Abstract
For more than a half century, autoimmunity has been linked to a diverse array of heart diseases including rheumatic carditis, myocarditis, Chagas’ cardiomyopathy, post-myocardial infarction (Dressler’s) syndrome, and idiopathic dilated cardiomyopathy. Why the heart is targeted by autoimmunity in these seemingly unrelated conditions has remained enigmatic. Here, we discuss our recent studies indicating that this susceptibility is mediated by impaired negative selection of autoreactive α-myosin heavy chain -specific CD4+ T cells in the thymus of both mice and humans. We describe how this process may place the heart at increased risk for autoimmune attack following ischemic or infectious injury, providing a rationale for the development of antigen-specific tolerogenic therapies.
Introduction
Myocarditis, an inflammatory disease of heart muscle, is a common cause of heart failure in young patients(Andrews et al. 2008). Although cardiotropic viruses are considered to be the leading etiologic agents of myocarditis in North America and Europe(Cooper 2009), the cause of disease in most patients is unknown (Stewart et al. 2011). Approximately one third of cases resolve, but dilated cardiomyopathy (DCM) with chronic congestive heart failure is the major long-term sequelae of myocarditis (Cooper 2009). Chronic autoimmunity has long been postulated to underlie the progression from myocarditis to DCM. Until recently, the major focus of clinical investigation has been on the role of humoral immunity in DCM(Lappe et al. 2008). However, it has been unclear whether autoantibodies are primary disease mediators or markers of myocardial injury. From an immunological perspective, a major gap in our understanding the autoimmune basis of DCM is the identity of the specific self-antigen target(s) that elicits such destructive responses and the mechanism(s) by which self-tolerance is broken. In this review, we will discuss our recent findings showing that myocarditis is mediated by α-myosin heavy chain (α-myosin)-specific CD4+ T cells that escape thymic negative selection (‘central tolerance’), a major barrier against autoimmunity. In addition, our group has found that humans also lack expression of α-myosin in thymus and, accordingly, healthy individuals show relatively large numbers of α-myosin-specific T cells in peripheral blood, with markedly increased frequencies of autoreactive T cells in patients with myocarditis and inflammatory DCM. We will discuss how impaired central tolerance may place the heart at risk not only for primary autoimmune myocarditis, but also for autoimmune attack following infection by cardiotropic viruses or the protozoan Trypanosoma cruzi (T. cruzi). In addition, we will discuss our most recent studies demonstrating that ischemic heart injury alone – in the absence of microbial agents – can trigger chronic myocardial autoimmunity in autoimmune-prone mice and humans(Gottumukkala et al. 2012). Finally, we will discuss how the impaired tolerance to α-myosin provides a unifying explanation of why the heart is so commonly targeted by autoimmunity, and how these new insights have direct relevance to the diagnosis and treatment of autoimmune heart disease.
Critical role of the thymus in establishing self-tolerance
The primary role of the immune system is to protect the body from infectious agents. To handle potential challenges from an enormously diverse and continuously evolving microbial universe, T lymphocytes of the adaptive immune system must exhibit immense variability in their antigen receptor specificity. Because the T cell receptor diversification process in thymus is random, it inevitably gives rise to T cells that react to self-antigens. The immune system has devised numerous mechanisms to eliminate these self-reactive cells that can potentially cause autoimmunity. Most T cells with self-reactive receptors are induced to die in the thymus through an apoptotic process called negative selection. This process (‘central tolerance’) represents the first and most important mechanism by which autoimmune reactions are prevented and self-tolerance is established (von Boehmer & Melchers 2010).
It is now well established that a subset of stromal cells in the thymus, called medullary thymic epithelial cells, have the unusual ability to express transcripts encoding a wide range of tissue-specific antigens previously considered to be expressed exclusively in peripheral tissues(Derbinski et al. 2001). Relatively subtle variations in the thymic level of tissue-specific antigen expression can profoundly modulate susceptibility to various organ-specific autoimmune diseases(Kyewski & Klein 2006). This is exemplified by type 1 diabetes, in which inherited promoter variants that decrease expression of insulin transcripts in thymus of humans (IDDM2) are associated with increased susceptibility to disease (Pugliese et al. 1997, Vafiadis et al. 1997). Similar inverse correlations were observed between thymic expression levels of the α-subunit of muscle acetycholine receptor, the pathogenic autoantigen in myasthenia gravis, and the incidence of clinical disease(Giraud et al. 2007).
However, the process of central tolerance is not fool-proof as all individuals harbor circulating lymphocytes that are capable of reacting to self-antigens. This leakiness of thymic tolerance can be controlled by various ‘peripheral tolerance’ mechanisms (occurring in spleen, lymph node, or the target tissue itself) (Mueller 2010). It also needs to be appreciated that not all autoreactive T cell responses – or autoantibodies – are necessarily detrimental: they may bind autoantigens with too low an affinity to trigger an autoimmune tissue damage, but may bind strongly enough to invading pathogens to exert a protective host-defense effect(von Boehmer & Melchers 2010).
Why the Heart may be Particularly Vulnerable to T Cell-Mediated Autoimmune Attack
Until recently, the majority of published studies on the pathogenesis of DCM have involved the experimental induction of disease, whereby myocarditis is induced by infection with Coxsackievirus B3 (CB3) or by immunization with cardiac myosin, or peptides unique to α-myosin, in bacteria-containing adjuvant. These studies have yielded important insights into how viral injury can trigger inflammatory heart disease. However, several lines of evidence suggest that a subgroup of patients develop myocarditis of primary autoimmune origin, i.e., in the absence of co-existing infection or pathological injury trigger. For example, it has been reported that patients with biopsy-proven lymphocytic myocarditis and circulating autoantibodies to cardiac tissues, but without evidence of viral genome in the myocardium, may benefit from immunosuppression(Frustaci et al. 2003). In addition, autoantibodies against cardiac myosin have been found in subgroups of DCM patients and their first-degree relatives, suggesting familial clustering(Caforio et al. 1994), a characteristic feature of organ-specific autoimmune disease. Interestingly, 19% of patients with giant-cell myocarditis have coexistent autoimmune disorders suggesting clustering of autoimmune disorders within individuals(Cooper 2009). Furthermore, giant-cell myocarditis can recur in transplanted hearts(Cooper 2009); disease recurrence has also been noted following pancreas transplantation in type 1 diabetes(Tyden et al. 1996).
Direct experimental evidence that myocarditis can be of primary autoimmune origin was provided when we and another group serendipitously discovered that transgenic non-obese diabetic (NOD) mice expressing the high risk type 1 diabetes-associated human major histocompatibility complex (MHC) class II allele, HLA-DQ8 (hereafter, DQ8), instead of the endogenous murine class II gene, I-Ag7, spontaneously developed autoimmune myocarditis with high-titer autoantibodies to cardiac myosin and premature death due to congestive heart failure, resembling human myocarditis(Elliott et al. 2003, Taylor et al. 2004). Of note, DQ8 is known to be in strong linkage disequilibrium with HLA-DR4, which has also been linked to susceptibility to DCM(Carlquist et al. 1991, McKenna et al. 1997). It was shown that spontaneous myocarditis in DQ8+NOD mice was primarily mediated by CD4+ T cells and not autoantibodies. This model demonstrated for the first time that myocarditis could arise spontaneously, in the absence of microbial or pathological injury triggers(Elliott et al. 2003, Taylor et al. 2004). Furthermore, our group found that myocarditis did not develop when the same panel of DQ8 transgenes were expressed on another genetic background (i.e., C57BL/6), indicating that both MHC and non-MHC genes are required for the development of this disease process(Taylor et al. 2004), similar to other organ-specific autoimmune diseases(Gregersen & Olsson, 2009).
The development of a spontaneous myocarditis model stimulated a reevaluation of the mechanism by which cardiac self tolerance is abrogated. More recently, our group showed that α-myosin is the earliest detectable target of autoantibodies in DQ8+NOD mice and that CD4+ T cell clones specific for α-myosin adoptively transferred myocarditis into immunodeficient hosts, demonstrating the importance of this autoantigen in the disease process(Lv et al. 2011). In searching a publically available microarray database [the National Center for Biotechnology Information (NCBI) gene expression omnibus (GEO), accession number GSE85)], we found that α-myosin transcripts were barely at detection limits in medullary thymic epithelial cells, whereas transcripts for the β-myosin heavy chain (β-myosin) – which is also expressed in skeletal muscle - were clearly present. Since it can be difficult with microarray analyses to interpret expression levels in the very low range, we then performed extensive RT-PCR analyses (including quantitative RT-PCR assays) on purified thymic stromal subsets. These studies confirmed that α-myosin expression was absent in medullary thymic epithelial cells (Lv et al., 2011). We further showed that when α-myosin expression was transgenically introduced into thymic epithelial cells, the DQ8+NOD mice became “tolerant” to α-myosin (i.e., autoantibody and autoreactive T cell responses to cardiac myosin were no longer detectable, suggesting that thymic negative selection induced deletion of α-myosin-specific T cells) and myocarditis was prevented(Lv et al. 2011). These studies thus clearly demonstrated that α-myosin is a primary (i.e., initiating) autoantigen in autoimmune myocarditis and pointed to a previously unrecognized role for central tolerance in conferring susceptibility to inflammatory cardiomyopathy(Lv et al., 2011). It is important to note that the lack of α-myosin expression in thymus was not unique to DQ8+NOD mice, but was also observed in other mouse strains (NOD and C57BL/6) indicating that lack of α-myosin expression in thymus is necessary, but not sufficient, for the development of myocarditis.
The relevance of these findings to humans was highlighted when we performed similar quantitative RT-PCR analyses on purified human thymic stromal cell subsets and discovered that humans also lack α-myosin in thymic medullary epithelial cells. These findings correlated with the presence of high frequencies of autoreactive α-myosin-specific T cells in the blood of healthy control subjects [as assessed by ex vivo interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assays], with markedly augmented responses in patients with myocarditis(Lv et al. 2011). Since α-myosin constitutes a minor fraction of the total myosin protein expressed in human ventricle (Miyata et al. 2000), these findings challenged the longstanding notion that cardiac myosin is targeted by the immune system primarily because of its cardiac abundance and instead pointed to impaired central tolerance mechanisms.
Other Heart Conditions in Which Cardiac Myosin-Reactive T cells Have Been Implicated
1. Postviral myocarditis
CB3 has traditionally been linked to myocarditis and Noel Rose and colleagues were the first to demonstrate infection of susceptible strains of mice with CB3 virus leads to chronic myocarditis that resembles the human form of the disease(Wolfgram et al. 1985). Both patients with myocarditis and CB3-infected mice were noted to have autoantibodies that react with various heart antigens, the most prominent of which is cardiac myosin (Caforio et al. 1992, Neu et al. 1987a).
Based on the hypothesis that post-viral myocarditis is triggered by autoimmune responses to cardiac myosin, an experimental autoimmune myocarditis (EAM) model was established by immunizing mice with purified cardiac myosin emulsified in complete Freund’s adjuvant(Neu et al. 1987b). EAM was antigen-specific in that it could be induced by cardiac, but not skeletal, myosin(Neu et al. 1987b, Smith & Allen 1991). Subsequent studies showed that EAM is mediated by CD4+ T cells with pathogenic epitopes of myosin residing in the sequences present in α- but not in β-myosin(Donermeyer et al. 1995, Eriksson et al. 2003, Pummerer et al. 1996). Given that these two cardiac myosin isoforms share 93% amino acid identity, the biologic basis for enhanced immunogenicity of α-myosin was unclear. Our recent studies showing lack of central tolerance to α-myosin may provide an explanation for the preferential T cell targeting of α-myosin in EAM and CB3 myocarditis (Lv et al., 2011, Gottumukkala et al., 2012).
Interestingly, it has been suggested that α- and β-myosin have equal capability to induce myocarditis in rat(Kohno et al. 2001). In addition, it has been shown that peptides corresponding to human β-myosin are capable of inducing myocarditis in rat(Galvin et al. 2002). The basis for this potential discrepancy is unclear but may relate to the different source of proteins for immunization (e.g., from tissue versus recombinantly produced in bacteria), species-specific differences in proteins (e.g., human versus rat), and/or host-specific differences in MHC class II molecules.
2. Chagas’ Disease
Chagas’ cardiomyopathy, caused by T. cruzi, is thought to be a form of chronic myocarditis in which the T-cell enriched myocardial infiltrate play a major pathogenic role(Marin-Neto et al. 2007). The scarcity of T. cruzi parasites in the intensely inflamed heart tissue has further supported a role for autoimmunity in the pathogenesis of Chagas’ cardiomyopthy. We postulate that lack of CD4+ T cell thymic tolerance to α-myosin may also explain how T. cruzi infection can induce chronic myocarditis. Of note, both T. cruzi-specific and cardiac myosin-specific CD4+ T cells are found in the myocardium of patients with Chagas’s cardiomyopathy, indicating that both antigens may contribute to inflammation-induced myocardial damage(Cunha-Neto et al. 1996, Marin-Neto et al. 2007). However, the T-cruzi-specific and cardiac myosin-specific autoimmune responses may not be mutually exclusive in the perpetuation of myocardial damage in Chagas disease(Marin-Neto et al. 2007). Experimental evidence also suggests that α-myosin is also a major autoantigen of pathogenic CD4+ T cells in a Chagas’ cardiomyopathy murine model(Rizzo et al. 1989). Interestingly, peripheral tolerance induction with a cardiac myosin-enriched cardiac homogenate resulted in reduced myocarditis and fibrosis in this Chagas model(Pontes-de-Carvalho et al., 2002). These studies suggest that antigen-specific tolerogenic therapies may not only useful in primary autoimmune myocarditis(Lv et al., 2011) but also in myocarditis secondary to infectious pathogens.
3. Post-Infarct Autoimmunity (“PIA”)
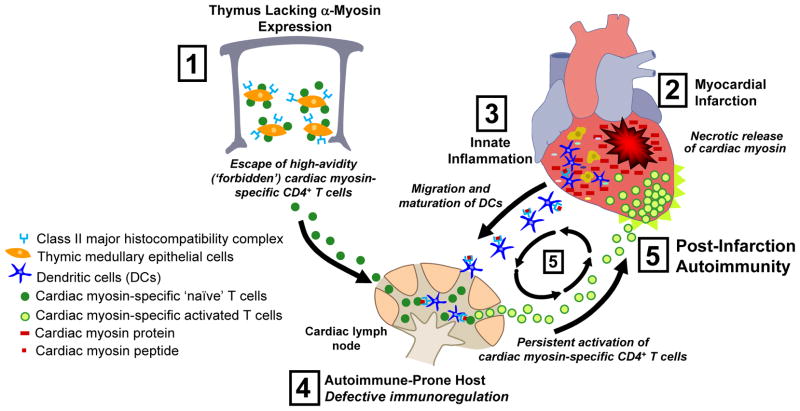
MI is known to trigger a profound innate inflammatory response with influx of dendritic cells and macrophages along with the production of proinflammatory cytokines that are crucial for cardiac repair (Fig. 1, Gottumukkala et al. 2012). While these innate immune responses are essential for repair of damaged heart tissue, these same cytokines and signals from necrotic cells have been shown to be potent maturation factors for dendritic cells, rendering them capable of activating adaptive immune responses(Gallucci et al. 1999). However, it has been argued that the endogenous (‘danger‘) signals generated by tissue damage should not normally activate adaptive immunity against released tissue antigens since T cells specific for these self-antigens would normally have been removed by thymic negative selection(Medzhitov 2008).
Figure 1. Proposed pathogenesis of post-infarction autoimmunity (PIA).
(1) Absence of α-myosin expression in thymic medullary epithelial cells leads to the escape of high-avidity cardiac myosin (CM)-specific naïve CD4+ T cells into the peripheral blood. (2) Myocardial infarction triggers the release of large amounts of CM, followed by (3) influx of innate immune cells including macrophages and dendritic cells (DCs) into the infarct zone, with engulfment of necrotic myocytes and activation (‘maturation’) of DCs. (4) Mature DCs then migrate to cardiac-draining lymph node and present CM peptides to naïve CD4+ T cells. In autoimmune-prone hosts, such as NOD mice or type 1 diabetes patients, CM-specific CD4+ T cells become persistently activated, egress out of cardiac LN, and migrate back into the injured heart, causing further injury. (5) This secondary injury forms a ‘positive feedback loop’ for the persistent activation and expansion of CM-specific CD4+ T cells, leading to chronic myocarditis with impaired infarct healing and adverse ventricular remodeling. This model may also be relevant to cardiac autoimmunity induced by pathogens such as Coxsackievirus B3 and T. cruzi.
As discussed above, our group has shown that α-myosin is absent in human thymus and that this is associated with a peripheral T cell repertoire highly enriched in reactivities against cardiac myosin(Lv et al., 2011) (Fig. 1). One implication of these findings is that individuals in the general population might be at high risk for developing cardiac autoimmunity after heart injury. Indeed, over 50% of people in the general population have been reported to develop autoantibodies and augmented T cell reactivity to cardiac myosin after acute myocardial infarction, but these responses are transient and resolve once the damaged heart heals(Dressler 1956, Moraru et al. 2006). It therefore seemed plausible that in autoimmune-prone individuals, for example patients with type 1 diabetes, these same reactions might become self-perpetuating and amplified, with pathological consequences (Gottumukkala et al. 2012).
Indeed, our group has recently shown that experimental induction of MI in NOD mice - the most widely used animal model of human type 1 diabetes - results in a severe post-infarction autoimmune (PIA) syndrome characterized by destructive lymphocytic infiltrates in the myocardium, infarct expansion, along with sustained autoantibody production and proinflammatory Th1 effector cell responses against cardiac myosin(Gottumukkala et al. 2012). Furthermore, PIA was prevented by inducing thymic tolerance to α-myosin, demonstrating that immune responses to cardiac myosin are essential for this disease process. We further identified shared cardiac myosin autoantibody “signatures” between post-MI T1D patients and non-diabetic patients with acute myocarditis, and confirmed the presence of myocarditis in an autoantibody-positive type 1 diabetic patient by cardiac magnetic resonance imaging techniques(Gottumukkala et al. 2012). These findings provide experimental and clinical evidence for a post-MI autoimmune syndrome in type 1 diabetes that may contribute to the poor cardiovascular disease outcomes observed in these patients.
Concluding Remarks
Our discovery of impaired central tolerance to α-myosin provides a novel mechanistic framework for an array of seemingly unrelated forms of heart diseases in which autoimmunity has been postulated to play a role. Further work needs to be performed to determine whether the presence of augmented T cell responses to cardiac myosin in patients with myocarditis is predictive of progression to DCM. These studies highlight a potential role for antigen-specific tolerogenic therapies in inflammatory heart disease. This could be an especially attractive option in the setting of acute MI in patients with type 1 diabetes, in whom therapy to block α-myosin autoreactivity would only be required during a limited time window (i.e., months) to enable the infarcted heart tissue to properly heal, and would not need to be administered lifelong. Although the initial focus of our MI studies has been on patients with type 1 diabetes, these findings could have broad relevance to individuals with other autoimmune disorders, who comprise 5–8% of the general population.
Acknowledgments
This work was supported by the NIDDK, NHLBI, Seaver Institute and American Diabetes Association (to M.A.L.). H.L. is a recipient of a Postdoctoral Fellowship Award from the Juvenile Diabetes Research Foundation International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews RE, Fenton MJ, Ridout DA, Burch M. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United kingdom and Ireland. Circulation. 2008;117:79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- Caforio AL, Grazzini M, Mann JM, et al. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85:1734–1742. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- Caforio AL, Keeling PJ, Zachara E, et al. Evidence from family studies for autoimmunity in dilated cardiomyopathy. Lancet. 1994;344:773–777. doi: 10.1016/s0140-6736(94)92339-6. [DOI] [PubMed] [Google Scholar]
- Carlquist JF, Menlove RL, Murray MB, O’Connell JB, Anderson JL. HLA class II (DR and DQ) antigen associations in idiopathic dilated cardiomyopathy: Validation study and meta-analysis of published HLA association studies. Circulation. 1991;83:515–522. doi: 10.1161/01.cir.83.2.515. [DOI] [PubMed] [Google Scholar]
- Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Neto E, Coelho V, Guilherme L, Fiorelli A, Stolf N, Kalil J. Autoimmunity in Chagas’ disease. Identification of cardiac myosin-B13 Trypanosoma cruzi protein cross reactive T cell clones in heart lesions of a chronic Chagas’ cardiomyopathy patient. J Clin Invest. 1996;98:1709–1712. doi: 10.1172/JCI118969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Donermeyer DL, Beisel KW, Allen PM, Smith SC. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. J Exp Med. 1995;182:1291–1300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler W. A post-myocardial infarction syndrome: preliminary report of a complication resembling idiopathic, recurrent, benign pericarditis. J Am Med Assoc. 1956;160:1379–1383. doi: 10.1001/jama.1956.02960510005002. [DOI] [PubMed] [Google Scholar]
- Elliott JF, Liu J, Yuan ZN, et al. Autoimmune cardiomyopathy and heart block develop spontaneously in HLA-DQ8 transgenic IAbeta knockout NOD mice. Proc Natl Acad Sci U S A. 2003;100:13447–13452. doi: 10.1073/pnas.2235552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U, Ricci R, Hunziker L, et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nature medicine. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Chimenti C, Calabrese F, Pieroni M, Thiene G, Maseri A. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation. 2003;107:857–863. doi: 10.1161/01.cir.0000048147.15962.31. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nature medicine. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. 2002;160:297–306. doi: 10.1016/S0002-9440(10)64373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud M, Taubert R, Vandiedonck C, et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature. 2007;448:934–937. doi: 10.1038/nature06066. [DOI] [PubMed] [Google Scholar]
- Gottumukkala R, Lv H, Cornivelli L, et al. Myocardial Infarction Triggers Chronic Cardiac Autoimmunity in Type 1 Diabetes. Sci Transl Med. 2012;4:138ra80. doi: 10.1126/scitranslmed.3003551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen PK, Olsson LM. Recent advances in the genetics of autoimmune disease. Annu Rev Immunol. 2009;27:363–391. doi: 10.1146/annurev.immunol.021908.132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K, Takagaki Y, Aoyama N, Yokoyama H, Takehana H, Izumi T. A peptide fragment of beta cardiac myosin heavy chain (beta-CMHC) can provoke autoimmune myocarditis as well as the corresponding alpha cardiac myosin heavy chain (alpha-CMHC) fragment. Autoimmunity. 2001;34:177–185. doi: 10.3109/08916930109007382. [DOI] [PubMed] [Google Scholar]
- Kyewski B, Klein L. A Central Role for Central Tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Pelfrey CM, Tang WH. Recent insights into the role of autoimmunity in idiopathic dilated cardiomyopathy. J Card Fail. 2008;14:521–530. doi: 10.1016/j.cardfail.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Havari E, Pinto S, et al. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011;121:1561–1573. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- McKenna CJ, Codd MB, McCann HA, Sugrue DD. Idiopathic dilated cardiomyopathy: familial prevalence and HLA distribution. Heart. 1997;77:549–552. doi: 10.1136/hrt.77.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–390. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- Moraru M, Roth A, Keren G, George J. Cellular autoimmunity to cardiac myosin in patients with a recent myocardial infarction. International journal of cardiology. 2006;107:61–66. doi: 10.1016/j.ijcard.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- Neu N, Beisel KW, Traystman MD, Rose NR, Craig SW. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987a;138:2488–2492. [PubMed] [Google Scholar]
- Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987b;139:3630–3636. [PubMed] [Google Scholar]
- Pontes-de-Carvalho L, Santana CC, Soares MB, Oliveira GG, Cunha-Neto E, Ribeiro-dos-Santos R. Experimental chronic Chagas’ disease myocarditis is an autoimmune disease preventable by induction of immunological tolerance to myocardial antigens. J Autoimmun. 2002;18:131–138. doi: 10.1006/jaut.2001.0574. [DOI] [PubMed] [Google Scholar]
- Pugliese A, Zeller M, Fernandez A, Jr, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- Pummerer CL, Luze K, Grassl G, et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo LV, Cunha-Neto E, Teixeira AR. Autoimmunity in Chagas’ disease: specific inhibition of reactivity of CD4+ T cells against myosin in mice chronically infected with Trypanosoma cruzi. Infect Immun. 1989;57:2640–2644. doi: 10.1128/iai.57.9.2640-2644.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–2147. [PubMed] [Google Scholar]
- Stewart GC, Lopez-Molina J, Gottumukkala RV, et al. Myocardial parvovirus B19 persistence: lack of association with clinicopathologic phenotype in adults with heart failure. Circ Heart Fail. 2011;4:71–78. doi: 10.1161/CIRCHEARTFAILURE.110.958249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Havari E, McInerney MF, Bronson R, Wucherpfennig KW, Lipes MA, et al. A spontaneous model for autoimmune myocarditis using the human MHC molecule HLA-DQ8. J Immunol. 2004;172:2651–2658. doi: 10.4049/jimmunol.172.4.2651. [DOI] [PubMed] [Google Scholar]
- Tyden G, Reinholt FP, Sundkvist G, Bolinder J. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. New Engl J Med. 1996;335:860–864. doi: 10.1056/NEJM199609193351205. [DOI] [PubMed] [Google Scholar]
- Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- Wolfgram LJ, Beisel KW, Rose NR. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. 1985;161:1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]