. The adjusted association of AKIN stage with death at discharge remained significant and minimally changed (Figure 3).
Fig. 3. Acute Kidney Injury Network (AKIN) stage association with hospital mortality.
Numbers at the top of each bar represent the total number of subjects for each stage. Stage 0 represents no AKI. aOdds ratio for death is adjusted for age and non-renal APACHE III score.
Discussion
Our primary objective in this study was to determine novel baseline characteristics associated with AKI in critically ill trauma patients. Knowledge of such characteristics may improve our understanding of how the AKI risk imparted by severe injury is modified by patient-level factors. This may aid both in identifying new areas for studying the pathophysiology of AKI and in predicting AKI risk after trauma.
Strengths of our study include the use of a population with a broad mix of trauma mechanisms, substantial representation of African Americans, extensive baseline information, and urine output data to enhance detection of AKI. Two prior studies identified independent risk factors for RIFLE-defined AKI in trauma populations [4,5]. The first, a retrospective database study limited to road traffic injury patients in China, had no data on underlying medical conditions [4]. The second included only blunt trauma patients, had limited racial heterogeneity, and did not focus on exploring the relevance of identified risk factors to AKI pathophysiology [5]. Both of these studies used RIFLE creatinine-only criteria applied over a lengthy time frame (at least 28 days of hospitalization) during which the effects of risk factors for AKI measured immediately after trauma may have been diluted by late complications like sepsis.
Few prior studies have reported an association of race with AKI. While African American race is well known to be associated with CKD and progression to renal failure [19,20], the only prior reports of its association with AKI did not persist in multivariable analysis [2,21,22]. African American race was common in our cohort, unlike prior studies of trauma-associated AKI [3–5,7], which may explain our ability to detect this as a risk factor. The significant association of AKI with mortality regardless of race suggests that such creatinine increases were as clinically meaningful in African Americans as non-African Americans. Our finding may reflect a social effect of race related to culture, lifestyle, socioeconomic status, and other non-genetic factors. Such an effect could manifest as lack of prior diagnosis (due to inadequate access to primary care) of comorbidities which make the kidneys more susceptible to acute injury. A genetic predisposition among African Americans, while less likely, represents another possible reason for this association. Distinguishing between inherent and social effects is not possible in this study, but may be worthy of further investigation, particularly if this finding is replicated in other representative cohorts. The disproportionate impact of trauma on African Americans in the United States makes this novel association particularly noteworthy [23].
It is unclear if the association of BMI with AKI represents a causal link. AKIN urine output criteria are normalized to weight (Table 2). It is not clear, though, that the association of weight and urine output is strictly linear, especially at extremes of weight. An obese person may therefore be inappropriately held to a higher standard for urine production and more easily classified as having AKI despite, in fact, maintaining normal glomerular filtration rate. In our analysis, however, BMI maintained an association with AKI defined by creatinine criteria only. Such an association has also been reported in general ICU populations [24]. A pathophysiologic contribution of adiposity to AKI is plausible: excess adipose tissue results in a chronic systemic inflammatory state, and proinflammatory cytokines have been linked to AKI in plasma studies of critically ill patients [25,26]. Obesity also has known associations with chronic glomerulopathy that may predispose to AKI [25]. Whether the BMI-AKI association reflects a pathogenetic role of obesity or an artifact driven by the method of defining AKI remains to be determined and is worthy of further study.
Diabetes mellitus, often overlooked in other studies due to inadequate patient-level data, has not been previously reported as a risk factor for AKI in trauma. Diabetes may simply be a marker of subclinical CKD, though there was no significant difference in admission creatinine between diabetic and non-diabetic subjects. Use of intravenous contrast for computed tomography is common in the initial evaluation of patients with severe trauma. While diabetes in the absence of CKD has a questionable association with contrast-induced nephropathy [27], it is possible that it could potentiate contrast nephropathy in the setting of the additional renal insults associated with critical illness. Of note, the diagnosis of diabetes in this study was made by chart review. However, the data were collected prospectively without awareness of AKI status, so any misclassification most likely would have been nondifferential and, therefore, not expected to bias toward finding an association of diabetes with AKI.
Our finding that blood product transfusion is associated with AKI has several potential pathophysiologic explanations. Blood product administration is a marker for hypovolemia from hemorrhage and resultant ischemic renal injury. It is also possible that transfusions may be exerting an injurious effect on the kidney through over-resuscitation and tissue edema with propagation of the systemic inflammatory response, and also via cytokine-mediated nephrotoxicity [28]. Similarly, intra-abdominal hypertension from aggressive resuscitation may compromise renal function. The finding that unmatched PRBC had a much stronger association with AKI than did cross-matched PRBC may reflect that use of unmatched blood products identifies the most critically ill, rapidly hemorrhaging patients in a way that APACHE III or ISS cannot. However, it remains possible that certain characteristics of unmatched blood (e.g., immunologic incompatibility with recipient or age of products) may result in a direct nephrotoxic effect. These findings are consistent with those of Bihorac et al [5], who previously reported that PRBC transfusion during resuscitation confers increased AKI risk in blunt trauma patients. We expanded upon the prior findings by showing that unmatched PRBC have a stronger association with AKI than matched PRBC, cumulative PRBC are most strongly associated with early AKI (days 1–3), FFP and platelet transfusions also increase AKI risk, and risk associated with blood products may extend to the penetrating trauma population. The impact of different ratios of these blood products on AKI risk could not be determined in this study given the resuscitation protocol for transfusions at our institution.
The association of older age with AKI in the non-BMI model (Supplemental Table 2) has been noted in several prior studies of trauma patients [3–5,7,8] and is not surprising given the increased likelihood of subclinical renal dysfunction and undiagnosed comorbidities with advancing age [29]. Increased abdominal injury severity, also reported as a risk factor for AKI after road-traffic injuries [4], may predispose to AKI by causing abdominal compartment syndrome or significant hemorrhage into the retroperitoneal or intraperitoneal space with resultant hypovolemia.
In secondary analyses, we found a strong association of AKIN-defined AKI with mortality in trauma patients. RIFLE stage was shown to be associated with mortality in previous studies of trauma patients, though two used creatinine criteria only [4,5], and a third focused on incidence and outcomes of AKI during the first 24 hours only [3]. Both methods may miss a substantial number of AKI cases. Of the 147 cases of AKI in our study, over one third were defined by urine output criteria alone, and nearly half developed between days 2 and 5 (and had the greatest associated mortality risk). AKIN modifications of RIFLE may be particularly appropriate to use in this population since the majority of trauma patients present shortly following event onset, making the initial serum creatinine a useful estimate of baseline renal function. This method may result in less misclassification of AKI than that of RIFLE, which frequently necessitates using an estimated baseline creatinine that assumes all patients have a glomerular filtration rate of 75 ml/min/1.73 m2, an assumption recently shown to be inaccurate [30].
This study has several limitations. It is a single-center study in an urban level one trauma center and is not likely to represent all critically ill trauma populations. We did not have data to detect 6-hour urine output declines (AKIN Stage 1 criteria). However, because transient decreases in urine output are recognized as the least specific AKIN criterion [11] this limitation was unlikely to contribute dramatically to misclassification bias in our cohort. Though the competing risk of death theoretically could have misclassified as non-AKI subjects those who might have developed AKI had they lived longer, 6 of the 8 included subjects who died in the first 5 days had already developed AKI. Similarly, the clinical importance of AKI diagnoses we missed in subjects who left the ICU before day 5 is less clear given 100% hospital survival in this group. Missed AKI diagnoses in a group that recovers so quickly are also less likely to be related to the acute traumatic insult and immediately subsequent pathophysiologic processes, which are the foci of the current study. Finally, with the exception of hospital survival, no data beyond the fifth ICU day were available, which limited our ability to fully stage subjects who developed AKI. However, it is unlikely that a shift of several subjects from lower to higher AKIN stage would have made a significant impact on the convincing association of stage with mortality.
In conclusion, acute kidney injury in critically ill trauma patients, as defined by AKIN criteria, is common, occurring in 36.8% of our study population within the first five days of admission. AKIN stage was independently associated with mortality. We identified novel AKI risk factors in this population—African American race, obesity, and diabetes mellitus—and shed further light on the association of blood products with AKI. Future investigations determining the mechanisms that underlie these observed associations will have the potential to shed light on AKI pathophysiology in trauma patients and possibly identify new targets for intervention.
Supplementary Material
Acknowledgments
Financial support provided by National Institutes of Health grants P50-HL60290, P01-HL079063, K12-HL090021, and T32-HL07891-11.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].National Center for Injury Prevention and Control, CDC: 10 Leading Causes of Death by Age Group, United States -- 2007. http://www.cdc.gov/injury/wisqars/pdf/Death_by_Age_2007-a.pdf.
- [2].Hoste EAJ, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Critical Care. 2006;10 doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bagshaw SM, George C, Gibney RTN, et al. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren Fail. 2008;30:581–589. doi: 10.1080/08860220802134649. [DOI] [PubMed] [Google Scholar]
- [4].Yuan F, Hou FF, Wu Q, et al. Natural history and impact on outcomes of acute kidney injury in patients with road traffic injury. Clin Nephrol. 2009;71:669–679. doi: 10.5414/cnp71669. [DOI] [PubMed] [Google Scholar]
- [5].Bihorac A, Delano MJ, Schold JD, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252:158–165. doi: 10.1097/SLA.0b013e3181deb6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Morris JA, Jr, Mucha P, Jr, Ross SE, et al. Acute posttraumatic renal failure: A multicenter perspective. J Trauma. 1991;31:1584–1590. doi: 10.1097/00005373-199112000-00003. [DOI] [PubMed] [Google Scholar]
- [7].Vivino G, Antonelli M, Moro ML, et al. Risk factors for acute renal failure in trauma patients. Intensive Care Med. 1998;24:808–814. doi: 10.1007/s001340050670. [DOI] [PubMed] [Google Scholar]
- [8].Plurad D, Brown C, Chan L, et al. Emergency Department Hypotension is not an independent risk factor for post-traumatic acute renal dysfunction. Journal of Trauma - Injury, Infection and Critical Care. 2006;61:1120–1127. doi: 10.1097/01.ta.0000244737.54032.98. [DOI] [PubMed] [Google Scholar]
- [9].Brown CVR, Dubose JJ, Hadjizacharia P, et al. Natural History and Outcomes of Renal Failure after Trauma. J Am Coll Surg. 2008;206:426–431. doi: 10.1016/j.jamcollsurg.2007.09.011. [DOI] [PubMed] [Google Scholar]
- [10].Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11 doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lopes JA, Jorge S, Resina C, et al. Acute renal failure in patients with sepsis. Critical Care. 2007;11 doi: 10.1186/cc5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shah CV, Localio AR, Lanken PN, et al. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med. 2008;36:2309–2315. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- [14].Waydhas C, Nast-Kolb D, Jochum M, et al. Inflammatory mediators, infection, sepsis, and multiple organ failure after severe trauma. Archives of Surgery. 1992;127:460–467. doi: 10.1001/archsurg.1992.01420040106019. [DOI] [PubMed] [Google Scholar]
- [15].Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- [16].Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- [17].Singer JD, Willett JB. Applied Longitudinal Data Analysis : Modeling Change and Event Occurrence. Oxford University Press; Oxford ; New York: 2003. [Google Scholar]
- [18].Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: Risk prediction of hospital mortality for critically III hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- [19].Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- [20].Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wyatt CM, Arons RR, Klotman PE, et al. Acute renal failure in hospitalized patients with HIV: Risk factors and impact on in-hospital mortality. AIDS. 2006;20:561–565. doi: 10.1097/01.aids.0000210610.52836.07. [DOI] [PubMed] [Google Scholar]
- [22].Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrology Dialysis Transplantation. 1999;14:1158–1162. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- [23].Arthur M, Hedges JR, Newgard CD, et al. Racial disparities in mortality among adults hospitalized after injury. Med Care. 2008;46:192–199. doi: 10.1097/MLR.0b013e31815b9d8e. [DOI] [PubMed] [Google Scholar]
- [24].Druml W, Metnitz B, Schaden E, et al. Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med. 2010;36:1221–1228. doi: 10.1007/s00134-010-1844-2. [DOI] [PubMed] [Google Scholar]
- [25].Honiden S, McArdle JR. Obesity in the Intensive Care Unit. Clin Chest Med. 2009;30:581–599. doi: 10.1016/j.ccm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- [26].Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–2761. [PMC free article] [PubMed] [Google Scholar]
- [27].Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- [28].Gosling P. The cellular, immune, and metabolic response to trauma. Crit Rev Clin Lab Sci. 1998;35:59–112. [PubMed] [Google Scholar]
- [29].Anderson S, Brenner BM. Effects of aging on the renal glomerulus. Am J Med. 1986;80:435–442. doi: 10.1016/0002-9343(86)90718-7. [DOI] [PubMed] [Google Scholar]
- [30].Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clinical Journal of the American Society of Nephrology. 2010;5:1165–1173. doi: 10.2215/CJN.08531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.