Abstract
In normal and diseased vascular smooth muscle (SM), the RhoA pathway, which is activated by multiple agonists through G protein-coupled receptors (GPCRs), plays a central role in regulating basal tone and peripheral resistance. Multiple RhoA GTP exchange factors (GEFs) are expressed in SM raising the possibility that specific agonists coupled to specific GPCRs may couple to distinct RhoGEFs and provide novel therapeutic targets. This review will focus on the function and mechanisms of activation of p63RhoGEF (Arhgef 25; GEFT) recently identified in SM and its possible role in selective targeting of RhoA-mediated regulation of basal blood pressure through agonists that couple through Gαq/11.
Physiology and Pathophysiology of RhoA signaling in smooth muscle
Smooth muscle (SM) cell contraction and motility is activated when Ser19 of the myosin regulatory light chain (RLC20) is phosphorylated by the calcium-calmodulin dependent myosin light chain kinase (MLCK) resulting in an increase in actin-activated ATPase activity and crossbridge cycling. Relaxation occurs upon Ser19 dephosphorylation by myosin light chain phosphatase (MLCP) consisting of a regulatory subunit (MYPT1), a catalytic subunit (PP1C) and a small subunit (M21) implicated in microtubule dynamics and possibly contraction (Takizawa et al. 2003; Zhou et al. 1999). Thus, the contractile state reflects the ratio of activities of MLCK to MLCP. A second level of regulation is through G protein coupled receptor (GPCR) activation of other kinases that phosphorylate and modulate the activities of MLCK and MLCP. For example, Ca2+-sensitization occurs through the activation of the small GTPase, RhoA and its effector Rho kinase (ROCK). ROCK phosphorylates and inhibits MLCP activity resulting in increased phosphorylation of RLC20 in the presence of either basal Ca2+ or increased MLCK activity (Somlyo and Somlyo 2003). Aberrations of this signaling pathway occur in hypertension, asthma, cerebral vasospasm, and in some dysfunctional gastrointestinal diseases (de Godoy and Rattan 2011; Gohla et al. 2000; Loirand and Pacaud 2010; Moriki et al. 2004; Seasholtz and Brown 2004; Seko et al. 2003; Uehata et al. 1997). ROCK inhibitors have played a major role in defining the contribution of RhoA/ROCK signaling under normal physiological conditions and in these disease states (Uehata et al. 1997). More recently RhoA GTP exchange factors (GEFs) are being identified in SM, opening up a new and exciting opportunity for selective targeting of specific RhoA functions. This review will focus on a recently identified RhoGEF, p63RhoGEF (Arhgef 25; GEFT) and its possible role in selective targeting of RhoA-mediated regulation of blood pressure through agonists that couple through Gαq/11.
RhoGEFs
RhoGEFs up-regulate RhoA activity by catalyzing the exchange of GTP for GDP in response to upstream receptor signaling. The conformation induced by small GTPases when bound to the GEF Dbl homology (DH) domain results in loss of bound GDP and Mg2+, allowing subsequent binding of the more abundant GTP in the cell, thus driving nucleotide exchange. There are ~70 GEFs in the human genome; some have demonstrated or potential activities towards RhoA. An intriguing question is which of these RhoGEFs are present and functional in SM and why are there so many? Accumulating evidence suggests that different agonists through different GPCRs may select for different RhoA mediated end points such as salt- or non-salt-induced hypertension or basal blood pressure (Guilluy et al. 2010; Momotani et al. 2011; Wirth et al. 2007).
p63RhoGEF, the focus of this review, is a RhoGEF found to be the most highly expressed in an mRNA screen for 20 RhoGEFs in mouse portal vein. It was also detected in large arteries and in small resistance vessels involved in regulation of blood pressure, with its protein-level expression confirmed in a range of blood vessels and gastrointestinal SM (Momotani et al. 2011). Of note, amino acid sequences of p63RhoGEF across species are highly conserved with different amino-termini giving potential size differences (580–619 amino acids). The human isoform, used in the majority of studies, consists of 580 amino acids (aa) (e.g. NCBI Reference Sequence: NP_891992), a longer human p63RhoGEF isoform (predicted) has 619 aa (NCBI Reference Sequence: NP_001104740), while the mouse has 618 aa (GenBank: AAO49464) and the rat 613 aa (GenBank: EDM16490). For example, residue 1–32 of human p63RhoGEF is different from residue 1–72 of mouse p63RhoGEF suggesting amino-terminal splice variants, while the downstream sequences are highly conserved. The mouse and human long isoforms do not have the cysteine residues crucial for palmitoylation and membrane localization. To date, no in-depth profiling of expression patterns of these isoforms have been done.
Mechanism of Regulation of p63RhoGEF
p63RhoGEF has been shown to have specific activity towards RhoA but not Rac1, Cdc42 or RhoG. This was first demonstrated by induction of RhoA-mediated stress fiber formation upon over-expression of p63RhoGEF (Lutz et al. 2004; Souchet et al. 2002). Based on sequence homology, p63RhoGEF belongs to the Dbl protein family of RhoGEFs having the typical tandem DH and pleckstrin homology (PH) domains but lacking other functional domains. For example, p63RhoGEF, unlike the well known regulator of G-protein signaling (RGS) family of RhoGEFs, p115RhoGEF, leukemia- associated RhoGEF (LARG) and PDZ-RhoGEF, lacks this RGS domain that interacts with Gα12/13 resulting in GTP exchange and activation of RhoA (Lutz et al. 2004; Lutz et al. 2005; Rojas et al. 2007). Importantly, studies using selective expression of Gα subunits have demonstrated that Gαq/11 subunits could activate RhoA independently of Gα12/13 activation (Barnes et al. 2005; Chikumi et al. 2002; Dutt et al. 2002; Katoh et al. 1998; Vogt et al. 2003) but a specific Gαq/11 activated RhoGEF was unknown at that time. Subsequently, p63RhoGEF was shown to be activated by direct protein-protein interaction with Gαq, thus selectively linking Gαq/11-coupled receptors to RhoA activation (Lutz et al. 2005). Furthermore, Gαq activation of p63RhoGEF was shown to occur independently of its well-known effector, phospholipase C β (PLCβ) (Lutz et al. 2005; Vogt et al. 2003). On the other hand, p63RhoGEF and PLCβ were shown to compete for binding to Gαq/11. This was based on the finding that carbachol/Gαq/RhoA mediated gene transcription was suppressed upon co-expression of p63RhoGEF and PLCβ (Lutz et al. 2005; Vogt et al. 2003). Subsequently, “shielding” of active Gαq from PLC by p63RhoGEF has been proposed based on prolonged dissociation of GTP-bound Gαq from its heterotrimeric partners Gβ and Gγ subunits in the presence of over-expressed p63RhoGEF following stimulation by histamine (Adjobo- Hermans et al. 2011). p63RhoGEF has also been shown to interact with another Gαq family member, Gα16 and to compete with PLCβ2 for binding to Gα16 (Yeung and Wong 2010). Although it is likely that PLCβ and p63RhoGEF are both activated with physiological stimuli in SM as increases in RhoA activity and Ca2+ occur through receptors coupled to Gαq family members, these findings further demonstrate the intriguing possibility for regulation of downstream signaling through competition of effectors for activated Gαq. As p63RhoGEF is palmitoylated at cysteine residues close to the amino-terminus and localized to the plasma membrane (Aittaleb et al. 2011) its concentration and localization relative to membrane associated GPCRs, Gαq/11 and PLCβ will impact this competition for Gαq. Whether these cysteine residues and thereby this localization mechanism are conserved across the different isoforms in different species awaits analysis of their amino-termini and determination of expression levels of the long p63RhoGEF isoforms lacking cysteine residues.
The regulatory mechanism of p63RhoGEF through autoinhibition was initially suggested based on the observation that over-expression of p63RhoGEF lacking the PH domain showed enhanced RhoA mediated-gene transcription activity compared to that of full-length p63RhoGEF (Lutz et al. 2004). Later an extension of the DH-associated PH domain was shown to play an important role in the “off state” of p63RhoGEF. In this case activated Gαq relieved the basal autoinhibitory state of p63RhoGEF by interacting with a highly conserved carboxyl-terminal extension of the PH domain (Rojas et al. 2007). The exchange activity of the DH domain alone was approximately 10-fold greater than that of the DH-PH constructs with carboxyl-terminal extension showing that the PH domain negatively regulates the exchange activity of p63RhoGEF. In vitro assays using active Gαq subunits demonstrated a 26-fold stimulation of exchange activity of autoinhibited DH-PH extended protein compared with the spontaneous exchange rate of RhoA alone (Rojas et al. 2007). Importantly, this activation was specific for Gαq when compared with other Gα subunits (GαI, Gαo, Gαs, Gαt, Gα13). The potential importance of the autoinhibitory mechanism in the regulation of vascular SM contractility was later shown by suppression of agonist-induced Ca2+-sensitized force upon introduction of recombinant PH domain to permeabilized blood vessels (Momotani et al. 2011).
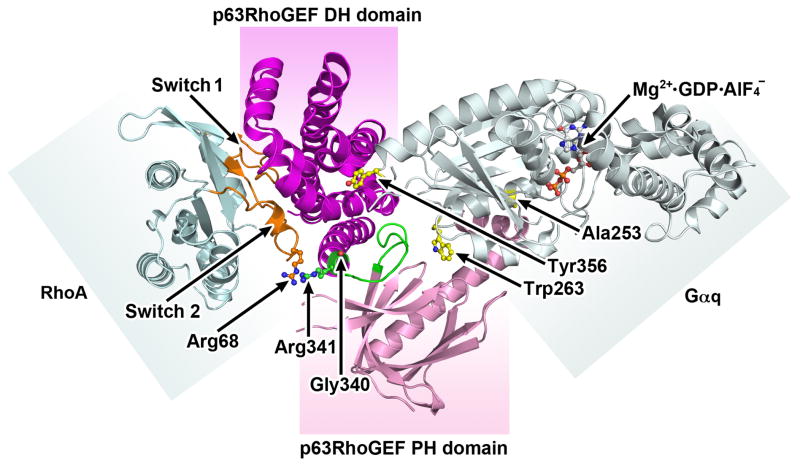
Biochemical data using purified and mutated proteins, and crystal structure analysis of a complex of p63RhoGEF with activated Gαq and RhoA (Fig. 1) have provided important insights. They show activation occurring through an allosteric mechanism, and the importance of the α6-αN linker connecting the DH and PH domain functioning as a conformational switch (green in Fig. 1) (Lutz et al. 2007; Shankaranarayanan et al. 2010). In the autoinhibited state, the conformation of the α6-αN linker is influenced by residues Gly340 and Arg341. These residues do not interact with RhoA nor do they contribute to the mechanism of activation by Gαq. In the basal state the conformation of the PH domain prevents RhoA from binding but the exact conformation has not been solved. In the active state, Gαq engages both the DH and PH domains (Fig. 1) altering the conformation of the α6-αN linker to favor nucleotide exchange but does not engage RhoA. Mutation of residues Ala253 and Trp263 in Gαq at an interface that contacts the PH domain leads to the loss of the ability of Gαq to activate p63RhoGEF. These residues are thought to constitute the selectivity of p63RhoGEF for Gαq. Tyr356 is another critical residue in Gαq that interfaces with the DH domain (Lutz et al. 2007; Rojas et al. 2007; Shankaranarayanan et al. 2010)) and when mutated results in loss of activation of p63RhoGEF ((Lutz et al. 2007; Rojas et al. 2007; Shankaranarayanan et al. 2010). Altogether, these authors propose that the p63RhoGEF α6-αN linker, when activated forms direct contacts with RhoA acting as a conformational switch with different residues controlling the autoinhibitory mechanisms and the activation by Gαq. Nonetheless, the exact molecular mechanism underlying the activation of GEF activity remains to be determined as a basal state structure of p63RhoGEF has not been solved.
Fig. 1.
Crystal structure of Gαq-p63RhoGEF-RhoA complex (PDB ID:2RGN) showing how Gαq allosterically activates and relieves autoinhibition of p63RhoGEF. In this structure Gαq •GDP•AlF-4 allosterically relieves the autoinhibition of the catalytic DH domain by the PH domain. The DH and PH domains are colored purple and pink respectively. The α6-αN linker (shown in green) between the DH and PH domain acts as a conformational switch with different residues controlling the autoinhibitory mechanisms and the activation by Gαq. RhoA is shown in blue with gold switch 1 and switch 2 regions. Gαq is colored grey with Mg2+•GDP•AlF-4 in the active site shown as a ball and stick model. Residues Ala253 and Trp263 in Gαq at an interface that contacts the PH domain are critical for the ability of Gαq to activate p63RhoGEF. These residues contribute to the selectivity of p63RhoGEF. Tyr356 is another critical residue in Gαq that interfaces with the DH domain (Lutz et al. 2007; Rojas et al. 2007; Shankaranarayanan et al. 2010)) and when mutated results in loss of activation of p63RhoGEF. The relative positions of Arg341 and Gly340, in the α6-αN linker region are necessary for the basal inhibited state and interact with Arg68 in switch 2 of RhoA. Modified from (Shankaranarayanan et al. 2010).
Role of p63RhoGEF in smooth muscle
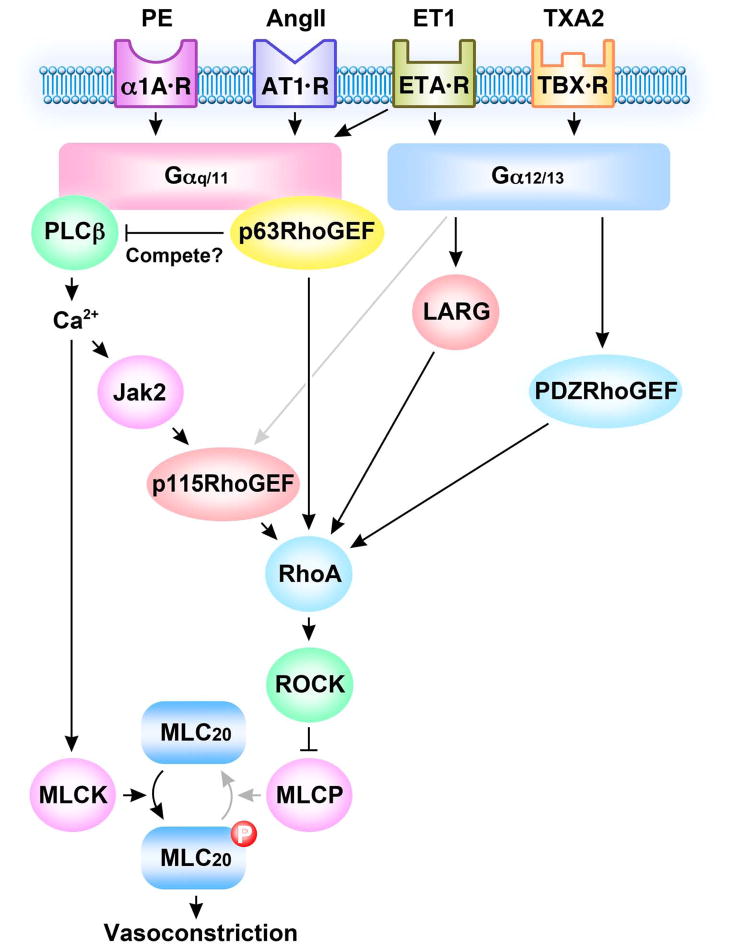
Two recent studies that build on the strong foundation established through in vitro biochemical, crystallographic and over-expression studies now establish a physiological function for p63RhoGEF in cultured aortic SM cells and in blood vessels (Momotani et al. 2011; Wuertz et al. 2011). In these studies, involvement of p63RhoGEF in Gαq/11-mediated SM contraction induced by agonists, such as phenylephrine, angiotensin II (AngII), and endothelin-1 (ET-1), has been demonstrated. In view of the importance of AngII in hypertension and vascular remodeling (Cat and Touyz 2011), Wuertz and colleagues focused on AngII showing that it selectively couples through Gαq/11 rather than Gα12/13 to activate RhoA (but not Rac1). This is in keeping with earlier studies showing that AngII was unable to contract the aorta of Gαq-Gα11 deficient mice but gave near full contraction in the absence of Gα12-Gα13 (Wirth et al. 2008). Loss of function studies through suppression of Gαq/11 or p63RhoGEF in early passaged cultured rat aortic SM cells markedly decreased AngII-induced RhoA activation, cell proliferation, stress fiber formation and contraction of collagen gels embedded with aortic SM cells in which p63RhoGEF was knocked down by approximately 67% (Wuertz et al. 2011). Interestingly, not every agonist that signals through Gαq/11 to increase RhoA is mediated by p63RhoGEF, as sphingosine-1-phosphate (S1P)-induced activation of RhoA was refractory to silencing of p63RhoGEF (Wuertz et al. 2011). S1P induced activation of RhoA is transduced through both Gαq/11 and Gα12/13 and is independent of Ca2+ and PLCβ (Takashima et al. 2008). On the other hand, AngII is well known to increase Ca2+ through the traditional activation of PLCβ to activate MLCK, phosphorylate RLC20 and contract SM. A recent study has also shown another potentially important AngII signaling pathway for the control of vascular tone and blood pressure (Guilluy et al. 2010). In this case, the AngII/Gαq/11 mediated increase in Ca2+ leads to Ca2+dependent Janus kinase 2 (Jak2) activation that in turn phosphorylates p115RhoGEF, increasing RhoA activity and Ca2+-sensitized force (Fig. 2). Silencing of p115RhoGEF in SM suppressed AngII-dependent hypertension in mice but did not affect normal blood pressure (Guilluy et al. 2010). We propose, that in view of its response to phenylephrine, that p63RhoGEF may predominantly control basal blood pressure in support of the hypothesis that different RhoGEFs regulate specific RhoA functions. Regarding AngII stimulation, why are there so many signaling modes to regulate tone, contraction and hypertension in SM? These pathways may be redundant reflecting the physiological importance of AngII signaling in regulating the contractile state. Alternatively, these different signaling pathways may act in concert in time and space to sustain an AngII induced tonic contraction (Guilluy et al. 2010; Wuertz et al. 2011). In aortic SM cells p63RhoGEF activation of RhoA occurred within the first 30 seconds (Wuertz et al. 2011). PLCβ-induced Ca2+ release by AngII Gαq/11 is likely very rapid while Ca2+ activation of Jak2 and p115RhoGEF and AngII activation of Gα12/13 comes on later to maintain RhoA activity (Fig. 2).
Fig. 2.
Scheme illustrating signaling pathways for p63RhoGEF, p115RhoGEF, LARG and PDZRhoGEF leading to RhoA mediated vasoconstriction. GPCRs for phenylephrine (PE), angiotensin (AngII), endothelin (ET-1) and thromboxane (TXA2) couple through Gαq/11 and or Gα12/13 to activate varieties RhoGEFs. Activated RhoGEFs differently or synergistically catalyze GTP loading and activation of RhoA. RhoA activates Rho kinase (ROCK) to phosphorylate and inhibit myosin light chain phosphatase (MLCP) activity resulting in an increase in myosin regulatory light chain phosphorylation (MLC20) and vasoconstriction. Gαq/11 selectively activates p63RhoGEF and phospholipase C β (PLCβ) that may compete for Gαq/11. PLCβ increases cytosolic Ca2+ to activate myosin light chain kinase (MLCK) and to activate Jak2 that in turn activates p115RhoGEF to activate RhoA.
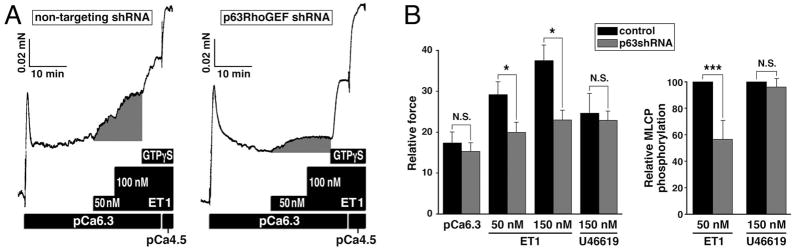
In our study, a physiological role for p63RhoGEF as a mediator of RhoA activation in response to stimulation by Gαq/11 linked agonists was directly demonstrated in blood vessels (Momotani et al. 2011). This study focused on three agonists, ET-1 which couples predominantly to Gαq/11, the thromboxane A2 analogue U46619 in which Gαq/11 plays a lesser role and the α1 adrenergic agonist phenylephrine shown to couple exclusively to Gαq/11 (Wirth et al. 2007). p63RhoGEF was partially silenced in isolated portal veins and the ability of ET-1 and U46619 to induce Ca2+-sensitized force was directly measured using an established protocol which retains agonist-GPCR coupling to RhoA under conditions where the concentration of Ca2+ is clamped. Ca2+-sensitization of contractile force and phosphorylation of the ROCK target MYPT1 at Thr853 in response to ET-1 but not U46619 were significantly reduced when p63RhoGEF expression was suppressed (Fig. 3). The remaining component of RhoA mediated Ca2+-sensitized force reflected by the subsequent magnitude of the response to GTPγS was increased in vessels where p63RhoGEF expression was reduced, consistent with p63RhoGEF mediating ET-1 activation of RhoA. Further support for p63RhoGEF coupling selectively through Gαq/11 was demonstrated by the ability of a recombinant PH domain fragment (residues 331–580) of p63RhoGEF to relax phenylephrine-induced Ca2+-sensitized force and reduce activation of RhoA and RLC20 phosphorylation. Evidence for an in vitro interaction between the DH and PH domain fragments (351–477/502) of purified p63RhoGEF was not found by Shankaranarayanan and colleagues, unlike the findings of the Rojas group (Rojas et al. 2007). The reason for this difference is not clear. It could be that the constructs used were different or that the PH domain interacts with some other region of full-length p63RhoGEF. A longer PH domain fragment (331–580) was shown to associate with full-length p63RhoGEF when co-expressed in mammalian cells (Momotani et al. 2011). This fragment (331–580) was also shown to associate with constitutively active Gαq/11 (Q206L). The association with full-length p63RhoGEF and constitutively active Gαq/11 (Q206L) together provide in cellulo evidence supporting the molecular mechanism regulating p63RhoGEF inferred from the biochemical and crystallographic studies described above.
Fig. 3.
Inhibition of ET1-induced Ca2+-sensitized force and phosphorylation of MCLP (RhoA/ROCK substrate) when p63RhoGEF is partially silenced in mouse portal vein. A. ET1-, GTPγS- (i.e. maximal activation of RhoA/Ca2+-sensitized force) and Ca2+-induced force in permeabilized mouse portal veins following treatment with shRNA targeting p63RhoGEF or a non-targeting control. Note that ET1-induced Ca2+-sensitized force (shaded component) is inhibited by partial silencing of p63RhoGEF at constant Ca2+ concentration (pCa 6.3). GTPγS-induced force is a measure of the residual component of Ca2+-sensitization. pCa 4.5: maximal force. B. Summary of changes in ET1- (50 nM and subsequent addition of 100 nM; i.e. 150 nM final concentration) induced Ca2+-sensitized force as shown in panels A, in thromboxane analogue U46619- (150 nM) induced Ca2+-sensitized force, and in phosphorylation of the RhoA/ROCK substrate MLCP (i.e. phosphorylation of the MLCP subunit MYPT1 at Thr853) using the protocol shown in panel A. The reduction in force, normalized to maximal force induced by pCa 4.5, for 50 nM ET-1 (IC50 ) was 29.0±3.4 vs 19.7±2.6 respectively and at maximal ET-1 (150 nM) was 38.1±4.0% vs 23.0±2.5% respectively; no significant change in U46619-induced force between control and treated samples (mean values ± S.E. n = 12–14, * P < 0.01). Relative phosphorylations of MLCP: mean values ± S.E. n = 4, *** P < 0.02. From (Momotani et al. 2011).
Questions and future directions
A fundamental question is whether different agonists through different GPCRs select for particular RhoGEFs to regulate different RhoA mediated functions such as salt-induced hypertension, basal blood pressure or SM proliferation, and if so how does the SM cell discriminate between these different sources of RhoA? One possibility is that a given agonist may in addition activate or inhibit other critical molecules that set up the cell to favor a particular outcome. The association of RhoGEFs with scaffold proteins that associate with distinct cassettes of signaling molecules to govern signal output is an attractive hypothesis. Selectivity through regulation has been shown for MAPK and FAK that regulate GEFH1 activity but not LARG but little is known for p63RhoGEF (Guilluy et al. 2011). Also, regulator of G-protein signaling (RGS) protein when bound to Gαq comes into the proximity of p63RhoGEF when p63RhoGEF is in a complex with Gαq (Shankaranarayanan et al. 2008). This is consistent with findings that RGS2 serves as a negative regulator for the AngII type 1 receptor and with RGS2-null mice showing increased sensitivity and prolonged responsiveness to vasoconstrictors and having hypertension (Thoonen et al. 2011).
No information is yet available on the role of p63RhoGEF in human physiology or pathophysiology. Identification of dominant positive mutations in p63RhoGEF such as in the α6-αN linker in patients with diseases associated with hypercontractility or its reduced expression in Bartter’s/Gitelman’s syndrome need to be explored (Calo et al. 2011).
p63RhoGEF partners and effectors are just beginning to be identified. p63RhoGEF has been shown to interact with MLK3 (mixed lineage kinase 3) where MLK3 serves as a negative regulator of p63RhoGEF (Swenson-Fields et al. 2008) but a role in regulation of SM contractility and the identification of other possible kinases and phosphatases that regulate p63RhoGEF activity are unknown. It is also not clear whether p63RhoGEF is localized at the membrane to a scaffold of proteins that locally regulate its activity and accessibility to Gαq/11 or its potential competitor PLCβ. Future studies of SM should also take into account the other p63RhoGEF family members Trio and Kalirin (Rojas et al. 2007; Lutz et al. 2007) that unlike p63RhoGEF have multiple amino-terminal domains for specialized regulation. The carboxyl-terminal RhoA specific DH-PH cassette of Trio like p63RhoGEF is activated by Gαq (Rojas et al. 2007) and Trio is a major Gαq effector together with PLCβ driving locomotion, egg laying and growth in C. elegans (Williams et al. 2007). Much remains to be learned about selective regulation of p63RhoGEF in SM, especially in humans, and possible differences in its regulation in different SMs and diseases of SM. The identification and distinct functions of GTPase activating proteins (GAPs) and their regulation in time and space should also provide a fertile ground for exploration in normal and diseased SM.
Summary
Altogether, these results suggest that Gαq/11 coupling to p63RhoGEF mediates RhoA activation by agonists such as AngII, phenylephrine and ET-1 that solely or partially couple through Gαq/11 and that these signaling pathways in conjunction with PLCβ-induced Ca2+ release contribute to basal and stimulated increases in blood pressure and vessel remodeling (Fig. 2). These findings raise the possibility of selective targeting of p63RhoGEF for therapeutic use.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01DK088905 and R01GM86457). We are very grateful to Dr. Ulla Derewenda for the design of Fig. 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adjobo-Hermans MJ, Goedhart J, van Weeren L, Nijmeijer S, Manders EM, Offermanns S, Gadella TW., Jr Real-time visualization of heterotrimeric G protein Gq activation in living cells. BMC Biol. 2011;9:32. doi: 10.1186/1741-7007-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aittaleb M, Nishimura A, Linder ME, Tesmer JJ. Plasma membrane association of p63 Rho guanine nucleotide exchange factor (p63RhoGEF) is mediated by palmitoylation and is required for basal activity in cells. J Biol Chem. 2011;286:34448–56. doi: 10.1074/jbc.M111.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. ®-Arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–50. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- Calo LA, Davis PA, Pessina AC. Does p63RhoGEF, a new key mediator of angiotensin II signalling, play a role in blood pressure regulation and cardiovascular remodelling in humans? J Renin Angiotensin Aldosterone Syst. 2011;12:634–6. doi: 10.1177/1470320311407232. [DOI] [PubMed] [Google Scholar]
- Cat AND, Touyz RM. Cell Signaling of Angiotension II on Vascular Tone: Novel Mechanisms. Curr Hypertens Rep. 2011;13:122–128. doi: 10.1007/s11906-011-0187-x. [DOI] [PubMed] [Google Scholar]
- Chikumi H, Vazquez-Prado J, Servitja JM, Miyazaki H, Gutkind JS. Potent activation of RhoA by Gαq and Gq-coupled receptors. J Biol Chem. 2002;277:27130–4. doi: 10.1074/jbc.M204715200. [DOI] [PubMed] [Google Scholar]
- de Godoy MA, Rattan S. Role of rho kinase in the functional and dysfunctional tonic smooth muscles. Trends Pharmacol Sci. 2011;32:384–93. doi: 10.1016/j.tips.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt P, Kjoller L, Giel M, Hall A, Toksoz D. Activated Gαq family members induce Rho GTPase activation and Rho-dependent actin filament assembly. FEBS Lett. 2002;531:565–9. doi: 10.1016/s0014-5793(02)03625-6. [DOI] [PubMed] [Google Scholar]
- Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res. 2000;87:221–7. doi: 10.1161/01.res.87.3.221. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16:183–90. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–26. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Yamaguchi Y, Kitano Y, Ichikawa A, Negishi M. Constitutively active Gα12, Gα13, and Gα induce Rho-dependent neurite retraction through different signaling pathways. J Biol Chem. 1998;273:28700–7. doi: 10.1074/jbc.273.44.28700. [DOI] [PubMed] [Google Scholar]
- Loirand G, Pacaud P. The role of Rho protein signaling in hypertension. Nat Rev Cardiol. 2010;7:637–47. doi: 10.1038/nrcardio.2010.136. [DOI] [PubMed] [Google Scholar]
- Lutz S, Freichel-Blomquist A, Rumenapp U, Schmidt M, Jakobs KH, Wieland T. p63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same gene. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:540–6. doi: 10.1007/s00210-004-0926-5. [DOI] [PubMed] [Google Scholar]
- Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, Wieland T. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem. 2005;280:11134–9. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, et al. Structure of Gαq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318:1923–7. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- Momotani K, Artamonov MV, Utepbergenov D, Derewenda U, Derewenda ZS, Somlyo AV. p63RhoGEF couples Gα (q/11)-mediated signaling to Ca2+ sensitization of vascular smooth muscle contractility. Circ Res. 2011;109:993–1002. doi: 10.1161/CIRCRESAHA.111.248898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriki N, Ito M, Seko T, Kureishi Y, Okamoto R, Nakakuki T, Kongo M, Isaka N, Kaibuchi K, Nakano T. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens Res. 2004;27:263–70. doi: 10.1291/hypres.27.263. [DOI] [PubMed] [Google Scholar]
- Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. Gαq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem. 2007;282:29201–10. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seasholtz TM, Brown JH. RHO SIGNALING in vascular diseases. Mol Interv. 2004;4:348–57. doi: 10.1124/mi.4.6.8. [DOI] [PubMed] [Google Scholar]
- Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–8. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- Shankaranarayanan A, Boguth CA, Lutz S, Vettel C, Uhlemann F, Aittaleb M, Wieland T, Tesmer JJ. Gαq allosterically activates and relieves autoinhibition of p63RhoGEF. Cell Signal. 2010;22:1114–23. doi: 10.1016/j.cellsig.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaranarayanan A, Thal DM, Tesmer VM, Roman DL, Neubig RR, Kozasa T, Tesmer JJ. Assembly of high order Gαq-effector complexes with RGS proteins. J Biol Chem. 2008;283:34923–34. doi: 10.1074/jbc.M805860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Souchet M, Portales-Casamar E, Mazurais D, Schmidt S, Leger I, Javre JL, Robert P, Berrebi-Bertrand I, Bril A, Gout B, et al. Human p63RhoGEF, a novel RhoA-specific guanine nucleotide exchange factor, is localized in cardiac sarcomere. J Cell Sci. 2002;115:629–40. doi: 10.1242/jcs.115.3.629. [DOI] [PubMed] [Google Scholar]
- Swenson-Fields KI, Sandquist JC, Rossol-Allison J, Blat IC, Wennerberg K, Burridge K, Means AR. MLK3 limits activated Gαq signaling to Rho by binding to p63RhoGEF. Mol Cell. 2008;32:43–56. doi: 10.1016/j.molcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Sugimoto N, Takuwa N, Okamoto Y, Yoshioka K, Takamura M, Takata S, Kaneko S, Takuwa Y. G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on Rho but not Rho kinase. Cardiovasc Res. 2008;79:689–97. doi: 10.1093/cvr/cvn118. [DOI] [PubMed] [Google Scholar]
- Takizawa N, Schmidt DJ, Mabuchi K, Villa-Moruzzi E, Tuft RA, Ikebe M. M20, the small subunit of PP1M, binds to microtubules. Am J Physiol Cell Physiol. 2003;284:C250–62. doi: 10.1152/ajpcell.00153.2002. [DOI] [PubMed] [Google Scholar]
- Thoonen R, Zhang H, Abe Y, Nickerson H, Aronovitz M, Chambon P, Karas RH, Mendelsohn ME. Smooth muscle cell specific deletion of the PKGlα target RGS2 induces vascular dysfunction and hypertension. BMC Pharmacology. 2011;11:P72. [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Vogt S, Grosse R, Schultz G, Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J Biol Chem. 2003;278:28743–9. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, Tesmer JJ, Jorgensen EM, Wieland T, Miller KG. Trio’s Rho-specific GEF domain is the missing Gαq effector in C. elegans. Genes Dev. 2007;21:2731–46. doi: 10.1101/gad.1592007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–8. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, et al. G(12)-G(13)-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2007 doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- Wuertz CM, Lorincz A, Vettel C, Thomas MA, Wieland T, Lutz S. p63RhoGEF--a key mediator of angiotensin II-dependent signaling and processes in vascular smooth muscle cells. FASEB J. 2011;24:4865–76. doi: 10.1096/fj.10-155499. [DOI] [PubMed] [Google Scholar]
- Yeung WW, Wong YH. Gα16 interacts with Class IA phosphatidylinositol 3-kinases and inhibits Akt signaling. Cell Signal. 2010;22:1379–87. doi: 10.1016/j.cellsig.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nishimuta J, Hirano K, Kanaide H. The exogenously added small subunit of smooth muscle myosin phosphatase increases the Ca2+ sensitivity of the contractile apparatus in the permeabilized porcine renal artery. Biochem Biophys Res Commun. 1999;254:158–163. doi: 10.1006/bbrc.1998.9915. [DOI] [PubMed] [Google Scholar]