Abstract
Serotonin-1B (5-HT1B) autoreceptors are located in serotonin (5-HT) terminals along with serotonin transporters (SERT), and play a critical role in autoregulation of serotonergic neurotransmission, and are implicated in disorders of serotonergic function, particularly emotional regulation. SERT modulates serotonergic neurotransmission by high-affinity reuptake of 5-HT. Alterations in SERT activity are associated with increased risk for depression and anxiety. Several neurotransmitter receptors are known to regulate SERT Km and Vmax, and previous work suggests that 5-HT1B autoreceptors may regulate 5-HT reuptake, in addition to modulating 5-HT release and synthesis. We used rotating disk electrode voltammetry to investigate 5-HT1B autoreceptor regulation of SERT-mediated 5-HT uptake into synaptosomes. The selective 5-HT1B antagonist SB224289 decreased SERT activity in synaptosomes prepared from wild-type but not 5-HT1B knockout mice, whereas SERT uptake was enhanced after pre-treatment with the selective 5-HT1B agonist CP94253. Furthermore, SERT activity varies as a function of 5-HT1B receptor expression—specifically, genetic deletion of 5-HT1B decreased SERT function, while viral-mediated overexpression of 5-HT1B autoreceptors in rat raphe neurons increased SERT activity in rat hippocampal synaptosomes. Considered collectively, these results provide evidence that 5-HT1B autoreceptors regulate SERT activity. Since SERT clearance rate varies as a function of 5-HT1B autoreceptor expression levels and is modulated by both activation and inhibition of 5-HT1B autoreceptors, this dynamic interaction may be an important mechanism of serotonin autoregulation with therapeutic implications.
Keywords: SERT, 5-HT, uptake, knockout, overexpression, serotonergic, voltammetry, 1B, CP94253, SB224289
INTRODUCTION
Serotonin-1B (5-HT1B) autoreceptors are located in serotonin (5-HT) terminals (Riad et al., 2000),, along with serotonin transporters (SERT), and play a critical role in autoregulation of serotonergic neurotransmission, and are implicated in disorders of serotonergic function, particularly emotional regulation (Clark and Neumaier, 2001; McDevitt and Neumaier, 2011; Sari, 2004). Activation of 5-HT1B autoreceptors reduces 5-HT synthesis and release (Hjorth et al., 1995; Hoyer and Middlemiss, 1989), providing a negative feedback mechanism for serotonergic neurotransmission. Data from studies using 5-HT1B knockout mice further substantiate their important role in behavior and regulation of serotonin neurotransmission in different brain regions (Gardier, 2009; Scearce-Levie et al., 1999).
Another potential mechanism by which 5-HT1B autoreceptors may regulate serotonergic neurotransmission is by modulating its reuptake. Serotonin reuptake is mediated by SERT and other transporters, including the plasma membrane monoamine transporter (PMAT) or the organic cation transporter 3 (OCT3) (Daws, 2009). SERT modulates the strength and duration of serotonergic neurotransmission by high-affinity 5-HT uptake (Blakely et al., 1994; Iversen, 1971; Quick, 2003; Rudnick, 2006), and is regulated by several rapidly acting post-translational mechanisms (Blakely et al., 1998; Steiner et al., 2008). Additionally, SERT is a primary site of action for many antidepressants (Blakely et al., 1998; Sulzer and Edwards, 2005) as well as drugs of abuse (Amara and Sonders, 1998). These aspects of serotonin transporter biology have been investigated as potential targets for antidepressant drug development (See Review by (White et al., 2005). Convergent signals from several stress-related receptor and cytokine systems appear to integrate at the level of the 5-HT terminal and alter SERT activity (Bruchas et al., 2011; McDevitt and Neumaier, 2011; Zhu et al., 2006; Zhu et al., 2010; Zhu et al., 2007).
Multiple lines of evidence suggest the possibility of a direct 5-HT1B-SERT interaction in regulating central nervous system serotonin dynamics. First, in vivo chronoamperometric studies show that 5-HT1B antagonists decrease 5-HT clearance rates in hippocampus (Daws et al., 1999; Daws et al., 2000). Because this technique measures multiple mechanisms of clearance (Daws and Toney, 2007), these studies cannot definitively conclude that the effects of 5-HT1B antagonists on clearance are mediated by SERT. Also, because 5-HT1B receptors are expressed throughout the brain and act as both auto- and heteroreceptors (Morikawa et al., 2000), it is also difficult to attribute the observed effects to 5-HT1B autoreceptors. Second, certain behavioral effects of systemic 5-HT1B agonism are attenuated by pharmacological or genetic inactivation of SERT (Shanahan et al., 2009). While this study establishes that these effects of 5-HT1B drugs are mediated by SERT, it cannot be concluded whether the effects were due to direct 5-HT1B-SERT interactions or via other indirect mechanisms – for example, by activating 5-HT1B heteroreceptors on glutamatergic afferents to the dorsal raphe nucleus (DRN) (Lemos et al., 2006). Interpretation of both sets of studies is limited by the selectivity of the 5-HT1B ligands used, most of which are only partially selective (Stamford et al., 2000). Third, a study using molecular tools to selectively isolate 5-HT1B, cultured HEK cells co-transfected with SERT and 5-HT1B cDNAs displayed enhanced 5-HT uptake following 5-HT pre-treatment – presumably due to interactions between 5-HT1B and SERT (Xie et al., 2008). Finally, several studies suggest that 5-HT1B antagonists may be effective adjunctive therapies for depression (Artigas et al., 2001; Artigas et al., 1994; Hjorth et al., 2000). It is also noteworthy that two additional studies have demonstrated 5-HT1B autoreceptor regulation of SERT in pulmonary vasculature tissue (Lawrie et al., 2005; Morecroft et al., 2005).
The goal of the present study was to extend previous work—particularly that of Daws and colleagues (Daws et al., 1999; Daws et al., 2000)—and determine whether the 5-HT1B autoreceptor regulates SERT function in brain tissue. To specifically manipulate 5-HT1B autoreceptor function, we used a combination of pharmacologic and genetic approaches. To measure SERT-mediated 5-HT uptake, we used rotating disk electrode voltammetry (RDEV) in whole-brain and hippocampal synaptosomes. We have demonstrated that RDEV detects SERT activity with excellent kinetic resolution, sensitivity, and discrimination from other 5-HT clearance mechanisms (Hagan et al., 2010; Hagan et al., 2011). These are the first studies examining SERT function and kinetics in 5-HT1B knockout mice and the first studies using RDEV to look at brain region-specific SERT function (rat hippocampus). The specific aims of this study were to determine the extent to which SERT function could be decreased via pharmacologic blockade or genetic deletion of 5-HT1B autoreceptors, to determine the extent to which SERT function could be enhanced by pharmacologic activation or viral overexpression of 5-HT1B autoreceptors, and to determine whether the kinetic mechanism involved 5-HT1B effects on SERT Km or Vmax.
MATERIALS AND METHODS
Animals
5-HT1B autoreceptor knockout (−/−) mice were obtained from the Dr. Rene Hen at Columbia University and crossed onto the 129SvEvTac wild-type (+/+) background. All mouse experiments were carried out with synaptosomes isolated from adult male and female −/− and +/+ mice, in groups that were age-matched with littermate controls. No significant uptake differences between males and females were observed. Mice ranged in size from 25 – 30 grams and were between 3 – 6 months old at the time of experiments. Mice were group housed and fed ad libitum. Adult male Sprague-Dawley rats (Charles River Laboratories, Hollister, California) were housed two per cage with access to food and water available ad libitum. Rats weighed 275 to 350 g at the time of euthanasia. All rodents were kept in a temperature-controlled vivarium with a 12:12 light cycle (lights on 0600). Efforts were made to reduce the number of animals used and to minimize animal stress and discomfort during experimental procedures. Experiments were performed exactly as approved by the University of Washington Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Solutions and Chemicals
Solutions were made with water purified by a Millipore (Billerica, MA) Milli-Q ultrapurification system. The KCl and MgSO4 were purchased from Fisher Scientific (Pittsburg, PA). The NaCl, KH2PO4, NaHCO3, CaCl2, were purchased from JT Baker Chemical Co. (Philipsburg, NJ). The sucrose, glucose, HEPES, serotonin hydrochloride, paroxetine hydrochloride, GBR 12935, nisoxetine hydrochloride, and sumitriptan succinate were purchased from Sigma (St. Louis, MO). CP94253 was purchased from Tocris Bioscience (Bristol, UK). Drugs were diluted into physiological buffer, except 5-HT, SB224289, and CP94253. 5-HT was diluted in pH 7.2 phosphate buffered saline (PBS) to improve its stability (Huang and Kissinger, 1996). SB224289 was solubilized in DMSO and matching vehicle controls were prepared. The final percentage of DMSO in the assay was 0.0125%. CP94253 was diluted into water. The sucrose buffer contained 300 mM sucrose and 10 mM HEPES (pH 7.4). The physiological buffer contained 124 mM NaCl, 1.8 mM KCl, 1.3 mM MgSO4, 1.24 mM KH2PO4, 2.5 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose (saturated with 95% O2, 5% CO2 for at least 10 minutes before use, pH 7.4).
Rotating Disk Electrode Voltammetry
RDEV and synaptosome preparations were carried out as previously described (Hagan et al., 2010). Briefly, a Pine Instruments Inc. (Grove City, PA) AFMD03 glassy carbon electrode was connected to a Pine Instruments MSRX high-precision rotator and rotated at 3000 RPM in synaptosomes in a custom-made cylindrical glass chamber maintained at 37 °C by a Polyscience (Niles, IL) Series 8000 water recirculator. A constant potential of 550 mV relative to an Ag/AgCl reference electrode was applied with a Bioanalytical Systems LC-4B amperometric detector (West Lafayette, IN) with a laboratory-modified time constant (20 ms). Each experiment was performed on 0.5 mL of fresh synaptosomes to which exogenous 5-HT was added (more details in Uptake Studies section, below). While the electrode is not specifically selective for 5-HT we performed RDEV under conditions that optimized the sensitivity for 5-HT and after a stabilized baseline was reached. The measured current was exactly proportionate to the concentration of exogenously added 5-HT (Hagan et al., 2010). All experiments were performed in the presence of 100 nM nisoxetine and 1 μM GBR 12935 to minimize non-specific 5-HT uptake through the norepinephrine transporter (NET) and the dopamine transporter (DAT), respectively. Specific transport via SERT is accomplished using pharmacologic approaches that we have previously shown are highly specific to SERT (Hagan et al., 2010; Hagan et al., 2011). Detection currents were recorded digitally on a PC computer with an ITC-18 analog-digital converter and Ecell software from HEKA (formerly InstruTECH, Bellmore, New York). Data was acquired at a 1000 Hz sample rate with a 60 Hz digital notch filter. The data was converted to a waveform and background corrected for analysis using Igor Pro software from WaveMetrics (Portland, OR). Statistical analyses were performed with Graphpad Prism 5 (San Diego, CA).
Surgical Procedures
Surgical procedures were carried out as previously described (Clark et al., 2002). Rats were anesthetized with isoflurane (1–3%) and secured in a Stoelting stereotaxic device. Scalp fur was shaved and the underlying skin was disinfected with Betadine. The scalp was incised and bregma and lambda were visualized and used to guide injections. A small hole was bored at the site of injection. A 27-gauge needle was stereotaxically directed at an angle of 25° off midline towards the following coordinates: −8.3mm from bregma, midline, −7.2mm. These coordinates are ventral to those previously used for targeting caudal DRN alone (McDevitt et al., 2011); injection of virus at these coordinates was found to infect both caudal DRN and median raphe nucleus (MRN), which are raphe subregions that innervate the hippocampus (Leranth and Vertes, 1999; Vertes, 1991; Vertes et al., 1999).
Viral-Mediated Gene Overexpression
We used herpes simplex virus (HSV) viral vectors to express hemagglutinin-tagged 5-HT1B and green fluorescent protein (GFP) from separate transcription cassettes on the viral amplicon (HA1B/GFP) and compared these with GFP-only controls (Clark et al., 2002). These vectors are neuron-selective and peak expression is reached after about 3–4 days (Barot et al., 2007; Ferguson et al., 2008). Virally expressed HA1B receptors exhibit normal 5-HT1B function in vitro and in vivo (Clark et al., 2002; Clark et al., 2004; Riegert et al., 2008). HA1B/GFP and GFP amplicons were packaged into viral particles to a concentration of approximately 10^8 infective units/ml (Clark et al., 2002). Viral particles were loaded into the needle at a rate of 50 nl/s, flow was verified, and the needle was placed into the caudal DRN/MRN junction (coordinates above). Virus was injected at 200 nl/min using a microprocessor controlled pump (World Precision Instruments, Sarasota, FL). A volume of 2 μl of HA1B/GFP or GFP virus was injected (over 10 min), after which the needle was left in place for 5 min then withdrawn slowly over ~20 s. The skin was closed with sutures and rats monitored until recovery.
GFP was visualized after euthanasia to confirm delivery of virus to the targeted brain region. Midbrain tissue from rats was post-fixed at least 24 h in 4% paraformaldehyde. Infection of dorsal and median raphe nuclei was verified by observation of GFP fluorescence in 60 μm tissue sections as previously described (McDevitt et al., 2011).
Preparation of synaptosomes
Unanesthetized mice and rats were decapitated and brains (in mice) or hippocampi (in rats) were rapidly dissected out and homogenized in 10 volumes (w/v) of ice-cold sucrose buffer with a Potter Elvehjem homogenizer rotated at 540 RPM by a 10” Ryobi drill press. The whole-brain or hippocampal homogenate was centrifuged (1000 × g for 10 min at 4 °C) in an Eppendorf 5804R (Westbury, NY) to pellet and remove cellular debris (P1). The resulting supernatants were centrifuged (15,900 × g for 20 min at 4 °C) to obtain the crude synaptosomal pellet (P2). P2 was rinsed twice by resuspending in 15 mL physiological buffer pre-oxygenated with 95% O2, 5% CO2 gas, and recentrifuging (15,900 × g for 5 min at 4 °C) to remove any remaining sucrose. The rinsed P2 was resuspended in 5 mL oxygenated physiological buffer and maintained with 95% O2, 5% CO2 gas in a 50 mL polypropylene conical tube on ice. For rats, the P2 pellet was resuspended in 2.5 mL physiological buffer. The tissue was gently vortexed between assays before aliquoting tissue into the electrochemical chamber. Between assays, the glass chamber was gently wiped with a cotton-tipped applicator and rinsed with 70% ethanol followed by several (7–10) water rinses. The electrode was gently rinsed with 70% ethanol and water and rotated while in contact with a damp brown velvet electrode polishing pad from a Bioanalytical Systems electrode polishing kit (West Lafayette, IN).
Rats were sacrificed three days after surgery. Euthanasia was performed between 6AM and 11 AM (early in the light cycle), since previous work has suggested that autoreceptor vulnerability to desensitization may be enhanced in the mid-to late phase of the light cycle (Sayer et al., 1999). Midbrain tissue was dissected and postfixed in paraformaldehyde for GFP visualization.
[3H]-Citalopram Binding
Binding was performed on synaptosomes from RDEV experiments stored at −80 C following a modified protocol (Sexton et al., 1999). Synaptosomes were resuspended and sonicated in hypotonic Modified Krebs Buffer (MKB). The buffer contained 50 mM Tris, 5 mM KCl, and 120 mM NaCl, pH 7.4. Protein was quantified with the Pierce BCA kit and samples containing 25 μg of total protein were incubated in triplicate with 4 nM [3H]-citalopram with or without 1 μM paroxetine to define non-specific binding for an hour at room temperature. Bound radioactivity was separated from free radioactivity by vacuum filtration using a Unifilter-96 Perkin Elmer Harvester (Waltham, MA) on to 0.3% polyethylenimine (PEI) presoaked GF-C UniFilter-96 plates. Plates were dried overnight then read on a Packard Top-Count (Meriden, CT) microplate liquid scintillation counter after addition of 30 μL MicroScint-O cocktail (PerkinElmer; Waltham, MA) to each well.
Uptake Studies
For all experiments except the substrate-velocity kinetic analysis of 5-HT1B +/+ and −/− synaptosomes, 100 nM of 5-HT was added because it is close to the RDEV-measured Km for SERT (Hagan et al., 2010), and thus is highly sensitive to changes in SERT function. For 5-HT1B antagonist studies, 5-HT1B +/+ and −/− synaptosomes were tested with three concentrations of the 5-HT1B antagonist SB224289: 10 nM (low), 100 nM (medium), and 1000 nM (high). For agonist studies, in vitro and in vivo experiments were performed. The effect of 10 nM sumitriptan was investigated in vitro in wild-type and 5-HT1B −/− synaptosomes from male mice (all other mouse studies used both males and females). The effect of 1 mg/kg CP94253 given in vivo was investigated in wild-types, with injections given intraperitoneally 30 minutes before euthanasia. The selection of the agent and the dose was based on previous behavioral work from our lab (McDevitt et al., 2011). The substrate-velocity kinetic analysis involved addition of 30, 100, 300, 500, and 1000 nM 5-HT to 5-HT1B +/+ and −/− synaptosomes. All drugs were allowed to incubate with the synaptosomes during baseline acquisition for approximately 15 minutes prior to addition of 5-HT.
Data Analysis
In all studies, 1 μM paroxetine was used to define SERT function, as described in previous studies (Hagan et al., 2010; Hagan et al., 2011). Initial velocities of 5-HT uptake were calculated from the linear slope of a tangent line of the initial [steepest] portion of a plot of [5-HT] versus time as described previously (Hagan et al., 2010). Electrode drift was subtracted and data were normalized to protein concentration, which was quantified with a bicinchoninic acid (BCA) colorimetric based assay from Pierce (Rockford, IL). SERT function is expressed as its initial velocity measured in femtomoles of 5-HT uptake per second per milligram of protein (fmol 5-HT / (s * mg)). SERT function is calculated by subtracting the initial velocity in the presence of 1 μM paroxetine from the initial velocity in the presence of vehicle. Statistical analyses were performed with Graphpad Prism 5 (San Diego, CA), except where indicated otherwise.
In experiments comparing SERT clearance in 5-HT1B +/+ vs. −/− synaptosomes, SERT function was analyzed with a t-test. Serotonin clearance can vary significantly among individual animals. Synaptosomes from each animal are subjected to treatment with vehicle or drug; thus, each animal can serve as its own control. Normalization enables effects to be observed independent from the inter-animal variability in clearance velocities; however, many effects in these studies are robust and apparent without normalization of the data. Experiments testing the effects of drugs in vitro (e.g. SB224289, sumitriptan) tested four conditions: control (vehicle), drug, paroxetine, and drug+paroxetine. SERT function in the absence of drug was calculated as (initial velocity of control) – (initial velocity of paroxetine). Each individual animal produces a sufficient volume of synaptosomes (~5 mL, with 0.5 mL used for each assay) to test multiple drug conditions and serves as its own control for calculations of SERT function. SERT function in the presence of drug was calculated as (initial velocity of drug) – (initial velocity of drug+paroxetine). Each independent observation represents measurements from a single animal.
For the experiments testing low, medium, and high SB224289 on 5-HT1B +/+ vs. −/− synaptosomes, data were normalized to % of control and two analyses were performed: a two-way ANOVA with a Dunnett post-test, and a linear regression of initial rates as a function of the log of the drug concentration was performed in SPSS (Chicago, IL). An IC50 of SB224289 was also calculated. Studies with sumitriptan were analyzed by two-way ANOVA. SERT function was compared between the pre-treatment mouse studies with CP94253 and in rat studies by t-test. The Km and Vmax for SERT in 5-HT1B +/+ and −/− synaptosomes was determined using curve-fitting procedures to fit SERT uptake velocity versus 5-HT concentration to the Michaelis–Menten equation as described in our previous studies (Hagan et al., 2010). Photomicrographs were acquired on a Bio-Rad (Hercules, CA) Radiance 2000 confocal imaging system and were not altered other than cropping in Photoshop Elements from Adobe (San Jose, CA). Kinetic constants are reported as mean ± SER (standard error of regression). Other data are reported as the average + SEM, except for the substrate-velocity plot and IC50 calculation, which present data as mean ± SEM. The significance level was set at p<0.05.
RESULTS
Genetic deletion and pharmacologic blockade of 5-HT1B receptor both decrease SERT function
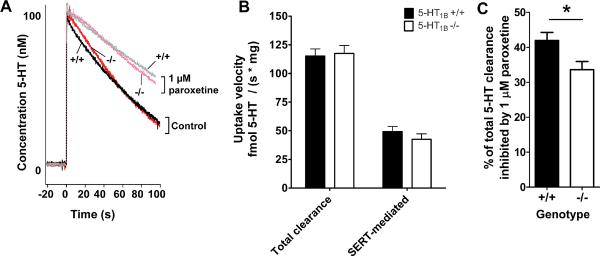
Initial rates of serotonin clearance were visibly more rapid in wild-type than in 5-HT1B −/− synaptosomes, as shown in the raw data traces in Figure 1A. While there was no significant effect for enhanced SERT-dependent uptake in wild-types synaptosomes over 5-HT1B −/−, when taken as an average (p = 0.380, Figure 1B), when the data are normalized for each individual animal's SERT uptake, a significant effect of genotype emerges (p = 0.0159, Figure 1C). SERT function varies considerably among individual animals, which can mask subtle alterations in function. The normalization of the data was intended to unmask any subtle difference in basal uptake rates between the genotypes.
Figure 1. SERT function is diminished in 5-HT1B −/− synaptosomes relative to +/+ controls.
A. Representative raw data traces from voltammetry experiments with wild-type and 5-HT1B −/− mice. Solid traces show uptake of 100 nM added 5-HT in +/+ (black) and −/− (red) synaptosomes. Dotted light grey and light pink traces show the effect of 1 μM paroxetine. The control trace in +/+ has enhanced SERT-mediated clearance relative to −/− synaptosomes during the linear phase (e.g. the initial rate) of uptake (approximately the first 10 – 15 seconds). There are no significant differences in total uptake rates between wild-type and 5-HT1B −/−, as shown in panel B. SERT function varies considerably across individuals, and while absolute values of SERT uptake rates do not differ significantly, when the data are normalized for each individual animal's SERT uptake, a modest but significant effect of genotype emerges, as shown in C (t-test, p = 0.0159); n = 20 wild-type and 19 5-HT1B −/− animals in both B and C. Data are expressed as mean + SEM, *p<0.05.
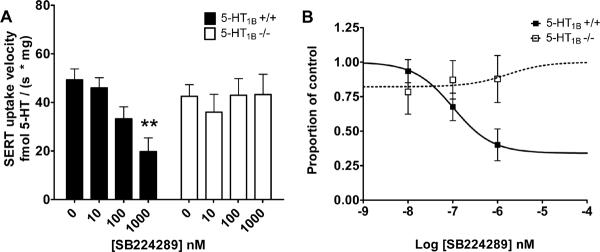
We next explored the sensitivity of 5-HT uptake in the presence or absence of a highly selective 5-HT1B antagonist, SB224289. Addition of SB224289 inhibited SERT function in a dose and genotype-dependent manner (Figure 2A). One-way ANOVA revealed a significant drug effect in wild-type synaptosomes (p = 0.0026), with a Dunnett post-test revealing the effect at the 1000 nM concentration of SB224289. The drug had no effect in 5-HT1B knockouts (p = 0.9610), indicating that the drug effect required the expression of 5-HT1B receptors. The IC50 for SB224289 was calculated in wild-type synaptosomes at 102 nM (95% confidence interval 20–524) (Figure 2B). A linear regression of SERT activity as a function of the log of the drug concentration showed the genotype effect was significant (p < 0.01) and that effect varies by drug dose in wild-type but not knockout synaptosomes (p<0.0001).
Figure 2. Pharmacologic antagonism of 5-HT1B autoreceptors decreases SERT function in +/+ but not −/− synaptosomes.
A. SERT function was measured by adding 100 nM 5-HT in the presence of low (10 nM), medium (100 nM) and high (1000 nM) concentrations of the 5-HT1B antagonist SB224289 in both 5-HT1B +/+ (wild-type) and 5-HT1B −/− synaptosomes. Data were analyzed by a one-way ANOVA with a post hoc Dunnett's multiple comparisons tests comparing each treatment to control; for wild-type p = 0.0026, for knockout, p = 0.9011. B. In a dose-response plot of SB224289 in +/+ vs. −/− synaptosomes, the IC50 was determined with a non-linear sigmoidal dose-response curve to be 102 nM (95% confidence interval 20–524 nM). The bottom of the curve was estimated at 0.34 ± 0.14. n = 6–7 per treatment.
Pharmacologic activation of 5-HT1B receptors with pre-treatment and viral overexpression of 5-HT1B autoreceptors both increase SERT function
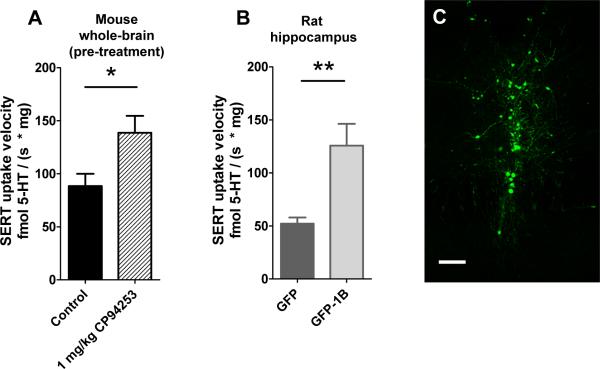
No enhancement of SERT function was observed with 10 nM sumitriptan given in vitro in either wild-type or knockouts. In wild-types, control and sumitriptan SERT initial velocities were, respectively, 111 ± 22 and 125 ± 27 fmol 5-HT / (s * mg); while, in knockouts, control and sumitriptan SERT initial velocities were, respectively, 102 ± 41; and 80 ± 41 (p=0.668, n = 4 per genotype). We reasoned that high levels of 5-HT exposure during synaptosome preparation may interact with 5-HT1B receptors and interfere with subsequent attempts to further stimulate these receptors with agonist in vitro; therefore, we adopted a 5-HT1B agonist pre-treatment strategy. Pre-treating mice with the selective, brain-penetrant 5-HT1B agonist CP94253 (1 mg/kg) significantly enhanced SERT function by 57% (p=0.028) (Figure 3A). To assess whether increased expression of 5-HT1B autoreceptors alters the extent of SERT regulation, we manipulated 5-HT1B autoreceptor expression by injecting a viral vector that expresses hemagglutinin-tagged 5-HT1B and GFP (HA1B/GFP) or a GFP-only control vector into caudal raphe, infecting both dorsal and median raphe, as previously described (Clark et al., 2002; Clark et al., 2004; McDevitt et al., 2011). We then compared SERT activity in hippocampal synaptosomes (an area that receives strong 5-HT innervation from the injected region of raphe) between groups of rats expressing the GFP-only control virus or the HA1B/GFP virus. SERT function was significantly increased in hippocampal synaptosomes from animals with increased 5-HT1B autoreceptor expression by 141% (p=0.008) (Figure 3B).
Figure 3. Pharmacologic activation and viral overexpression of 5-HT1B enhance SERT function.
A. Wild-type mice (n=14) pre-treated with 1 mg/kg CP94253 given intraperitoneally 30 minutes before euthanasia have increased SERT function relative to vehicle-treated controls (n=10). The uptake of 100 nM added 5-HT was measured. Data were analyzed by a t-test; p = 0.028. B. Overexpression of 5-HT1B receptors by viral mediated gene transfer enhances hippocampal SERT function in Sprague-Dawley rats (n = 5/group, 100 nM 5-HT added to experiments). C. Viral-mediated expression of green fluorescent protein in caudal dorsal raphe nucleus. Scale bar = 200 μm. Data were analyzed by a t-test; p = 0.008. Data are expressed as mean + SEM, *p < 0.05, **p < 0.01.
Neither deletion nor overexpression of 5-HT1B receptor change SERT binding
There were no differences in specific [3H]-citalopram binding to SERT between 5-HT1B +/+ and −/− mouse synaptosomes (600 ± 50 vs. 510 ± 60 counts per minute (cpm); p=0.2550) or between GFP and HA1B/GFP rat synaptosomes (190 ± 40 vs. 140 ± 60 cpm; p=0.5173), suggesting that the increased SERT activity in hippocampus from rats with increased 5-HT1B autoreceptors reflected a change in SERT function rather than SERT density.
Deletion of 5-HT1B receptor may impact SERT Km to a greater extent than Vmax
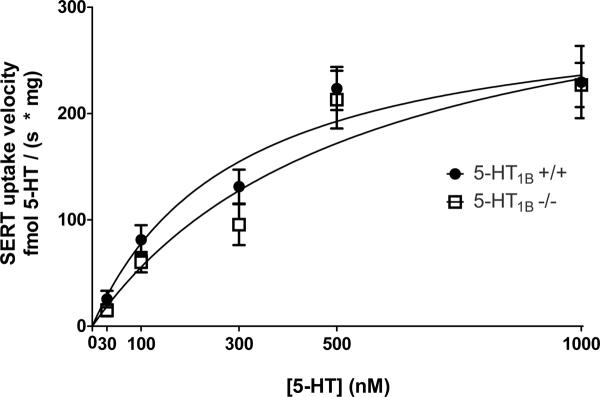
To assess the possible kinetic mechanism by which 5-HT1B autoreceptors regulate SERT activity, we examined SERT-mediated uptake over a range of concentrations of added 5-HT. Deletion of 5-HT1B induces a slight, though not statistically significant right-ward shift in the substrate-velocity plot of SERT function (Figure 4). A greater impact of the 5-HT1B–SERT interaction was observed at lower extracellular concentrations of 5-HT (Table 1),
Figure 4.
Substrate-velocity plot of SERT function in 5-HT1B −/− mice relative to +/+ (wild-type) controls. The rightward shift in the plot for 5-HT1B −/− mice suggests a reduction in Km but not Vmax. The Km in +/+ synaptosomes was 291 ± 141 nM and the Km in −/− synaptosomes was 548 ± 250 nM. The Vmax in +/+ synaptosomes was 305 ± 52 fmol 5-HT / (s * mg) and the Vmax in −/− synaptosomes was 361 ± 74 fmol 5-HT / (s * mg); n = 8–18 determinations per concentration per genotype. Data are shown on the graph as mean ± SEM; kinetic constants are presented as mean ± SER (standard error of regression).
Table 1.
SERT velocities in 5-HT1B +/+ and −/− synaptosomes (shown in Figure 4).
| 5-HT1B +/+ (wild-type) | 5-HT1B −/− (knockout) | |||
|---|---|---|---|---|
| [5-HT] nM | fmol 5-HT / (s * mg) | n | fmol 5-HT /(s * mg) | n |
| 30 | 26 ± 8 | 9 | 15 ± 6 | 8 |
| 100 | 81 ± 14 | 12 | 60 ± 10 | 12 |
| 300 | 131 ± 16 | 10 | 96 ± 19 | 10 |
| 500 | 224 ± 20 | 10 | 213 ± 27 | 9 |
| 1000 | 230 ± 34 | 17 | 227 ± 21 | 18 |
DISCUSSION
These experiments produced three major findings. First, SERT function in synaptosomes can be decreased by genetic deletion of 5-HT1B or by pharmacologic blockade of 5-HT1B autoreceptors. Second, SERT function can be enhanced by treatment of mice with the selective, brain penetrant 5-HT1B agonist just prior to sacrifice or by increasing 5-HT1B autoreceptor expression using viral-mediated gene transfer. Finally, 5-HT1B autoreceptors appear to increase SERT function by altering SERT Km rather than Vmax.
Several aspects of our experimental approach were selected to increase the sensitivity to detect changes in SERT function and to discern contributions of 5-HT1B autoreceptors. RDEV was used because of several advantages offered by this method, including decreased vulnerability to `fouling' by oxidized 5-HT products, precise control of analyte and drug concentrations, and improved sensitivity to differences in 5-HT uptake, such as the gene-dose effect between SERT +/+ and +/− synaptosomes, which cannot be detected using radioligand-based uptake assays (Hagan et al., 2010). While the gold standard for demonstrating biological relevance remains in vivo studies, RDEV can provide complementary information because the precise concentration of analytes in the assay (e.g., 5-HT and other ligands) are controlled. The use of a synaptosomal preparation favors the likelihood that 5-HT1B autoreceptors, as opposed to heteroreceptors, were involved since only synaptosomes formed from serotonergic neurons will express both 5-HT1B and SERT. Since it is very unlikely that any actions at 5-HT1B heteroreceptors on nonserotonergic synaptosomes could be transmitted to serotonergic synaptosomes where SERT resides, we infer that the data in these experiments involves 5-HT1B autoreceptors only. The addition of 100 nM 5-HT was used in assays because this concentration is close to the Km of SERT (99 ± 35 nM) as measured previously using rotating disk electrode voltammetry (Hagan et al., 2010).
SERT function differed between 5-HT1B +/+ and −/− synaptosomes. The raw data traces in Figure 1A show comparable slopes between the +/+ and −/− control traces over the initial linear portion of the uptake plot, though, in the traces shown, the uptake in the knockout “lags” slightly behind the wild-type trace. When SERT-mediated uptake was normalized to total uptake for each animal, a significant effect of 5-HT1B autoreceptors on SERT function was apparent. Total clearance did not differ between genotypes; thus, the data also suggest that non-SERT clearance mechanisms in 5-HT1B knockout animals were enhanced. Total clearance involves a combination of high affinity uptake (by SERT), low affinity, high capacity uptake transporters including the extraneuronal monoamine transporter (EMT), the plasmalemmal monoamine transporter (PMAT) and the organocation transporters—OCT1, OCT2, and OCT3 (Baganz et al., 2008; Feng et al., 2005; Gasser et al., 2006; Schmitt et al., 2003). Contributions from the dopamine and norepinephrine transporters (DAT and NET, respectively) can be excluded because experiments were performed in the presence of 1 μM GBR 12935 (a DAT blocker) and 100 nM nisoxetine (a NET blocker). The contributions of SERT and non-SERT uptake has been addressed in our previous work (Hagan et al., 2011) and OCT3, in particular, may be an interesting mechanism since it has been demonstrated that its function can be altered by stress (Baganz et al., 2010; Baganz et al., 2008; Gasser et al., 2006). Although the focus of this study was the regulation of SERT by 5-HT1B autoreceptors, future studies should address the role of 5-HT1B regulation of non-SERT transport mechanisms. These data also suggests that endogenous 5-HT1B autoreceptor tone may influence SERT function under basal conditions.
SB224289 exhibited drug dose and genotype-dependent inhibition of SERT function (Figure 2A). The IC50 calculated in wild-type synaptosomes was 102 nM (Figure 2B), which is very close to the IC50 previously reported for regulation of [3H]-5-HT release from rat striatal synaptosomes, 62 nM (Sarhan and Fillion, 1999). These findings clarify the results of previous studies showing that 5-HT1B antagonists can reduce serotonin uptake activity via 5-HT1B receptor inhibition. Daws and colleagues used in vivo chronoamperometry to observe that cyanopindolol inhibited 5-HT clearance in the CA3 hippocampal area and that this inhibition was additive with fluvoxamine at non-saturating concentrations (Daws et al., 2000). 5-HT-moduline, an allosteric 5-HT1B modulator, and methiothepin, a nonselective antagonist, also inhibited clearance (Daws and Toney, 2007). While these studies suggest a possible 5-HT1B – SERT interaction, a few methodological limitations prevented acquisition of definitive evidence that the observed 5-HT clearance decreases were due to 5-HT1B autoreceptor effects on SERT. First, most of the drugs used are only partially selective for 5-HT1B, a problem compounded by the fact that microinjection of drugs into brain tissue does not result in uniform concentrations of drug throughout the region of interest. Second, because in vivo chronoamperometry requires the local infusion of test ligands into tissue, there is some uncertainty regarding the site of action (e.g. 5-HT1B autoreceptors, heteroreceptors or other receptors). Finally, with in vivo chronoamperometry, 5-HT clearance is comprised of diffusion of injected 5-HT away from the electrode as well as multiple biological mechanisms of uptake. These studies resolve some of these issues by using a very selective antagonist, SB224289 (Gaster et al., 1998; Selkirk et al., 1998), at known concentrations, using genetic approaches including the 5-HT1B knockout, and by using RDEV and synaptosomes, the advantages of which are described above. The absence of an effect of SB224289 in 5-HT1B knockout mice even at very high concentrations confirms that 5-HT1D autoreceptors were not involved; thus, the data support the conclusion that 5-HT1B receptors are the primary form of serotonergic terminal autoreceptor found in mouse brain (Trillat et al., 1997).
The 5-HT1B agonist sumitriptan did not enhance SERT function in the in vitro studies. We used 100 nM 5-HT as the substrate for SERT-mediated reuptake, which could also strongly activate 5-HT1B autoreceptors (KD ~10 nM), and would be expected to be a confound if the receptor response time is on the order of seconds, since the measure of initial uptake rate occurs within the first 10–20 seconds following addition of 5-HT. A SERT substrate that could be measured by RDEV but does not activate 5-HT1B receptors would be ideal; however, one has not yet been identified. It is possible that endogenous 5-HT in the synaptosome preparations activates 5-HT1B autoreceptors, inducing a “ceiling effect” limiting additional receptor activation; however, this seems unlikely because the endogenous 5-HT was expected to have been significantly eliminated during multiple rinsings of the P2 pellet. It is unclear whether a biologic factor was masking the potential effects of any additional 5-HT1B agonist on SERT function, or whether we merely failed to identify an effective combination of agonist, concentration, and duration of treatment for observing effects on SERT function.
We recently circumvented a similar complication in measuring kappa opioid receptor regulation of SERT activity with RDEV. We found that the selective kappa agonist, U50,488 was effective at increasing SERT function when given prior to sacrifice but not directly in the in vitro RDEV assay (Bruchas et al., 2011). Therefore, we pre-treated mice with the 5-HT1B agonist CP94253 using a moderate dose that appears to be optimal for preferentially activating 5-HT1B autoreceptors (McDevitt et al., 2011; Sarhan et al., 1999). Indeed, enhanced SERT function was detectable in mice pre-treated with 1 mg/kg CP94253 (Figure 3A). Because this experimental design does not fully discriminate potential contributions of 5-HT1B auto- vs. heteroreceptors, we addressed this issue using a viral overexpression paradigm to test the hypothesis that increased 5-HT1B autoreceptors in serotonin terminals in the forebrain could increase SERT function.
We injected the viral vectors in a subregion of dorsal raphe known to innervate the hippocampus (Leranth and Vertes, 1999; Vertes, 1991; Vertes et al., 1999); the hippocampus was chosen because it could be discretely dissected and has significant serotonergic innervation and hence was a good source for synaptosome preparation. Recently we showed that 5-HT1B overexpression using the same viral vector injected using the same procedure into the same target has a significant impact on conditioned fear responses (McDevitt et al., 2011). Indeed, SERT activity in hippocampal synaptosomes after viral overexpression of 5-HT1B autoreceptors in caudal raphe increased nearly two-fold compared with GFP-only controls (Figure 3B). Though previous work has demonstrated that cultured HEK-293 cells transfected with 5-HT1B cDNA have enhanced 5-HT uptake over control (Xie et al., 2008), this study is the first demonstration that increased expression of 5-HT1B autoreceptors can enhance SERT function in brain tissue; this may have physiological implications since 5-HT1B autoreceptor expression is quite variable among individuals and this has behavioral impacts on stress reactivity (Kaiyala et al., 2003; Neumaier et al., 2002).
Two mechanisms that have previously been shown to regulate SERT activity include altering SERT trafficking to the synaptic membrane surface, which would produce a change in SERT Vmax, and increasing SERT's catalytic activity, which would produce a change in Km (Steiner et al., 2008). Our data do not rule out either as a possible mechanism. A more detailed kinetic analysis will be required in a future study. The changes in SERT activity observed in the present experiments do not appear to be due to differences in the amount of total SERT protein in the whole-brain synaptosomal preparations, as no significant differences in radioligand binding were observed between the experimental and control groups in either the rat or mouse samples. Estimates of the kinetic constants for SERT as measured in 5-HT1B −/− and wild-type synaptosomes suggest that Km may be altered to a greater extent than Vmax in knockouts (Figure 4), though there is not a statistically significant difference in either. Additionally, while Figure 4 should reflect SERT function, the SERT signal is subject to more noise with increasing added 5-HT; it is possible that non-SERT mechanisms of clearance may interfere the signal, particularly at higher concentrations of added 5-HT, where these mechanisms predominate in mediating uptake (Hagan et al., 2011).
This is the first study demonstrating that increases and decreases in 5-HT1B autoreceptor expression levels in brain tissue can increase or decrease SERT activity, respectively. Bidirectional regulation of SERT by 5-HT1B autoreceptors is an important finding, since both natural and experimental variations in 5-HT1B expression in dorsal raphe are associated with altered stress reactivity (Clark et al., 2002; Clark et al., 2004; Kaiyala et al., 2003; McDevitt et al., 2011; Neumaier et al., 2002). This is especially intriguing in the context of the relationship between environmental stress and SERT genotype, which has been implicated in altering the vulnerability to depression (Caspi et al., 2003; Daws and Gould, 2011). We have recently observed that p38 map kinase is involved in stress-mediated regulation of SERT activity (Bruchas et al., 2011). Moreover, p38 map kinase has the ability to catalytically activate SERT (Zhu et al., 2005). Perhaps the impact of 5-HT1B autoreceptors on stress vulnerability is mediated, at least in part, by an interaction with 5-HT reuptake. Additionally, a direct interaction could exist between 5-HT1B autoreceptors and SERT. These are possibilities to explore in future investigations.
In summary, SERT function can be dynamically influenced by increases or decreases in 5-HT1B autoreceptor activity. We found that 5-HT1B autoreceptors regulate SERT catalytic activity (Km). The molecular interactions between 5-HT1B autoreceptors and SERT may be an important mechanism by which serotonin neurotransmission is regulated in basal and high-stress circumstances and may provide a novel basis for synergistic pharmacological treatment of psychiatric disorders.
Acknowledgements
The authors sincerely thank Dr. Jim Schenk, Dr. Nicole Bjorklund, Dr. Michele Kelly, and Dr. Scott Ng-Evans for their feedback and technical advice, and Ms. Hannah DeMeritt for her technical assistance. We also thank Dr. Nicholas Poolos and Dr. Diane Lattemann for loaning equipment for these studies and Dr. Rene Hen for the 5-HT1B knockout mice. The authors also give a special thank you to Dr. Denny Liggitt and the University of Washington's Department of Comparative Medicine for supporting this work. This work was supported by NIH grants R01 MH63303, T32 RR07019, and K01 RR024471.
Footnotes
Present address: Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, USA.
The authors declare no conflicts of interest.
REFERENCES
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51(1–2):87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Artigas F, Celada P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends Pharmacol Sci. 2001;22(5):224–228. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51(3):248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci. 2010;30(45):15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105(48):18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25(10):3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44(3):169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71(3):498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clark MS, Neumaier JF. The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull. 2001;35(4):170–185. [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22(11):4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007(1–2):86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121(1):89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Gerhardt GA, Frazer A. 5-HT1B antagonists modulate clearance of extracellular serotonin in rat hippocampus. Neurosci Lett. 1999;266(3):165–168. doi: 10.1016/s0304-3940(99)00277-3. [DOI] [PubMed] [Google Scholar]
- Daws LC, Gould GG. Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther. 2011;131(1):61–79. doi: 10.1016/j.pharmthera.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75(5):2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- Daws LC, Toney GM. High-speed chronoamperometry to study kinetics and mechanisms for serotonin clearance in vivo. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. CRC Press; Boca Raton, FL: 2007. pp. 63–81. [PubMed] [Google Scholar]
- Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063(1):69–76. doi: 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF. Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry. 2008;63(2):207–213. doi: 10.1016/j.biopsych.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Gardier AM. Mutant mouse models and antidepressant drug research: focus on serotonin and brain-derived neurotrophic factor. Behav Pharmacol. 2009;20(1):18–32. doi: 10.1097/FBP.0b013e3283243fcd. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26(34):8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaster LM, Ham P, Joiner GF, King FD, Mulholland KR, Wyman PA, Hagan JJ, Price GW, Roberts C, Routledge C, Selkirk J, Slade PD, Middlemiss DN. The selective 5-HT1B receptor inverse agonist SB-224289, potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo. Ann N Y Acad Sci. 1998;861:270–271. doi: 10.1111/j.1749-6632.1998.tb10219.x. [DOI] [PubMed] [Google Scholar]
- Hagan CE, Neumaier JF, Schenk JO. Rotating disk electrode voltammetric measurements of serotonin transporter kinetics in synaptosomes. J Neurosci Methods. 2010;193(1):29–38. doi: 10.1016/j.jneumeth.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CE, Schenk JO, Neumaier JF. The contribution of low-affinity transport mechanisms to serotonin clearance in synaptosomes. Synapse. 2011;65(10):1015–1023. doi: 10.1002/syn.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol. 2000;14(2):177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19(3):170–176. doi: 10.1002/syn.890190304. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Middlemiss DN. Species differences in the pharmacology of terminal 5-HT autoreceptors in mammalian brain. Trends Pharmacol Sci. 1989;10(4):130–132. doi: 10.1016/0165-6147(89)90159-4. [DOI] [PubMed] [Google Scholar]
- Huang T, Kissinger PT. Liquid chromatographic determination of serotonin in homogenized dog intestine and rat brain tissue using a 2 mm i.d. PEEK Column. Current Separations. 1996;14(3/4):114–119. [Google Scholar]
- Iversen L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala KJ, Vincow ES, Sexton TJ, Neumaier JF. 5-HT1B receptor mRNA levels in dorsal raphe nucleus: inverse association with anxiety behavior in the elevated plus maze. Pharmacol Biochem Behav. 2003;75(4):769–776. doi: 10.1016/s0091-3057(03)00152-7. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, Harmar AJ, Schmidt AM, Lukanidin E, Rabinovitch M. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res. 2005;97(3):227–235. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Pan YZ, Ma X, Lamy C, Akanwa AC, Beck SG. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci. 2006;24(12):3415–3430. doi: 10.1111/j.1460-9568.2006.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Vertes RP. Median raphe serotonergic innervation of medial septum/diagonal band of broca (MSDB) parvalbumin-containing neurons: possible involvement of the MSDB in the desynchronization of the hippocampal EEG. J Comp Neurol. 1999;410(4):586–598. [PubMed] [Google Scholar]
- McDevitt RA, Hiroi R, Mackenzie SM, Robin NC, Cohn A, Kim JJ, Neumaier JF. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol Psychiatry. 2011;69(8):780–787. doi: 10.1016/j.biopsych.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, Neumaier JF. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: A behavioral perspective. J Chem Neuroanat. 2011;41(4):234–246. doi: 10.1016/j.jchemneu.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, Sheward J, Harmar A, MacLean MR. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther. 2005;313(2):539–548. doi: 10.1124/jpet.104.081182. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol. 2000;58(6):1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mrna regulation in two animal models of altered stress reactivity. Biol Psychiatry. 2002;51(11):902–908. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40(3):537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417(2):181–194. [PubMed] [Google Scholar]
- Riegert C, Rothmaier AK, Leemhuis J, Sexton TJ, Neumaier JF, Cassel JC, Jackisch R. Increased expression of 5-HT(1B) receptors by Herpes simplex virus gene transfer in septal neurons: New in vitro and in vivo models to study 5-HT(1B) receptor function. Brain Res Bull. 2008;76(4):439–453. doi: 10.1016/j.brainresbull.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G. Serotonin transporters--structure and function. J Membr Biol. 2006;213(2):101–110. doi: 10.1007/s00232-006-0878-4. [DOI] [PubMed] [Google Scholar]
- Sarhan H, Cloez-Tayarani I, Massot O, Fillion MP, Fillion G. 5-HT1B receptors modulate release of [3H]dopamine from rat striatal synaptosomes. Naunyn Schmiedebergs Arch Pharmacol. 1999;359(1):40–47. doi: 10.1007/pl00005321. [DOI] [PubMed] [Google Scholar]
- Sarhan H, Fillion G. Differential sensitivity of 5-HT1B auto and heteroreceptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;360(4):382–390. doi: 10.1007/s002109900067. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28(6):565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sayer TJ, Hannon SD, Redfern PH, Martin KF. Diurnal variation in 5-HT1B autoreceptor function in the anterior hypothalamus in vivo: effect of chronic antidepressant drug treatment. Br J Pharmacol. 1999;126(8):1777–1784. doi: 10.1038/sj.bjp.0702535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Chen JP, Gardner E, Hen R. 5-HT receptor knockout mice: pharmacological tools or models of psychiatric disorders. Ann N Y Acad Sci. 1999;868:701–715. doi: 10.1111/j.1749-6632.1999.tb11350.x. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71(5):701–709. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Scott C, Ho M, Burton MJ, Watson J, Gaster LM, Collin L, Jones BJ, Middlemiss DN, Price GW. SB-224289--a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br J Pharmacol. 1998;125(1):202–208. doi: 10.1038/sj.bjp.0702059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton TJ, McEvoy C, Neumaier JF. (+) 3,4-methylenedioxymethamphetamine ('ecstasy') transiently increases striatal 5-HT1B binding sites without altering 5-HT1B mRNA in rat brain. Mol Psychiatry. 1999;4(6):572–579. doi: 10.1038/sj.mp.4000574. [DOI] [PubMed] [Google Scholar]
- Shanahan NA, Holick Pierz KA, Masten VL, Waeber C, Ansorge M, Gingrich JA, Geyer MA, Hen R, Dulawa SC. Chronic reductions in serotonin transporter function prevent 5-HT1B-induced behavioral effects in mice. Biol Psychiatry. 2009;65(5):401–408. doi: 10.1016/j.biopsych.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Davidson C, McLaughlin DP, Hopwood SE. Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: parallel purposes or pointless plurality? Trends Neurosci. 2000;23(10):459–465. doi: 10.1016/s0166-2236(00)01631-3. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and - independent regulation of serotonin transport. Traffic. 2008;9(9):1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Edwards RH. Antidepressants and the monoamine masquerade. Neuron. 2005;46(1):1–2. doi: 10.1016/j.neuron.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Trillat AC, Malagie I, Scearce K, Pons D, Anmella MC, Jacquot C, Hen R, Gardier AM. Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: in vivo microdialysis studies. J Neurochem. 1997;69(5):2019–2025. doi: 10.1046/j.1471-4159.1997.69052019.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313(4):643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407(4):555–582. [PubMed] [Google Scholar]
- White KJ, Walline CC, Barker EL. Serotonin transporters: implications for antidepressant drug development. AAPS J. 2005;7(2):E421–433. doi: 10.1208/aapsj070242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Miller GM. Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes. J Pharmacol Exp Ther. 2008;325(2):629–640. doi: 10.1124/jpet.107.135079. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31(10):2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280(16):15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35(13):2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Steiner JA, Munn JL, Daws LC, Hewlett WA, Blakely RD. Rapid stimulation of presynaptic serotonin transport by A3 adenosine receptors. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]