Abstract
Background
Emotional lability (EL) is commonly seen in patients with Attention Deficit/Hyperactivity Disorder (ADHD). The reasons for this association are currently unknown. To address this question we examined the relationship between ADHD and EL symptoms, and performance on a range of neuropsychological tasks to clarify whether EL symptoms are predicted by particular cognitive and/or motivational dysfunctions and whether these associations are mediated by the presence of ADHD symptoms.
Methods
A large multi-site sample of 424 carefully diagnosed ADHD cases and 564 unaffected siblings and controls aged 6 to 18 years performed a broad neuropsychological test battery, including a Go/No-Go Task, a warned 4-choice Reaction Time task, the Maudsley Index of Childhood Delay Aversion, and Digit span backwards. Neuropsychological variables were aggregated as indices of processing speed, response variability, executive functions, choice impulsivity and the influence of energetic and/or motivational factors.
EL and ADHD symptoms were regressed on each neuropsychological variable in separate analyses controlling for age, gender and IQ, and, in subsequent regression analyses, for ADHD and EL symptoms respectively.
Results
Neuropsychological variables significantly predicted ADHD and EL symptoms with moderate to low regression coefficients. However, the association between neuropsychological parameters on EL disappeared entirely when the effect of ADHD symptoms was taken into account, revealing that the association between the neuropsychological performance measures and EL is completely mediated statistically by variations in ADHD symptoms. Conversely, neuropsychological effects on ADHD symptoms remained after EL symptom severity was taken into account.
Conclusions
The neuropsychological parameters examined here predict ADHD more strongly than EL. They cannot explain EL symptoms beyond what is already accounted for by ADHD symptom severity. The association between EL and ADHD cannot be explained by these cognitive or motivational deficits. Alternative mechanisms, including overlapping genetic influences (pleiotropic effects), and/or alternative neuropsychological processes need to be considered.
Keywords: ADHD, neuropsychological performance, emotional lability, executive functions, delay aversion
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is frequently accompanied by symptoms of emotional lability (EL) such as irritability, hot temper and sudden unpredictable shifts towards negative emotions (Sobanski et al., 2010; Surman et al., 2011; Biederman et al., 2011). The presence of EL is clinically relevant as it is associated with increased severity of ADHD core symptoms, particularly hyperactivity-impulsivity, elevated rates of comorbid conditions (such as oppositional defiant disorder, depression, anxiety, and substance abuse), more functional impairment and a worse long-term course (Barkley & Fischer, 2010; Hinshaw, 2003; Maedgen & Carlson, 2000; Sobanski et al., 2010; Spencer et al., 2011; Stringaris, Cohen, Pine, & Leibenluft, 2009; Stringaris & Goodman, 2009a).
EL can result from increased bottom-up emotional reactivity and/or reduced top-down emotional regulation skills, implicating possible dysfunctions of various subcortical (e.g. amygdala, hippocampus, and ventral striatum) and/or cortical brain regions or an altered connectivity between those regions (Meyer-Lindenberg et al., 2006). Bottom-up and top-down processes may be inseparable when examined at the behavioral level, but functional imaging, psychophysiological and animal studies show that they are distinguishable at a neural level (Wessa & Linke, 2009). The amygdala has a strategic role in emotional reactivity by modulating perceptual sensitivity to incoming information and generating an automatic, transient emotional response and subsequent subjective emotional experience, including emotional expressive behaviors and heightened autonomic reactivity. The up-and down-regulation of emotions associated with modulation of neural activity in the amygdala has been shown to be associated with a prefrontal network, including the lateral prefrontal cortex, the anterior cingulate cortex and the orbitofrontal cortex (Wessa & Linke, 2009). An alternative explanation implicated the insula with its rich connections to the ACC and OFC (Craig, 2008).
EL symptoms may reflect different underlying etiological mechanisms in the context of different disorders and normal development. The causes of the association between ADHD and EL are unknown. Earlier studies indicate that indeed both components of emotion processing, emotional reactivity and regulation skills, might be altered in children with ADHD (Maedgen & Carlson, 2000; Martel, 2009; Melnick & Hinshaw, 2000; Walcott & Landau, 2004). Recently, we showed that EL symptoms are not a mere epiphenomenon of ADHD symptom severity and can only partially be explained by the level of psychiatric comorbidity (Sobanski, et al., 2010). However, the frequent occurrence of EL in patients with ADHD might arise from risk factors or pathophysiological components that influence both ADHD and EL symptoms. Alterations in multiple brain networks and neuropsychological impairments have been implicated in the aetiology of ADHD. Thus, recent models posit the existence of multiple neurodevelopmental pathways to the disorder and of subgroups of patients with different profiles of neuropsychological dysfunctions (Nigg & Casey, 2005; Sonuga-Barke, 2002). These dysfunctions include a slightly lower general cognitive ability, executive function deficits (e.g. inhibitory control, working memory), reduced processing speed and efficiency (slow and variable reaction times), impulsive preference for immediate rewards, aversion to delay, and regulation skill deficits of psycho-physiological state during periods of under-or over-activation (Willcutt, Sonuga-Barke, Nigg, & Sergeant, 2008). While these neuropsychological alterations and the related brain dysfunctions are not specific to ADHD (Banaschewski et al., 2005), they might nevertheless explain the frequent co-occurrence of ADHD and EL symptoms.
Thus, both sets of symptoms have been suggested to result from executive dysfunctions caused by a primary inhibitory control deficit (Barkley, 2010). Contrary to this hypothesis, previous studies found that behavioural disinhibition predicted only a small amount of variability in emotional regulation in ADHD (Melnick & Hinshaw, 2000; Walcott & Landau, 2004). However, these studies investigated small samples and the contribution of deficits in different domains of executive functioning (e.g., inhibitory control, working memory) to EL have yet to be systematically investigated.
EL and ADHD symptoms might also be influenced by motivational alterations. One of the most robust motivational markers in ADHD is the preference for immediate smaller over delayed larger rewards in simple choice tasks in ADHD (Luman, Oosterlaan, & Sergeant, 2005; Marco et al., 2009; Paloyelis, Asherson, & Kuntsi, 2009; Sonuga-Barke, 2002; Tripp & Alsop, 2001). This has been argued to result from the combination of an impulsive drive for immediate reward and an emotional aversion to delay (hence: ‘delay aversion’; Marco, et al., 2009; Paloyelis, et al., 2009). Because ADHD has been associated with heightened levels of frustration during long and boring tasks (Bitsakou, Psychogiou, Thompson, & Sonuga-Barke, 2009) and with hyperactivation in the amygdala, when reward is delayed (Plichta et al., 2009), children with ADHD and EL might be characterized by a particularly strong negative emotional response during delay.
According to the cognitive-energetic model, children with ADHD might have particular difficulties in effectively allocating effort to regulate their sub-optimal psycho-physiological states during periods of under-or over-activation, which, for example, might be induced by changes in reward and/or event rates (Sergeant, 2000, 2005). Findings that show that the manipulation of task conditions such as event rate (Kuntsi, Wood, van der Meere, & Asherson, 2009; Sergeant, 2000, 2005; van der Meere, Marzocchi, & De Meo, 2005) or rewards (Kuntsi et al., 2009; Konrad, Gauggel, Manz, & Scholl, 2000; Uebel et al., 2010) or the combination of both factors (Kuntsi et al., 2009, 2010; Andreou et al., 2007) can substantially improve their reaction time performance, are consistent with this model. Psychophysiological under-or over-activation might also lead to increased emotional lability.
Slow and variable reaction times under slow unrewarded task conditions are two closely related variables that are among the best discriminating variables between ADHD and control samples (Kuntsi, Oosterlaan, & Stevenson, 2001; Kuntsi, et al., 2009; Kuntsi et al., 2010). The underlying processes and their relationship to emotion processing are currently unknown but it is feasible that the processes that lead to inconsistent reaction times could reflect general regulatory processes that impact in addition on emotion regulation (discussed in Skirrow et al., 2009). Recent theoretical models, which are not necessarily mutually exclusive, have proposed that increased response variability may reflect momentary attentional lapses, insufficient regulation of arousal, deficient extinction processes, or dysfunctional timing mechanisms (for a review see: Kuntsi & Klein, 2012).
Currently, it is unknown whether the frequent occurrence of EL in children with ADHD might be explained by the presence of particular cognitive and/or motivational dysfunctions. Here we studied the nature of the relationship between ADHD symptoms and EL, with a broad range of neuropsychological performance parameters implicated in ADHD, in a large sample of diagnosed ADHD cases, healthy controls, and siblings of both. Two main questions were addressed. First, whether EL symptoms are predicted by the neuropsychological alterations that have been previously shown to be implicated in ADHD; and second, whether ADHD symptoms explain the relationships between neuropsychological performance parameters and EL or whether there are independent effects of the cognitive functions on EL. We thus tested the statistical associations between neuropsychological variables and EL, and whether these become attenuated or remain stable when the effect of ADHD symptoms is taken into account.
METHODS
Sample
The sample consisted of 366ADHD probands and 359siblings,ascertained as part of the International Multicentre ADHD Genetics project, plus 263 controls including 99sibling pairs and 65singletons.IMAGE samples were excluded from these analyses if the ADHD, EL and neuropsychological data were not available. All participants were of European descent and aged 6 to 18 years. Probands had a research diagnosis of DSM-IV combined subtype ADHD. Siblings included both affected and unaffected individuals (for a detailed description see: Brookes et al., 2006; Chen et al., 2008; Müller et al., 2011a, 2011b). For this analysis we included only one sibling per proband family. Sibling selection was based first on gender and second on nearest age to the index proband. Controls were recruited from primary (ages 6–11 years) and secondary (ages 12–18 years) schools in the United Kingdom, Germany, and Spain, aiming for an age and gender match with the clinical sample. Case and control exclusion criteria were IQ <70, autism spectrum disorders, epilepsy, brain disorders, and genetic/medical disorders that might mimic ADHD (see also: Kuntsi et al., 2010).
The final sample in this study consisted of 988individuals: 411 were classified as combined subtype ADHD (including 45affected siblings), 13siblings who met criteria for the hyperactive-impulsive or inattentive subtypes, and 564individuals who were unaffected siblings and controls. Of the 411 individuals with combined-subtype ADHD, 103 had conduct disorder, 269 had oppositional defiant disorder, and 42 had possible mood disorder (excluding bipolar disorder). Ethical approval was obtained from local ethical review boards. Informed written consent was obtained from parents and children, respectively.
Measures
Clinical Symptoms
Diagnosis of ADHD and comorbid disorders according to DSM-IV-criteria were based on the Parental Account of Childhood Symptoms–Revised interview (Chen & Taylor, 2006); a semi-structured, standardized, investigator-based interview, assessing ADHD and other child psychiatric disorders according to DSM-IV, with good inter-rater reliability, predictive and discriminative validity (Taylor, Schachar, Thorley, & Wieselberg, 1986; Chen et al., 2008). Symptom ratings were based on the Conners’ Parent and Teachers Rating Scales-Revised (Conners, Sitarenios, Parker, & Epstein, 1998a, 1998b). Mean scores of the parent-and teacher-rated scales for ADHD total symptoms, inattention, hyperactivity/impulsivity, and emotional lability were computed as measures of the corresponding symptom dimensions.
Neuropsychological Tasks
Wechsler Intelligence Scales for Children, Third Edition
The vocabulary, similarities, picture completion, and block design subtests from the WISC-III (Wechsler, 1991) were used to obtain an IQ estimate (Sattler, 1992). Digit span backwards of the WISC-III was included as a measure of working memory.
The Go/No-Go Task
On each trial, 1 of 2 possible stimuli appeared for 300 milliseconds in the middle of the computer screen. Participants were instructed to respond as quickly as possible only to the “go” stimuli while maintaining a high level of accuracy. The proportion of “go” to “no-go” stimuli was 4:1. There were3 conditions (slow, fast, and slow-incentive), matched for task duration (Uebel, et al., 2010). The slow condition had an inter-stimulus interval (ISI) of 8 seconds (72 trials). The fast condition had an ISI of 1 second (462 trials). In the incentive condition participants could earn points for correct responses which were exchanged for real prizes after the game. The order of condition presentation varied randomly across participants. Dependent variables were mean reaction time (MRT), standard deviation of individual reaction times (RTV), commission, and omission errors.
The Fast Task
A standard warned 4-choice RT task (72 trials) was the baseline condition (Andreou, et al., 2007; Kuntsi, et al., 2010). At the start of the trial a warning signal (4 empty circles, arranged side by side) appeared on the screen. After8 seconds (presentation interval for the warning signal), the circle designated as the target signal for that trial was coloured in. Participants were asked to press the response key that corresponded in position to the location of the target. After a response, the stimuli disappeared from the screen and a fixed inter-trial interval of 2.5 seconds followed. Speed and accuracy were emphasized equally. If no response occurred within 10 seconds, the trial was terminated. Comparison conditions with a fast event rate (1 second instead of 8) and incentives followed the baseline condition. During the incentive condition participants could win smiley faces for quick response were exchanged for real prizes after the game (see: Andreou, et al., 2007).
In line with our previous analyses (Kuntsi et al., 2010), neuropsychological variables of the fast task and the Go/No-Go task were aggregated for subsequent analyses. Mean scores were obtained for: MRT and RTV across baseline conditions, as indices of ‘processing speed’ and ‘response variability’; and for omission and commission error rates, as indices of ‘attentional lapses’ and ‘inhibitory dysfunction’ on both tasks. Bivariate model-fitting analyses had indicated that the variables that were aggregated as mean scores show a large degree of familial overlap and that these mean scores yield valid measures (Kuntsi et al., 2010).
In addition, we included difference scores in terms of MRT and RTV between the a) fast and slow (baseline) conditions of the Go/No-Go Task, b) slow-incentive and slow (baseline) conditions of the Go/No-Go Task, and c) fast-incentive and slow (baseline) conditions of the Fast task, as measures of performance change across conditions induced by ‘energetic’ and/or motivational’ factors. The latter variables indicate performance dependency on extrinsic factors and thus self-regulation problems of arousal and activation states according to the task requirements.
The Maudsley Index of Childhood Delay Aversion (MIDA)
Participants choose between a smaller, immediate reward (one point involving a 2-second) and a larger delayed reward (two points involving a 30-second pre-reward delay) under two conditions (Marco, et al., 2009). In the no-post reward-delay condition, choosing the smaller reward led immediately to the next trial, reducing the overall length of task delay; in the post-reward delay condition, choosing the smaller reward led to a delay period of 30 seconds, whereas choosing the large reward led to a delay period of 2 seconds before the next trial (i.e., giving a constant trial length). The variables obtained from the task were the percentage of choices for the smaller, respectively larger reward, for each condition separately, controlling for total number of trials attempted. The percentage of choices of the smaller reward in the no-post-reward delay condition of the MIDA was used as an index of ‘choice impulsivity’; the percentage of choices of the smaller reward in the post-reward delay condition was used as an index of ‘impulsive drive to immediate reward’; and the difference in percentage of choices of the smaller reward between both the no-post-reward and post-reward delay conditions were computed as an index of ‘delay aversion’.
A minimum of a 48-h medication-free period was required prior to testing. Go/no-go data were available from 826, digit span backwards data from 854, fast task data from 823, and MIDA task data from 886 participants. Two of the sites did not administer the go/no-go task, two did not administer the fast task, and there were occasional technical problems with equipment.
Statistical analyses
To analyse the effects of neuropsychological variables on the ADHD score (mean parent and teacher, age and gender standardized, Conners’ ADHD score) and EL score (mean parent and teacher, age and gender standardized Conners’ EL score), general linear models (GLM) for correlated observations were applied to account for stochastic dependence of sibling data. For these analyses all variables (except gender) were standardized using the standard deviation of the control group. Thus, coefficients are comparable to beta coefficients. Separate GLM analyses were conducted to investigate the effect of ADHD on EL and the effects of each neuropsychological variable on ADHD and EL. Further, for each neuropsychological variable it was assessed whether the effect on EL remained significant when controlling for ADHD and vice versa.
Results
Background, clinical and neuropsychological variables for probands with ADHD, siblings of probands, and controls are given in Table 1. Correlations between processing speed, respectively response variability (measured as average MRT and RTV across Go/No-go task [slow condition] and Fast task [baseline condition]), and differences in MRT (ΔMRT) and RTV (ΔRTV) between incentive/fast and baseline condition (fast task) were as high as −.73 (ΔMRT) and −.58 (ΔRTV) for processing speed, respectively −.71 (ΔMRT) and −.78 (ΔRTV) for reaction time variability. All other correlations (between processing speed and response variability with performance change measures induced by either energetic or motivational factors) were in the range between −.38 and −.68.
Table 1.
Background, Clinical and Neuropsychological Variables
| Probands With ADHD | Siblings of Probands | Controls | ||||
|---|---|---|---|---|---|---|
| Gender (N; N males; %) | 366 | 325 (88.8) | 359 | 174 (48.5) | 263 | 186 (70.7) |
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Age (years) | 366 | 11.3 (2.6) | 359 | 11.3 (3.0) | 263 | 12.2 (2.5) |
| IQ | 366 | 102.8 (15.7) | 359 | 104.1 (13.4) | 263 | 107.6 (13.7) |
| Conners’ Emotional Lability | ||||||
| Mean1 | 366 | 69.1 (10.6) | 359 | 54.7 (10.5) | 263 | 51.2 (8.2) |
| Parent ratings | 366 | 69.0 (12.9) | 359 | 53.7 (12.3) | 263 | 52.7 (12.0) |
| Teacher ratings | 366 | 69.3 (14.5) | 359 | 55.8 (13.5) | 263 | 49.6 (8.1) |
| Conners’ DSM-IV ADHD | ||||||
| Mean1 | 366 | 75.1 (7.6) | 359 | 55.5 (11.3) | 263 | 51.1 (8.4) |
| Parent ratings | 366 | 78.7 (8.5) | 359 | 54.5 (13.4) | 263 | 52.1 (10.4) |
| Teacher ratings | 366 | 71.5 (10.2) | 359 | 56.5 (12.6) | 263 | 50.2 (9.1) |
| Neuropsychological variables | ||||||
| MRT2, ms | 142 | 764.1 (257.4) | 155 | 718.0 (259.6) | 215 | 592.2 (157.8) |
| RTV2, ms | 142 | 374.6 (232.5) | 155 | 285.8 (216.9) | 215 | 179.2 (129.2) |
| Omission errors, %3 | 288 | 15.3 (11.3) | 291 | 9.1 (8.8) | 234 | 5.6 (5.4) |
| Commission errors, %3 | 288 | 53.3 (18.6) | 291 | 43.5 (19.8) | 234 | 39.4 (18.3) |
| Digit span backwards | 302 | 4.6 (1.8) | 293 | 5.1 (2.0) | 259 | 5.6 (2.0) |
| Choice impulsivity %4 | 332 | 27.5 (32.3) | 317 | 23.8 (29.4) | 237 | 12.6 (22.3) |
| IDIR %5 | 324 | 15.4 (22.2) | 316 | 12.2 (19.5) | 239 | 6.7 (17.6) |
| Delay aversion %6 | 321 | 12.2 (24.4) | 309 | 11.3 (25.0) | 237 | 6.0 (19.5) |
| Go/no-go task | ||||||
| MRT (slow condition; ms) | 293 | 649.2 (235.1) | 297 | 590.0 (185.8) | 235 | 501.0 (123.3) |
| RTV (slow condition; ms) | 293 | 316.2 (223.3) | 297 | 228.5 (170.8) | 235 | 148.0 (105.9) |
| MRT (event rate effect; ms)7 | 288 | −228.7 (195.9) | 291 | −186.5 (140.2) | 234 | −141.4 (92.0) |
| RTV (event rate effect; ms)7 | 288 | −125.2 (200.3) | 291 | −81.5 (141.7) | 234 | −33.2 (100.9) |
| MRT (incentive effect; ms)8 | 153 | −43.0 (151.6) | 155 | −20.5 (115.9) | 218 | 8.4 (73.3) |
| RTV (incentive effect; ms)8 | 153 | −91.7 (183.0) | 155 | −57.5 (145.7) | 218 | −30.2 (103.7) |
| Fast task | ||||||
| MRT (slow condition; ms) | 184 | 931.4 (354.9) | 197 | 894.9 (409.6) | 242 | 683.4 (216.6) |
| RTV (slow condition; ms) | 184 | 461.9 (346.6) | 197 | 369.4 (331.0) | 242 | 209.3 (186.2) |
| MRT (event rate + incentive effect; ms)9 | 179 | −282.5 (233.1) | 194 | −244.8 (209.0) | 241 | −157.8 (118.0) |
| RTV (event rate + incentive effect; ms)9 | 179 | −239.1 (290.3) | 194 | −156.0 (249.3) | 241 | −81.9 (144.5) |
Abbreviations: MRT, mean reaction time; RTV, reaction time variability
Mean score (parent-& teacher-rated subscales)
Mean score (Go/No-go task, slow condition & Fast task, baseline condition)
Mean score of error percentage in the Go/no-go task, slow condition & fast condition;
Percentage of impulsive choices (MIDA task, no-postdelay condition)
Impulsive drive for immediate reward; percentage of impulsive choices (MIDA task, postdelay condition)
Difference of percentages of impulsive choices in no-postdelay condition vs. postdelay condition (MIDA task)
Go/No-go task; fast–slow condition difference
Go/No-go task; incentive – slow condition difference
Fast task; incentive/fast-baseline condition difference
We adopted a mediation model to delineate the degree to which ADHD symptoms might explain the effects of neuropsychological dysfunctions on EL symptoms, i.e., whether the statistical associations between neuropsychological variables and EL become attenuated or remain stable when the effect of ADHD symptoms are taken into account (Baron & Kenny, 1986). ADHD symptoms were regressed on each neuropsychological variable in separate analyses, controlling for age, gender and IQ (Table 2). All neuropsychological variables significantly predicted ADHD symptoms apart from the ‘delay aversion’ variable. Standardized regression coefficients (SRC) were moderate (>.35 and < .5) for MRT and RTV, and omission errors; and low (>.15 and < .35) for commission errors, digit span backwards, choice impulsivity, impulsive drive for immediate reward, and the effects of event rate and/or incentive change on MRT and RTV except for the effect of incentive change on MRT (Table 2a). Neuropsychological variables also predicted EL symptoms (Table 3), but with substantially lower standardized regression coefficients than found for ADHD symptoms. Coefficients were moderate (>.35 and < .5) for MRT and low (>.15 and < .35) for RTV, omission and commission errors, digit span, impulsive drive for immediate reward, and effects of event rate or incentive change on RTV and the combined effect of event rate with incentive change on MRT (Table 3a).
Tab. 2.
Effects of neuropsychological functions on ADHD symptoms, controlling for age, gender, and IQ (A) and for age, gender, IQ, and EL symptoms score (B)
| A | B | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | SRC (SE) | t | p | SRC (SE) | t | p | |
| MRT1 | 512 | 0.48 (0.06) | 8.54 | *** | 0.21 (0.04) | 5.24 | *** |
| RTV1 | 512 | 0.47 (0.05) | 10.04 | *** | 0.24 (0.03) | 7.42 | *** |
| Omission errors %2 | 813 | 0.39 (0.03) | 11.33 | *** | 0.18 (0.02) | 7.38 | *** |
| Commission errors %2 | 813 | 0.34 (0.06) | 6.11 | *** | 0.19 (0.04) | 5.09 | *** |
| Digit span backwards | 854 | −0.26 (0.07) | −3.92 | *** | −0.14 (0.04) | −3.16 | ** |
| Choice impulsivity %3 | 886 | 0.15 (0.05) | 3.25 | ** | 0.06 (0.03) | 2.12 | * |
| Immediate drive for impulsive reward4 | 879 | 0.20 (0.05) | 3.70 | *** | 0.08 (0.03) | 2,27 | * |
| Delay aversion5 | 867 | 0.04 (0.05) | 0.82 | ns | 0.01 (0.03) | 0.34 | ns |
| MRT (event rate effect; ms)6 | 813 | −0.19 (0.03) | −5.51 | *** | −0.08 (0.02) | −3.57 | *** |
| RTV (event rate effect; ms)6 | 813 | −0.19 (0.04) | −5.29 | *** | −0.08 (0.02) | −3.23 | ** |
| MRT (incentive effect; ms)7 | 526 | −0.13 (0.05) | −2.92 | ** | −0.04 (0.03) | −1.31 | ns |
| RTV (incentive effect; ms)7 | 526 | −0.19 (0.05) | −3.76 | *** | −0.07 (0.03) | −2.18 | * |
| MRT (event rate + incentive effect; ms)8 | 614 | −0.28 (0.04) | −6.86 | *** | −0.15 (0.03) | −5.44 | *** |
| RTV (event rate + incentive effect; ms)8 | 614 | −0.26 (0.04) | −6.78 | *** | −0.16 (0.03) | −6.28 | *** |
Abbreviations: EL, emotional lability; MRT, mean reaction time; RTV, reaction time variability
Mean score (Go/No-go task, slow condition & Fast task, baseline condition);
Mean score of error percentage in the Go/no-go task, slow condition & fast condition;
Percentage of impulsive choices (MIDA task, no-postdelay condition)
Percentage of impulsive choices (MIDA task, postdelay condition)
Difference of percentages of impulsive choices in no-postdelay condition vs. post delay condition (MIDA task)
Go/No-go task; fast -slow condition difference
Go/No-go task; incentive -slow condition difference
Fast task; incentive/fast -baseline condition difference
p ≤ 0.05,
p ≤ 0.01,
p≤0.001
The covariates age, gender and IQ explained 5.4% of EL variance (estimated from a subsample of uncorrelated observations, i. e. no sibling pairs)
Tab. 3.
Effects of neuropsychological functions on EL symptoms, controlling for age, gender, and IQ (A) and for age, gender, IQ, and ADHD symptoms score (B)
| A | B | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | SRC (SE) | t | p | SRC (SE) | t | p | |
| MRT1 | 512 | 0.36 (0.06) | 6.50 | *** | 0.01 (0.04) | 0.20 | ns |
| RTV1 | 512 | 0.30 (0.05) | 6.47 | *** | −0.05 (0.03) | −1.37 | ns |
| Omission errors2 | 813 | 0.28 (0.03) | 8.47 | *** | 0.00 (0.02) | 0.10 | ns |
| Commission errors2 | 813 | 0.19 (0.05) | 3.63 | *** | −0.05 (0.04) | −1.41 | ns |
| Digit span backwards | 854 | −0.15 (0.06) | −2.35 | * | 0.04 (0.04) | 0.97 | ns |
| Choice impulsivity3 | 886 | 0.11 (0.04) | 2.40 | * | 0.00 (0.03) | −0.14 | ns |
| Immediate drive for impulsive reward4 | 879 | 0.15 (0.05) | 3.03 | ** | 0.00 (0.03) | 0.15 | ns |
| Delay aversion5 | 867 | 0.03 (0.04) | 0.66 | ns | 0.00 (0.03) | 0.13 | ns |
| MRT (event rate effect; ms)6 | 813 | −0.14 (0.03) | −4.27 | *** | 0.00 (0.02) | −0.06 | ns |
| RTV (event rate effect; ms)6 | 813 | −0.15 (0.03) | −4.26 | *** | −0.01 (0.02) | −0.35 | ns |
| MRT (incentive effect; ms)7 | 526 | −0.12 (0.04) | −2.80 | ** | −0.02 (0.03) | −0.60 | ns |
| RTV (incentive effect; ms)7 | 526 | −0.15 (0.05) | −3.14 | ** | −0.01 (0.03) | −0.20 | ns |
| MRT (event rate + incentive effect; ms)8 | 614 | −0.17 (0.04) | −4.32 | *** | 0.04 (0.03) | 1.43 | ns |
| RTV (event rate + incentive effect; ms)8 | 614 | −0.13 (0.04) | −3.46 | *** | 0.07 (0.02) | 2.68 | ** |
Abbreviations: EL, emotional lability; MRT, mean reaction time; RTV, reaction time variability
Mean score (Go/No-go task, slow condition & Fast task, baseline condition);
Mean score of error percentage in the Go/no-go task, slow condition & fast condition;
Percentage of impulsive choices (MIDA task, no-postdelay condition)
Percentage of impulsive choices (MIDA task, postdelay condition)
Difference of percentages of impulsive choices in no-postdelay condition vs. postdelay condition (MIDA task)
Go/No-go task; slow-fast condition difference
Go/No-go task; slow-incentive condition difference
Fast task; incentive/fast-baseline condition difference
p ≤ 0.05,
p ≤ 0.01,
p≤0.001
The covariates age, gender and IQ explained 5.4% of EL variance (estimated from a subsample of uncorrelated observations, i. e. no sibling pairs)
The association between ADHD symptoms and neuropsychological impairments were substantially reduced for all neuropsychological variables, when EL was introduced as an additional covariate into the regression analyses, resulting in low (but statistically significant) coefficients (>.15 and < .35) for MRT, RTV, omission and commission errors, and event rate plus incentive change on MRT and RTV (Table 2b).
However, controlling for ADHD symptom severity in the mediational analysis completely removed the effects of the neuropsychological variables on EL and none of the associations remained significant, except for the combined effect of event rate plus incentive change on RTV (statistically significant, but below .15; Table 3b).
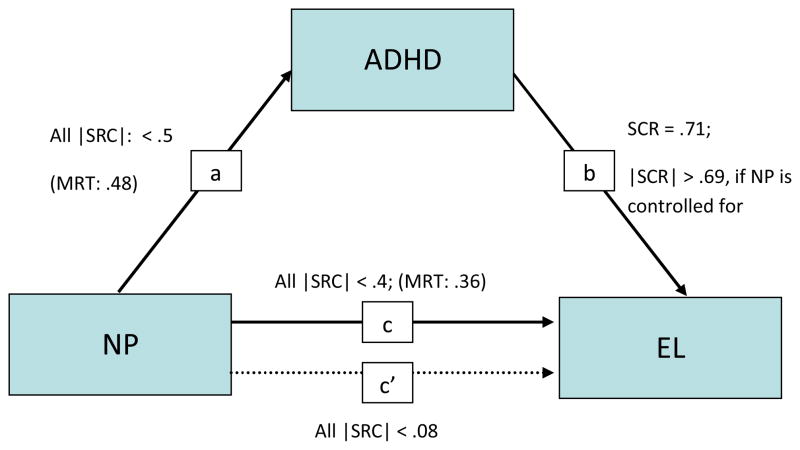
Importantly, the statistical association between ADHD and EL symptoms remained largely the same, whether or not the influence of any particular neuropsychological variables was taken into account (SCR = .71 vs >.69 for all variables); indicating that the cognitive dysfunctions included in this study do not explain the association of ADHD with EL.
Discussion
EL symptoms are commonly seen in patients with ADHD (Sobanski et al., 2010; Surman et al., 2011; Biederman et al., 2011) and are clinically relevant, since they predict functional impairment (Maedgen & Carlson, 2000; Melnick & Hinshaw, 2000; Stringaris, et al., 2009; Stringaris & Goodman, 2009b) and poorer adult psychosocial outcomes at 20-year follow-up (Stringaris, et al., 2009; Stringaris & Goodman, 2009a). The specific reasons for the association with ADHD are not well understood, but could arise from underlying neurobiological processes that influence both sets of symptoms.
The present study addressed two questions: first, whether the neuropsychological impairments previously implicated in ADHD predict EL symptoms; this would indicate that those neuropsychological functions and the related neuronal networks could be functionally involved in emotion. Second, to what extent ADHD symptoms might statistically mediate the relations between neuropsychological dysfunctions and EL symptoms.
Our results confirmed previous analyses of this dataset that found that EL symptoms are associated with ADHD severity (Sobanski, et al., 2010), and that ADHD symptoms are linked to neuropsychological dysfunctions (Andreou, et al., 2007; Kuntsi, et al., 2010; Marco, et al., 2009; Uebel, et al., 2010). ADHD symptoms were predicted by measures of neuropsychological functions, most strongly by processing speed and response variability; followed by measures of executive functions (commission and omission errors, digit span backwards) and performance change across conditions induced by energetic and/or motivational factors. The smallest association was with choice impulsivity and there was no association with delay aversion. The correlations of omission errors with RTV (.59), MRT (.51) and digit span backwards (−.38) underlines that omission errors could reflect short lapses of attention. The correlation between commission and omission errors reflecting inhibitory dysfunctions (.51) could therefore indicate that attentional processing deficits may cause secondary inhibition deficits and is in line with electrophysiological studies indicating that abnormal inhibitory processing in ADHD is typically preceded by attentional dysfunctions (Banaschewski et al., 2004; Brandeis et al., 1998; McLoughlin et al., 2010).
EL symptoms were also predicted by neuropsychological variables, in a similar rank order to that found to predict ADHD symptoms, i.e., most strongly by processing speed and response variability, followed by omission errors, and then by commission errors, digit span backwards, variables indicating state regulation problems and choice impulsivity. However, the effects of neuropsychological functioning on EL were substantially lower (small to moderate effect sizes) than on ADHD. This pattern of results is inconsistent with the hypothesis that EL symptoms in ADHD are mainly a consequence of an inhibitory deficit because commission errors, presumably reflecting this deficit, predicted EL to a substantially lesser degree than processing speed and response variability and omission errors (the latter presumably reflecting lapses of attention), and similarly well as measures of working memory and state regulation. The results also argue against a role of either choice impulsivity or delay aversion for EL symptoms. Processing speed and response variability seem to be the best predictors of ADHD and EL symptoms. The substantial correlations of processing speed and response variability with measures of performance change across conditions support the hypothesis that slow processing speed and increased response variability might at least partially reflecting state regulation difficulties (Kuntsi, et al., 2001; Sergeant, 2005).
However, any meaningful influence (SRC > .15) of neuropsychological parameters on EL disappeared entirely, when the effect of ADHD symptoms was taken into account; revealing that the association between the neuropsychological performance measures and EL is indirect, being statistically completely mediated by ADHD symptoms (Baron & Kenny, 1986). There were, therefore, no direct pathways of any of the neuropsychological functions on EL, independent of the link between neuropsychological dysfunctions and ADHD. Conversely, neuropsychological effects on ADHD symptoms remained after EL symptom severity was accounted for. Furthermore, the strength of the association between ADHD and EL remained similar whether or not the influence of any neuropsychological variable was controlled for (see Figure 1); therefore refuting the hypothesis that these neuropsychological functions might explain the association of ADHD with EL.
Figure 1.
Path diagram showing relations between ADHD symptoms, neuropsychological performance, and EL, controlling for the effects of gender, age and IQ; arrows indicate the directions predicted by the mediation model.
a) Effect of NP on ADHD symptoms
b) Effect of ADHD symptoms on EL
c) Effect of NP on EL
c′) Effect of NP on EL if ADHD symptoms are controlled for
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; EL, emotional lability; NP, neuropsychological performance; MRT, mean reaction time; SRC: standardized regression coefficients; |SRC|: SRC’s absolute value
Taken together, we found that the neuropsychological parameters investigated here predict ADHD symptoms more strongly than EL symptoms. The influence of these cognitive or motivational processes on EL symptoms is completely accounted for by ADHD, i.e., they are not predicting EL symptoms beyond what is already predicted by ADHD symptom severity. Our results also indicate that neuropsychological deficits do not explain the association between ADHD and EL. These findings are in line with recent results that neuropsychological deficits do not account for the link between deficient emotional self-regulation and ADHD in adults (Surman et al., in press). Therefore, alternative mechanisms, including overlapping genetic influences (pleiotropic effects) and/or alternative neuropsychological processes not measured in this study, need to be considered as factors explaining the association between ADHD and EL.
The lack of an association between ADHD symptom severity and delay a version in the current analysis was unexpected, although a general delay aversion tendency has not consistently been reported (Scheres et al., 2006; Paloyelis, et al., 2009). Our result is not in line with a previous report of significant case-control differences in both impulsive drive for immediate reward and delay aversion in the IMAGE sample, although the effect size is this study was relatively small (Marco, et al., 2009). Various factors might account for this discrepancy, including the use of different samples and analytical approaches. Here we used correlational approaches with dimensional measures of ADHD and a sample including the group of unaffected siblings and probands with sub-threshold symptoms. In the previous analysis, healthy controls were contrasted against diagnosed patients who met DSM-IV criteria for symptoms and impairment.
The study has a number of strengths: the very large multi-site sample of carefully diagnosed cases, siblings and controls provide strong evidence for the robustness of these effects across a broad age range and different cultural settings, and the use of tasks tapping a broad range of motivational and cognitive factors associated with ADHD. Some limitations should also be considered. The study was conducted in a clinical rather than an epidemiological ADHD sample, which potentially may have increased the associations between ADHD and EL symptoms, and neuropsychological measures. A limitation of the cross-sectional phenotypic design is in the interpretation of these data, since no causal mechanisms can be directly inferred. The association between ADHD, EL and the cognitive variables might all be accounted for by shared aetiological (genetic or environmental) factors with no direct causal pathways linking one with the other (Kendler & Neale, 2010). Multivariate genetic model fitting and longitudinal data would be necessary to further disentangle the causal relationship between ADHD and EL.
KEY POINTS.
Emotional lability (EL) symptoms are frequently present in patients with ADHD. They are clinically important since they predict psychosocial impairment and poorer outcome.
The causes for the association are unknown. EL symptoms are not a mere epiphenomenon of ADHD core symptom severity and can only partially be explained by the level of psychiatric comorbidity.
The neuropsychological alterations implicated in ADHD do predict EL symptoms; processing speed and response variability seem to be the best predictors. However, the association between EL and these cognitive or motivational dysfunctions is less strong than between neuropsychological alterations and ADHD symptoms.
Neuropsychological dysfunctions are likely not exerting a direct effect on emotion processing; rather, ADHD symptoms seem to mediate these links.
Acknowledgments
This work was supported in part by National Institute of Health (NIH) grants R01MH62873 andR01MH081803 to S. V. Faraone and, in London, by a UK Medical Research Council grant G03001896 to J. Kuntsi. We thank all the families who kindly participated in this research. Principal investigators for this study were P. Asherson, T. Banaschewski, S. V. Faraone, M. Gill, J. Kuntsi, I. Manor, A. Miranda, F. Mulas, R.D. Oades, A. Rothenberger, H. Roeyers and H.-C. Steinhausen. J. van der Meere contributed the go/no-go task. We thank further team members at data collection sites of Dublin, Essen, Ghent, Göttingen, London, Tel Aviv, Valencia and Zurich for their important contributions. Dr. Banaschewski served in an advisory or consultancy role for Bristol Myers-Sqibb, Develco Pharma, Lilly, Medice, Novartis, Shire and Viforpharma. He received conference attendance support and conference support or received speaker’s fee by Lilly, Janssen McNeil, Medice, Novartis and Shire. He is/has been involved in clinical trials conducted by Lilly and Shire. The present work is unrelated to the above grants and relationships.
Footnotes
Conflict of interest statement: The authors have no competing interests; potential conflicts are declared in the Acknowledgement section
Co-authors’ conflict of interest statements still need to be added.
References
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, Meidad S, Muller UC, Uebel H, Banaschewski T, Manor I, Oades R, Roeyers H, Rothenberger A, Sham P, Steinhausen HC, Asherson P, Kuntsi J. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med. 2007;37(12):1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Questioning inhibitory control as the specific deficit of ADHD--evidence from brain electrical activity. J Neural Transm. 2004;111(7):841–864. doi: 10.1007/s00702-003-0040-8. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Hollis C, Oosterlaan J, Roeyers H, Rubia K, Willcutt E, Taylor E. Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Developmental science. 2005;8(2):132–140. doi: 10.1111/j.1467-7687.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Deficient emotional self-regulation: a core component of attention-deficit/hyperactivity disorder. J ADHD Relat Disord. 2010;1(2):5–37. [Google Scholar]
- Barkley RA, Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(5):503–513. doi: 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T, Lomedico A, Day H, Petty CR, Faraone SV. Deficient emotional self-regulation and pediatric attention deficit hyperactivity disorder: a family risk analysis. Psychological medicine. 2011:1–8. doi: 10.1017/S0033291711001644. [DOI] [PubMed] [Google Scholar]
- Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Delay Aversion in Attention Deficit/Hyperactivity Disorder: an empirical investigation of the broader phenotype. Neuropsychologia. 2009;47(2):446–456. doi: 10.1016/j.neuropsychologia.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Brandeis D, van Leeuwen TH, Rubia K, Vitacco D, Steger J, Pascual-Marqui RD, Steinhausen HC. Neuroelectric mapping reveals precursor of stop failures in children with attention deficits. Behavioural brain research. 1998;94(1):111–125. doi: 10.1016/s0166-4328(97)00174-5. [DOI] [PubMed] [Google Scholar]
- Brookes K the Image Consortium. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11(10):934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Chen W, Taylor E. Parental Account of Children’s Symptoms (PACS), ADHD Phenotypes and its application to molecular genetic studies. In: Oades R, editor. Attention-Deficit/Hyperactivity Disorder (ADHD) and the Hyperkinetic Syndrome (HKS): Current Ideas and Ways forward. Nova Science Publisher; 2006. pp. 3–20. [Google Scholar]
- Chen W the Image Consortium. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. American Journal of Medical Genetics. 2008;147B(8):1450–1460. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998a;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998b;26(4):279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Impulsivity, emotion regulation, and developmental psychopathology: specificity versus generality of linkages. Ann N Y Acad Sci. 2003;1008:149–159. doi: 10.1196/annals.1301.016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Molecular psychiatry. 2010;15(8):789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Lack of inhibition: a motivational deficit in children with attention deficit/hyperactivity disorder and children with traumatic brain injury. Child Neuropsychol. 2000;6(4):286–296. doi: 10.1076/chin.6.4.286.3145. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Klein C. Intraindividual Variability in ADHD and Its Implications for Research of Causal Links. Current topics in behavioral neurosciences. 2012:67–91. doi: 10.1007/7854_2011_145. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42(2):199–210. [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, Buitelaar JK, McLoughlin G, Rommelse NN, Sergeant JA, Sonuga-Barke EJ, Uebel H, van der Meere JJ, Banaschewski T, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone SV, Asherson P. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry. 2010;67(11):1159–1167. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Van Der Meere J, Asherson P. Why cognitive performance in ADHD may not reveal true potential: findings from a large population-based sample. J Int Neuropsychol Soc. 2009;15(4):570–579. doi: 10.1017/S135561770909081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Maedgen JW, Carlson CL. Social functioning and emotional regulation in the attention deficit hyperactivity disorder subtypes. J Clin Child Psychol. 2000;29(1):30–42. doi: 10.1207/S15374424jccp2901_4. [DOI] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U, Andreou P, Butler L, Christiansen H, Gabriels I, Medad S, Albrecht B, Uebel H, Asherson P, Banaschewski T, Gill M, Kuntsi J, Mulas F, Oades R, Roeyers H, Steinhausen HC, Rothenberger A, Faraone SV, Sonuga-Barke EJ. Delay and reward choice in ADHD: an experimental test of the role of delay aversion. Neuropsychology. 2009;23(3):367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042–1051. doi: 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, Kuntsi J. Electrophysiological evidence for abnormal preparatory states and inhibitory processing in adult ADHD. Behav Brain Funct. 2010;6:66. doi: 10.1186/1744-9081-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick SM, Hinshaw SP. Emotion regulation and parenting in AD/HD and comparison boys: linkages with social behaviors and peer preference. J Abnorm Child Psychol. 2000;28(1):73–86. doi: 10.1023/a:1005174102794. [DOI] [PubMed] [Google Scholar]
- Müller UC the Image Consortium. The impact of study design and diagnostic approach in a large multi-centre ADHD study. Part 1: ADHD symptom patterns. BMC Psychiatry. 2011a;11:54. doi: 10.1186/1471-244X-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller UC the Image Consortium. The impact of study design and diagnostic approach in a large multi-centre ADHD study: Part 2: Dimensional measures of psychopathology and intelligence. BMC Psychiatry. 2011b;11:55. doi: 10.1186/1471-244X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. J Child Psychol Psychiatry. 2006;47(3–4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, Fallgatter AJ, Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biological psychiatry. 2009;65(1):7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Kuntsi J. Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. J Am Acad Child Adolesc Psychiatry. 2009;48(8):837–846. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: WISC-III and WPPSI-R supplement. San Diego, CA: Jerome M. Sattler, Publisher, Inc; 1992. [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44(11):2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry. 2005;57(11):1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Skirrow C, McLoughlin G, Kuntsi J, Asherson P. Behavioral, neurocognitive and treatment overlap between attention-deficit/hyperactivity disorder and mood instability. Expert Rev Neurother. 2009;9(4):489–503. doi: 10.1586/ern.09.2. [DOI] [PubMed] [Google Scholar]
- Sobanski E, Banaschewski T, Asherson P, Buitelaar J, Chen W, Franke B, Holtmann M, Krumm B, Sergeant J, Sonuga-Barke E, Stringaris A, Taylor E, Anney R, Ebstein RP, Gill M, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone SV. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51(8):915–923. doi: 10.1111/j.1469-7610.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130(1–2):29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Faraone SV, Surman CB, Petty C, Clarke A, Batchelder H, Wozniak J, Biederman J. Toward defining deficient emotional self-regulation in children with attention-deficit/hyperactivity disorder using the Child Behavior Checklist: a controlled study. Postgraduate medicine. 2011;123(5):50–59. doi: 10.3810/pgm.2011.09.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166(9):1048–1054. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R. Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. J Am Acad Child Adolesc Psychiatry. 2009a;48(4):404–412. doi: 10.1097/CHI.0b013e3181984f30. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Goodman R. Mood lability and psychopathology in youth. Psychol Med. 2009b;39(8):1237–1245. doi: 10.1017/S0033291708004662. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Stahl D, Santosh P, Goodman R. Dimensions and latent classes of episodic mania-like symptoms in youth: an empirical enquiry. J Abnorm Child Psychol. 2011;39(7):925–937. doi: 10.1007/s10802-011-9520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surman CB, Biederman J, Spencer T, Yorks D, Miller CA, Petty CR, Faraone SV. Deficient emotional self-regulation and adult attention deficit hyperactivity disorder: a family risk analysis. Am J Psychiatry. 2011;168(6):617–623. doi: 10.1176/appi.ajp.2010.10081172. [DOI] [PubMed] [Google Scholar]
- Surman CB, Biederman J, Spencer T, Miller CA, Petty CR, Faraone SV. Neuropsychological Deficits are not predictive of Deficient Emotional Self Regulation in Adults with Attention Deficit Hyperactivity Disorder. Journal of attention disorders. doi: 10.1177/1087054713476548. (in press) [DOI] [PubMed] [Google Scholar]
- Taylor E, Schachar R, Thorley G, Wieselberg M. Conduct disorder and hyperactivity: I. Separation of hyperactivity and antisocial conduct in British child psychiatric patients. Br J Psychiatry. 1986;149:760–767. doi: 10.1192/bjp.149.6.760. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2001;42(5):691–698. [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, Christiansen H, Heise A, Kuntsi J, Schafer U, Andreou P, Manor I, Marco R, Miranda A, Mulligan A, Oades RD, van der Meere J, Faraone SV, Rothenberger A, Banaschewski T. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. J Child Psychol Psychiatry. 2010;51(2):210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meere J, Marzocchi GM, De Meo T. Response inhibition and attention deficit hyperactivity disorder with and without oppositional defiant disorder screened from a community sample. Developmental neuropsychology. 2005;28(1):459–472. doi: 10.1207/s15326942dn2801_1. [DOI] [PubMed] [Google Scholar]
- Walcott CM, Landau S. The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol. 2004;33(4):772–782. doi: 10.1207/s15374424jccp3304_12. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. UK. London: The Psychological Corporation; 1991. [Google Scholar]
- Wessa M, Linke J. Emotional processing in bipolar disorder: behavioural and neuroimaging findings. International review of psychiatry. 2009;21(4):357–367. doi: 10.1080/09540260902962156. [DOI] [PubMed] [Google Scholar]
- Willcutt E, Sonuga-Barke EJ, Nigg J, Sergeant JA. Recent Developments in Neuropsychological Models of Childhood Psychiatric Disorders. In: Banaschewski T, RLA, editors. Biological Child Psychiatry. Recent Trends and Developments. Vol. 24. Basel: Karger; 2008. pp. 195–226. [Google Scholar]