Abstract
We studied GVHD on relapse, transplant-related mortality (TRM), disease-free (DFS) and survival (OS) after allogeneic transplantation for AML (n=4224) and MDS (n=1517) in four groups: without GVHD, acute GVHD alone, chronic GVHD alone, and acute + chronic GVHD. Examining GVHD as time dependent covariate, after myeloablative conditioning (MAC), chronic and acute + chronic GVHD were associated with lower relapse (p<0.002). TRM was higher in all GVHD groups (p<0.0001); DFS and OS were lower with acute ± chronic GVHD (p<0.0001). After reduced intensity conditioning (RIC), relapse was lower in all GVHD groups (p<0.0001); TRM was increased and DFS and OS were reduced with GVHD (p<0.0001). In those surviving disease-free (≥1-year) following MAC, relapse risks were similar in all groups and TRM higher with any GVHD (p<0.0001). DFS and OS were lower with chronic and acute + chronic GVHD (p<0.0006). After RIC, relapse was lower (p=0.009) and TRM higher (p=0.002) only with acute + chronic GVHD. DFS was similar in all groups and OS worse with acute + chronic GVHD. After MAC, GVHD has an adverse effect on TRM with early modest augmentation of GVHD-associated graft-versus-leukemia (GVL). With RIC, GVHD-associated GVL may be important in limiting both early and late leukemia recurrence.
Introduction
Graft vs. host disease (GVHD) can complicate allogeneic hematopoietic cell transplantation (HCT) by inducing substantial morbidity or transplant related, non-relapse mortality (TRM). It can also, however, be associated with augmented antineoplastic potency thus limiting risks of relapse by this association with the graft vs. leukemia (GVL) effect [1,2]. The net impact on survival represents a differential in potency between these two parallel immunologic influences. In recent years, as older patients, or those with pre-HCT comorbidities, receive allografts following reduced intensity conditioning (RIC), the strength of the GVL effect has become more critical in limiting the hazards of relapse. Because older patients may also be more vulnerable to acute and/or chronic GVHD, we studied the effect of GVHD on relapse, TRM, disease-free survival (DFS) and overall survival (OS) following allogeneic HCT for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). The study goal was to identify any differential GVHD-associated GVL influences following transplants using either myeloablative (MAC) or RIC regimens. We report the impact of these immunologic influences on mortality and relapse in these two settings where less potent antineoplastic conditioning might allow persistence of more minimal residual disease (MRD) and render the need for extra GVL more critical for survival.
Patients and Methods
Data Source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally. All patients provided written informed consent. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Inclusion criteria
Patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) who received allogeneic HCT from 1997 to 2006 were eligible (N=5741). Recipients of MAC and RIC regimens were eligible while children under 16 years were excluded as were cases with ex vivo T-cell depleted grafts. Transplant regimens were defined as reduced intensity for busulfan dose <9 mg/kg and melphalan dose <150 mg/m2 and total body irradiation dose ≤500 cGy.
Outcomes
Transplant-related mortality (TRM) was defined as death not related to disease recurrence or progression and relapse was defined as disease recurrence based on morphological evaluation. Patients who received a second transplant or donor leukocyte infusion were censored at time of event. Disease-free survival (DFS) was defined as survival in continuous complete remission. Treatment failure was defined as either relapse or death from any cause; the inverse of DFS. OS was calculated from the date of transplant with censoring at the time of last contact for survivors.
Statistical Analysis
The characteristics of patients, their disease and transplantation are shown in Table 1. Variables related to patients, disease and transplantation were compared among the groups using the chi-square statistic. Incidences of grade II–IV acute GVHD and chronic GVHD were based on reports from each transplant center. Patients were divided into four groups: 1) those without GVHD, 2) those with acute GVHD alone, 3) those with chronic GVHD alone and 4) those with acute and chronic GVHD. To examine the influence of acute and chronic GVHD on TRM, relapse, DFS and OS, we conducted two separate analyses and all analyses were performed separately for recipients of MAC and RIC regimens. Cox regression models [3] were built to adjust for effects of other patient, disease and treatment variables for both analyses. The first analysis included all patients (N = 5791) and acute and chronic GVHD were treated as time dependent covariates. The second analysis was a landmark analysis which included patients who survived at least one-year disease-free after their transplantation (N= 2369). For the landmark analysis of one-year disease-free survivors, acute and/or chronic GVHD status was evaluated at one year by which time all acute and 95% of chronic GVHD had developed. The landmark analysis allowed us to examine any late effect of GVHD on those who were disease-free for at least one year while the analysis with all patients allowed examination of any early effect of GVHD, within one year. The results of multivariate analysis are expressed as hazard ratio with the 95% confidence interval. Factors considered in the multivariate models included age, gender, diagnosis and disease/remission status, donor type and HLA matching, CMV serostatus, performance status, year of transplant and GVHD prophylaxis along with the primarily analysis variables of acute and chronic GVHD. The probabilities of TRM and relapse were calculated using cumulative incidence [4–6]. In all analyses, data on patients without an event were censored at last follow-up. Probability of DFS and OS was calculated with the Kaplan-Meier estimator [7]. Analyses used SAS version 9.2 (Carey, NC).
Table 1.
Demographics of patients receiving allogeneic HCT for AML and MDS
| MAC | RIC | |||||
|---|---|---|---|---|---|---|
| All Cases (Time-dependent analysis cohort) |
Disease-Free survivors > 1 year (landmark study cohort) |
Died, relapsed or followup ≤ 1 year (excluded) |
All Cases (Time- dependent analysis cohort) |
Disease-Free survivors > 1 year (landmark study cohort) |
Died, relapsed or followup ≤ 1 year (excluded) |
|
| N=4,022 | N=1,739 | N=2,283 | N=1,719 | N=630 | N=1,089 | |
| Age, median (range) year |
42 (16–70) | 41 (16–66) | 43 (16–70) | 56 (18–70) | 56 (18–70) | 56 (19–70) |
| Male, N (%) | 2,160 (54%) | 927 (53%) | 1,233 (54%) | 1,004 (58%) | 363 (58%) | 641 (59%) |
| Disease: AML | 2,973 (74%) | 1,268 (73%) | 1,705 (75%) | 1,251 (73%) | 451 (72%) | 800 (73%) |
| MDS | 1,049 (26%) | 471 (27%) | 578 (25%) | 468 (27%) | 179 (28%) | 289 (27%) |
| Disease Stage* | ||||||
| Early | 1,328 (33%) | 757 (44%) | 571 (25%) | 522 (30%) | 225 (36%) | 297 (27%) |
| Intermediate | 648 (16%) | 297 (17%) | 351 (15%) | 304 (18%) | 126 (20%) | 178 (16%) |
| Advanced | 2,004 (50%) | 669 (38%) | 1,335 (58%) | 864 (50%) | 269 (43%) | 595 (55%) |
| Unknown | 42 (1%) | 16 (1%) | 26 (1%) | 29 (2%) | 10 (2%) | 19 (2%) |
| HLA-matched sibling donor |
2,650 (66%) | 1,189 (68%) | 1,461 (64%) | 1,027 (60%) | 365 (58%) | 662 (61%) |
| Unrelated donor 8/8 Matched |
1,266 (31%) | 515 (30%) | 751 (33%) | 631 (37%) | 243 (39%) | 388 (36%) |
| ≥1 loci Mismatched | 106 (3%) | 35 (2%) | 71 (3%) | 61 (3%) | 22 (3%) | 39 (3%) |
| CMV+ recipient | 2,363 (59%) | 983 (57%) | 1,380 (60%) | 1,076 (63%) | 401 (64%) | 675 (62%) |
| GVHD prophylaxis | ||||||
| CSA/tac ± mtx | 3,536 (88%) | 1,556 (89%) | 1,980 (87%) | 775 (45%) | 298 (47%) | 477 (44%) |
| CSA/tac ± MMF | 294 (7%) | 112 (7%) | 182 (8%) | 730 (42%) | 259 (41%) | 471 (43%) |
| Ex-vivo T cell depletion | 192 (5%) | 71 (4%) | 121 (5%) | 214 (13%) | 73 (12%) | 141 (13%) |
| KPS, 90–100% | 2,503 (62%) | 1,226 (71%) | 1,277 (56%) | 924 (54%) | 374 (59%) | 550 (51%) |
| Year of HCT | 2001 (97–06) | 2001 (97–06) | 2001 (97–06) | 2003 (97–06) | 2003 (97–06) | 2003 (97–06) |
| Acute GVHD Grade II–IV, N (%) |
1910 (47%) | 735 (42%) | 1175 (51%) | 754 (44%) | 274 (43%) | 480 (44%) |
| CIF** at 5 year | 48 (46–49)% | 42 (40–45)% | 52 (50–54)% | 44 (42–46)% | 44 (40–47)% | 44 (41–47)% |
| Chronic GVHD**, N (%) |
1613 (40%) | 1148 (66%) | 465 (20%) | 683 (40%) | 458 (73%) | 225 (21%) |
| CIF** at 5 year | 42 (40–43)% | 67 (64–69)% | 22 (20–23)% | 43 (40–45)% | 75 (71–79)% | 23 (20–25)% |
Shown are the characteristics of all patients transplanted as well as those alive disease-free at 1 year included in the landmark analysis, and the excluded others.
Early = AML in CR1 or refractory anemia; Intermediate = CR 2–3; Advanced = relapsed leukemia or RAEB (refractory anemia with excess blasts), RAEB-T.
Cumulative incidence function (CIF) (95% confidence interval).
MAC = myeloablative conditioning; RIC = reduced intensity conditioning; MDS = myelodysplastic syndrome; CSA = cyclosporine; tac = tacrolimus; Mtx = methotrexate; MMF = mycophenolate mofetil; KPS = Karnofsky performance score
Results
Patients
Details of patients’ demographics and disease characteristics pre-transplantation are shown including all patients, the one-year disease-free survivors included in the landmark analysis and those excluded from the landmark analysis by death, relapse or duration of follow-up < 1 year (Table 1). Forty-three percent (1739 of 4022) of MAC transplants and 37% (630 of 1719) of RIC recipients were alive and disease-free at one year after HCT. The median time to acute GVHD onset after MAC was 23 days and after RIC, 28 days. The median times to chronic GVHD onset were 4.7 months and 4.9 months after MAC and RIC transplants, respectively. Clinical characteristics were similar across all groups except that MAC recipients were younger than those receiving RIC. Approximately 75% of patients had AML and the remaining, MDS. A third of patients were in first complete remission (CR), 15%, in second CR and the remaining patients, in relapse at transplantation. Median follow-up was 4 years.
Relapse, transplant-related mortality and disease-free survival: All patients
Results of multivariate analysis of the entire cohort with acute and chronic GVHD modeled as time-dependent covariates are shown in Table 2. Among recipients of MAC transplants, compared to patients without GVHD, relapse risks were significantly lower in patients with chronic GVHD either alone or with acute plus chronic GVHD. Relapse risks were similar in patients without GVHD or with only acute GVHD. However, compared to patients without GVHD, TRM was significantly higher in patients with any GVHD. Consequently, treatment failure and survival risks were significantly higher in patients with acute ± chronic GVHD.
Table 2.
Influence of GVHD on outcome after HCT: Time-dependent multivariate analysis of all patients
| Relapse: | ||
|---|---|---|
| MAC: | HR (95% CI) | P |
| <0.001*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 0.90 (0.78 – 1.04) | 0.16 |
| Chronic GVHD only | 0.68 (0.56 – 0.83) | 0.0002 |
| Acute & chronic GVHD | 0.67 (0.55 – 0.82) | <0.0001 |
| RIC: | ||
| <0.0001*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 0.63 (0.51 – 0.77) | <0.0001 |
| Chronic GVHD only | 0.53 (0.39 – 0.71) | <0.0001 |
| Acute & chronic GVHD | 0.39 (0.28 – 0.54) | <0.0001 |
| TRM: | ||
| MAC: | RR (95% CI) | P |
| <0.0001*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 2.51 (2.18 – 2.89) | <0.0001 |
| Chronic GVHD only | 2.24 (1.78 – 2.81) | <0.0001 |
| Acute & chronic GVHD | 3.71 (3.03 – 4.54) | <0.0001 |
| RIC: | ||
| <0.0001*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 3.66 (2.88 – 4.65) | <0.0001 |
| Chronic GVHD only | 2.10 (1.45 – 3.04) | 0.0001 |
| Acute & chronic GVHD | 4.64 (3.37 – 6.40) | <0.0001 |
| Treatment failure: | ||
| MAC: | RR (95% CI) | P |
| <0.0001*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 1.52 (1.38 – 1.67) | <0.0001 |
| Chronic GVHD only | 1.13 (0.98 – 1.32) | 0.10 |
| Acute & chronic GVHD | 1.50 (1.31 – 1.72) | <0.0001 |
| RIC: | ||
| 0.0007*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 1.25 (1.08 – 1.44) | 0.003 |
| Chronic GVHD only | 0.85 (0.67 – 1.07) | 0.17 |
| Acute & chronic GVHD | 1.19 (0.96 – 1.47) | 0.11 |
| Survival: | ||
| MAC: | RR (95% CI) | P |
| <0.0001*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 1.71 (1.55 – 1.89) | <0.0001 |
| Chronic GVHD only | 0.99 (0.86 – 1.15) | 0.90 |
| Acute & chronic GVHD | 1.37 (1.21 – 1.57) | <0.0001 |
| RIC: | ||
| <0.0001*** | ||
| No GVHD | 1.00 | |
| Acute GVHD only | 1.52 (1.31 – 1.75) | <0.0001 |
| Chronic GVHD only | 0.74 (0.60 – 0.92) | 0.007 |
| Acute & chronic GVHD | 1.13 (0.93 – 1.37) | 0.22 |
For all HCT (MAC 4022; RIC 1719), shown are the hazard ratio (RR, 95% confidence intervals) of the impact of acute and/or chronic GVHD on the incidence of relapse, non-relapse treatment related mortality (TRM), treatment failure (relapse or death) and survival. Regression models were adjusted as needed for age, performance status, diagnosis and disease status, year of transplant and CMV serostatus.
All cases are included using the development of acute and/or chronic GVHD as a time-dependent covariate.
Three degree-of-freedom test
Among recipients of RIC transplants, compared to patients without GVHD, patients with any GVHD had significantly lower relapse risks. TRM risks were also significantly higher in patients with any GVHD. In contrast to the findings after MAC transplants following RIC, compared to patients without GVHD patients with acute GVHD alone had significantly more treatment failure. Treatment failure risks were similar in patients without GVHD and those with chronic GVHD with or without preceding acute GVHD. Overall survival was worse in those with either acute or chronic GVHD.
Relapse, transplant-related mortality and disease-free survival: landmark analysis
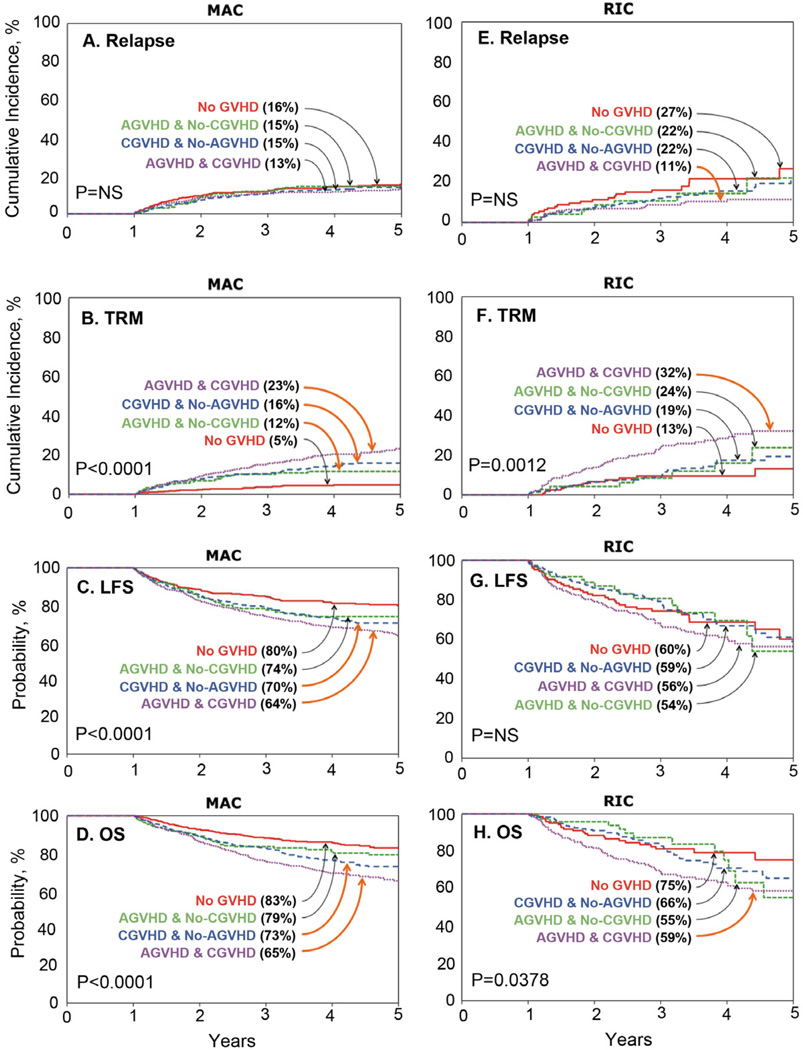
Multivariate analyses of the one-year disease free survivor cohort are shown in Table 3. Following MAC transplants, subsequent relapse risks among one-year disease free survivors were not significantly different amongst all four GVHD groups (Figure 1A). In contrast, TRM was higher in patients with any GVHD (Figure 1B), leading to higher subsequent treatment failure and lower DFS in those with chronic GVHD and with acute + chronic GVHD compared to those with no GVHD (Figure 1C). Similarly, overall survival was worse with chronic or with acute + chronic GVHD. Following RIC transplants, subsequent relapse risks among one-year disease free survivors were not significantly influenced by isolated acute or chronic GVHD, but risks were significantly lower in those with both acute and chronic GVHD (Figure 1D). TRM (Figure 1E) was also higher only in those both acute and chronic GVHD. Consequently, treatment failure and DFS were similar in all groups (Figure 1F) while survival was impaired only in those with both acute and chronic GVHD.
Table 3.
Influence of GVHD on outcome after HCT: landmark multivariate analysis among one-year disease-free survivors.
| Relapse: | |||
|---|---|---|---|
| MAC: | N | HR (95% CI) | P |
|
Disease-Free Survivors at 1 yeara (N=1,739): |
0.9608*** | ||
| No GVHD | 511 | 1.00 | |
| Acute GVHD only | 198 | 0.94 (0.60 – 1.46) | 0.77 |
| Chronic GVHD only | 524 | 0.95 (0.68 – 1.33) | 0.75 |
| Acute & chronic GVHD | 506 | 0.91 (0.65 – 1.28) | 0.60 |
| RIC: | |||
| Disease-Free Survivors at 1 yeara (N=630): | 0.0716*** | ||
| No GVHD | 130 | 1.00 | |
| Acute GVHD only | 79 | 0.61 (0.28 – 1.32) | 0.21 |
| Chronic GVHD only | 228 | 0.72 (0.42 – 1.23) | 0.23 |
| Acute and chronic GVHD | 193 | 0.44 (0.24 – 0.82) | 0.009 |
| TRM: | |||
| MAC: | N | RR (95% CI) | P |
|
Disease-Free Survivors at 1 yeara (N=1,739): |
<0.0001*** | ||
| No-GVHD | 511 | 1.00 | |
| Acute GVHD only | 198 | 2.34 (1.32 – 4.15) | 0.0038 |
| Chronic GVHD only | 524 | 3.28 (2.08 – 5.18) | <0.0001 |
| Acute & chronic GVHD | 506 | 4.54 (2.92 – 7.04) | <0.0001 |
| RIC: | |||
| Disease-Free Survivors at 1 yeara (N=630): | 0.0009*** | ||
| No GVHD | 130 | 1.00 | |
| Acute GVHD only | 79 | 1.24 (0.51 – 3.04) | 0.64 |
| Chronic GVHD only | 228 | 1.33 (0.68 – 2.60) | 0.41 |
| Acute and chronic GVHD | 193 | 2.69 (1.44 – 5.04) | 0.0020 |
| Treatment failure: | |||
| MAC: | N | RR (95% CI) | P |
|
Disease-Free Survivors at 1 yeara (N=1,739): |
<0.0001*** | ||
| No GVHD | 511 | 1.00 | |
| Acute GVHD only | 198 | 1.30 (0.92 – 1.83) | 0.13 |
| Chronic GVHD only | 524 | 1.57 (1.21 – 2.04) | 0.0006 |
| Acute & chronic GVHD | 506 | 1.83 (1.42 – 2.36) | <0.0001 |
| RIC: | |||
| Disease-Free Survivors at 1 yeara (N=630): | 0.3286*** | ||
| No GVHD | 130 | 1.00 | |
| Acute GVHD only | 79 | 0.88 (0.49 – 1.57) | 0.66 |
| Chronic GVHD only | 228 | 0.91 (0.60 – 1.38) | 0.67 |
| Acute & chronic GVHD | 193 | 1.23 (0.82 – 1.83) | 0.32 |
| Survival: | |||
| MAC: | N | RR (95% CI) | P |
|
Disease-Free Survivors at 1 yeara (N=1,739): |
<0.0001*** | ||
| No-GVHD | 511 | 1.00 | |
| Acute GVHD only | 198 | 1.24 (0.85 – 1.82) | 0.27 |
| Chronic GVHD only | 524 | 1.74 (1.31 – 2.30) | 0.0001 |
| Acute & chronic GVHD | 506 | 2.20 (1.68 – 2.87) | <0.0001 |
| RIC: | |||
| Disease-Free Survivors at 1 yeara (N=630): | 0.0277*** | ||
| No GVHD | 130 | 1.00 | |
| Acute GVHD only | 79 | 0.99 (0.51 – 1.94) | 0.98 |
| Chronic GVHD only | 228 | 1.16 (0.72 – 1.86) | 0.56 |
| Acute & chronic GVHD | 193 | 1.74 (1.10 – 2.76) | 0.018 |
Shown are the hazard ratio (HR, 95% confidence intervals) of the impact of acute and/or chronic GVHD on the incidence of relapse, non-relapse treatment related mortality (TRM), treatment failure (relapse or death) and survival after MAC or RIC HCT. Regression models were adjusted as needed for age, diagnosis and disease status, GVHD prophylaxis and year of transplant.
Fixed time effect of GVHD where acute and/or chronic GVHD status evaluated at 1 year after HCT. MAC = myeloablative conditioning; RIC = reduced intensity conditioning
Three degree-of-freedom test
Figure 1.
1A,1E: The 5-year cumulative incidence of relapse in patients surviving disease-free at one year posttransplant without GVHD (red), with acute GVHD (green), with chronic GVHD (blue) and with acute and chronic GVHD (purple) after MAC (A) or RIC (D) transplantation
1B, 1F: The 5-year cumulative incidence of TRM in patients surviving disease-free at one year posttransplant without GVHD (red), with acute GVHD (green), with chronic GVHD (blue) and with acute and chronic GVHD (purple) after MAC (B) or RIC (E) transplantation
1C, 1G: The 5-year probability of DFS in patients surviving disease-free at one year posttransplant without GVHD (red), with acute GVHD (green), with chronic GVHD (blue) and with acute and chronic GVHD (purple) after MAC (C) or RIC (F) transplantation
1D, 1H: The 5-year probability of survival in patients surviving disease-free at one year posttransplant without GVHD (red), with acute GVHD (green), with chronic GVHD (blue) and with acute and chronic GVHD (purple) after MAC (D) or RIC (H) transplantation
Groups with statistically significant outcomes are marked with an orange arrow.
Discussion
The potency of allogeneic HCT in preventing relapse has long been attributed to a T cell-mediated antitumor effect targeting histocompatibility or tumor-associated antigens expressed on the target neoplastic cells [1,2,8]. A second contention, that GVHD was directly associated with the antineoplastic effect was reported in a landmark paper which included only a modest number of patients with leukemia [1]. In 1990, Horowitz et al., described a stepwise, more evident protection against relapse associated with acute, chronic or acute plus chronic GVHD. All transplants with GVHD were found to associate with more potent GVL than either syngeneic or GVHD-free transplantation in a population that included sibling donor MAC transplantation for patients with AML, chronic myeloid leukemia and acute lymphoblastic leukemia but not MDS [2]. Additionally, only a minority of patients in that early report received double agent therapy for post-transplant immune suppression, which might potentially alter the GVL effect. Despite these differences, after MAC, the findings in the early study are similar to the MAC transplants in the current study with a statistically significant GVL effect associated with acute plus chronic GVHD, but no significant effect of isolated acute GVHD. However, no protection against relapse had been seen with isolated chronic GVHD, although only few (n=54) patients were in that group. In the current analysis, chronic GVHD alone or with acute GVHD was associated with relapse protection, but only in the first post transplant year. In patients receiving RIC regimens, the situation is different as both acute and chronic GVHD, alone or in combination, had protective effects on relapse.
The current analysis, by including a landmark analysis of one-year disease free survivors, allowed us to specifically examine later effects on relapse, beyond 1 year. Here we observed further divergence between GVHD and GVL with MAC and RIC regimens. After MAC we observed substantial higher late TRM risks with any GVHD, but no greater protection against relapse, suggesting that any GVL effects have already been manifest in the first year. GVHD, therefore, led to a substantially higher risk of late treatment failure. In contrast, among patients who had received RIC regimens, one-year disease free survivors with both acute and chronic GVHD continue to have a significant GVL benefit, suggesting an ongoing active anti-leukemia process. The increase in late TRM associated with GVHD was restricted to patients with both acute and chronic GVHD and, although statistically significant, was of a lesser magnitude in the cohort receiving RIC versus MAC regimens. Consequently, there was no higher risk of late treatment failure associated with any GVHD in patients receiving RIC. These data suggest that following RIC, a setting where late GVHD-associated mortality is ameliorated, its potent association with GVL might improve outcomes and increase the fraction of leukemia patients surviving disease-free.
It is difficult to predict, however, the effect GVHD on overall relapse and survival. Several recent reports could not document augmented antineoplastic protection with either partially matched related or unrelated donor transplantation compared to HLA matched sibling transplantation, circumstances where greater histoincompatibility leads to more GVHD [[9–12]. This suggests that the clinical consequences of GVHD might not always be accompanied by sufficiently potent GVL effects to improve disease control and survival in patients with AML or MDS. These observations do not illuminate the mechanism underlying the immunologic cytolytic effects that may be coordinately operative in GVHD and GVL. T cell, NK cell or pro-apoptotic inflammatory responses may all limit persistence of neoplastic cells, yet not induce clinically uncontrollable or fatal GVHD [13–16]. What, therefore, are the clinical implications of these findings? One might infer that using RIC [17–20], where a greater residual tumor burden may persist following conditioning, clinically limited GVHD may convey a profound and important GVL response. Indeed, in vivo T cell depletion using anti-thymocyte globulin or alemtuzumab with RIC transplants has been reported to compromise GVL and overall survival [21]. In patients with no manifestations of GVHD, additional interventions including, for example, adoptive cellular therapy or antitumor vaccination might be warranted to limit the risks of leukemia recurrence. Conversely, following MAC, with an expectedly lower post-transplant residual disease burden, more stringent measures to limit GVHD might be of net clinical benefit [22,23]. Clinical strategies to balance these two effects, perhaps best combined with measures to better detect persisting post-transplant MRD, might be integrated to identify those most likely to benefit from additional post-transplant anti-leukemic interventions.
Acknowledgements
Supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases and a contract (SCTOD#HHSH234200637015C) from the Health Services and Resource Administration. The authors acknowledge the participation of numerous transplant and donor centers who collect and submit data to the CIBMTR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These data were presented in part at the 2011 American Society of Hematology meeting, San Diego, California.
Authorship Contributions
DW, MJZ and ME designed the study and completed the analysis.
All authors wrote the paper and approved the final submission.
Conflict of Interest
The authors all report no conflict of interest for any submitted information in this manuscript.
References
- 1.Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, Storb R. Antileukemic effect of graft-versus-host disease in human recipients of allogeneicmarrow grafts. N Engl J Med. 1979 May 10;300(19):1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 3.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 4.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 8.Fefer A, Buckner CD, Thomas ED, Cheever MA, Clift RA, Glucksberg H, Neiman PE, Storb R. Cure of hematologic neoplasia with transplantation of marrow from identical twins. N Engl J Med. 1977 Jul 21;297(3):146–148. doi: 10.1056/NEJM197707212970307. [DOI] [PubMed] [Google Scholar]
- 9.Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99(6):1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 10.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113(13):3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora M, Weisdorf DJ, Spellman SR, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27(10):1644–1652. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisdorf DJ, Nelson G, Lee SJ, et al. Sibling versus unrelated donor allogeneic hematopoietic cell transplantation for chronic myelogenous leukemia: refined HLA matching shows more graft-versus-host disease but not less relapse. Biol Blood Marrow Transplant. 2009;15(11):1475–1478. doi: 10.1016/j.bbmt.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 14.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 15.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(4):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110(7):2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giralt S, Logan B, Rizzo D, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: Long-term follow-up of the first 258 reported to the National Marrow Donor Program. Biol Blood Marrow Transplant. 2007;13(7):844–852. doi: 10.1016/j.bbmt.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;(17):2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14(2):246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-Tcell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011 Jun 23;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inamoto Y, Flowers ME, Lee SJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011;118(2):456–463. doi: 10.1182/blood-2011-01-330217. [DOI] [PMC free article] [PubMed] [Google Scholar]