Abstract
Podocyte detachment and reduced endothelial cell fenestration and relationships between these features and the classic structural changes of diabetic nephropathy have not been described in patients with type 2 diabetes. Here we studied these relationships in 37 Pima Indians with type 2 diabetes of whom 11 had normal albuminuria, 16 had microalbuminuria, and 10 had macroalbuminuria. Biopsies from ten kidney donors (not Americans Indians) showed almost undetectable (0.03%) podocyte detachment and 43.5% endothelial cell fenestration. In patients with type 2 diabetes, by comparison, the mean percentage of podocyte detachment was significantly higher in macroalbuminuria (1.48%) than in normal albuminuria (0.41%) or microalbuminuria (0.37%). Podocyte detachment correlated significantly with podocyte number per glomerulus and albuminuria. The mean percentage of endothelial cell fenestration was significantly lower in macroalbuminuria (19.3%) than in normal (27.4%) or microalbuminuria (27.2%) and correlated significantly with glomerular basement membrane thickness, albuminuria, fractional mesangial area, and the glomerular filtration rate (iothalamate clearance). Podocyte detachment and diminished endothelial cell fenestration were not correlated, but were related to classic lesions of diabetic nephropathy. Thus, our findings confirm the important role these injuries play in the development and progression of kidney disease in type 2 diabetes, just as they do in type 1 diabetes. Whether podocyte detachment creates conduits for proteins to escape the glomerular circulation and reduced endothelial fenestration lowers glomerular hydraulic permeability requires further study.
INTRODUCTION
Podocytes and glomerular endothelial cells are essential for normal glomerular capillary permeability. Derangements of the architecture of these cells have significant effects on kidney function [1, 2]. In diabetic kidney disease, podocytes and glomerular endothelial cells appear to participate in both the initiation and progression of nephropathy [3–9]. We reported previously that podocyte loss is associated with diabetic nephropathy [10], that it predicts progression of nephropathy at least as well as mesangial expansion [11], and that loss of podocytes assessed by serial kidney biopsy occurs during the transition from microalbuminuria to more advanced nephropathy [12].
Podocyte detachment (PD) and reduced endothelial cell fenestration (ECF) on electron microscopy and the quantitative relationships between these features and the classic structural changes of diabetic nephropathy have been described in persons with type 1 diabetes (T1DM) [9] but not in those with type 2 diabetes (T2DM). We used morphometric methods to examine the relationship between PD and reduced ECF and other structural and functional features of diabetic nephropathy in 37 Pima Indians with T2DM. Ten healthy kidney transplant donors, none of whom were American Indians, served as controls.
RESULTS
Clinical characteristics of the 10 normal kidney donors (6 Caucasians, 2 Asian, 2 African Americans) before donation, and the 37 diabetic subjects (all American Indians) at the renal clearance study closest to the kidney biopsy, are summarized in Table 1. Mean fasting plasma glucose concentration among the kidney donors was 87±8 mg/dl. Among the diabetic subjects, time between the clearance study and the biopsy averaged 0.22 years (range=0.02–0.55 years). Eleven subjects had normal urinary albumin excretion (albumin/creatinine ratio (ACR) <30 mg/g), 16 had microalbuminuria (ACR=30–299 mg/g), and 10 had macroalbuminuria (ACR ≥300 mg/g) at the time of the clearance study. Diabetes duration (p=0.002) and HbA1c (p=0.008) were greater in participants with microalbuminuria than in those with normoalbuminuria (p=0.008). Glomerular filtration rate (GFR) was highest in those with microalbuminuria (p=0.030 vs. normoalbuminuria, p=0.004 vs. macroalbuminuria). A minority of normoalbuminuric participants and a majority of micro- and macroalbuminuric participants (Table 1) were prescribed angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers. Some were also prescribed other antihypertensive medicines, especially diuretics.
Table 1.
Patient characteristics at biopsy from 37 Pima Indians with type 2 diabetes mellitus.
| Donors (n=10) |
Normo- (n=11) |
Micro- (n=16) |
Macro- (n=10) |
P value | |||
|---|---|---|---|---|---|---|---|
| Fraction female | 5/10 | 10/11 | 11/16 | 5/10 |
a0.064 d0.350 |
b0.425 e0.064 |
c1.000 f0.425 |
| Age (years) | 47±11.7 | 45.7±11.0 | 48.1±9.0 | 48.3±7.3 |
a0.959 d0.512 |
b0.806 e0.605 |
c1.000 f0.698 |
| BMI (kg/m2) | 25.2±3.2 | 35.9±7.7 | 35.5±8.5 | 34.5±8.0 |
a6.8·10−5 d0.716 |
b2.5·10−4 e0.512 |
c0.002 f0.897 |
| Duration (years) | N/A | 12.6±3.0 | 16.4±7.2 | 19.8±5.4 |
aN/A d0.178 |
bN/A e0.002 |
cN/A f0.100 |
| HbA1c (%) | N/A | 7.7±1.4 | 8.4±2.3 | 9.8±1.6 |
aN/A d0.181 |
bN/A e0.008 |
cN/A f0.205 |
| MAP (mmHg) | 92±11 | 88±8 | 94±9 | 98±15 |
a0.512 e0.131 |
b0.542 e0.173 |
c0.363 f0.525 |
| ACR (mg/g) | 5 (3–91) |
19 (7–26) |
61 (36–212) |
978 (358–3762) |
§ | § | § |
| GFR (ml/min) | 110±24 | 127±48 | 172±56 | 111±49 |
a0.191 d0.030 |
b0.002 e0.314 |
c0.670 f0.004 |
| Anti- hypertensive treatment (%)* |
0 | 27.3 | 62.5 | 60.0 |
a0.214 d0.120 |
b0.003 e0.198 |
c0.011 f1.000 |
P values are shown for
donors vs. normo-,
donors vs. micro-,
donors vs. macro-,
normo vs. micro-,
normo- vs. macro-, and
micro- vs. macroalbuminuric groups.
P values less than 0.05 are shown in bold.
Data are means ± SD, median (range), or percent. MAP, mean arterial pressure; ACR, albumin-to-creatinine ratio; GFR, glomerular filtration rate.
Anti-hypertensive treatment included angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers in any patients receiving therapy, including the drug provided as part of the trial; N/A, not applicable.
ACR values by group are different by definition.
The structural characteristics are described in Table 2. Among subjects with diabetes, those with macroalbuminuria had higher percentage of fractional interstitial area (FIA; p=0.01 vs. normoalbuminuria, p=9.1×10−4 vs. microalbuminuria), higher fractional mesangial area (p=3.8×10−4 vs. normoalbuminuria, p=5.6×10−4 vs. microalbuminuria), wider glomerular basement membranes (GBM; p=4.8×10−3 vs. normoalbuminuria, p=0.05 vs. microalbuminuria), lower filtration surface area density (p=4.8×10−3 vs. normoalbuminuria, p=4.1×10−3 vs. microalbuminuria), and lower total filtration surface area (p=0.03 vs. normoalbuminuria, p=8.7×10−3 vs. microalbuminuria). Podocyte number was lower with higher levels of albuminuria, but not significantly so.
Table 2.
Glomerular structural parameters from 10 normal kidney donors and 37 Pima Indians with type 2 diabetes mellitus.
| Donors (32 glomeruli) |
Normo- (39 glomeruli) |
Micro- (64 glomeruli) |
Macro- (39 glomeruli) |
P value | |||
|---|---|---|---|---|---|---|---|
| General structural variables | |||||||
| Global sclerosis (%) | 3.0±3.4 | 12±13 | 15±14 | 24±16 |
a0.20 d0.38 |
b3.8·10−3 e0.07 |
c5.9·10−3 f0.20 |
| Glomerular volume (× 106 μm3) | 2.7±1.0 | 4.8±1.2 | 5.5±1.8 | 6.1±3.0 |
a5.5·10−4 d0.37 |
b1.0·10−4 e0.43 |
c4.9·10−4 f0.94 |
| Fractional interstitial area (%) | 10±3 | 25±6 | 25±5 | 34±7 |
a5.7·10−6 d0.57 |
b3.8·10−7 e0.01 |
c1.1·10−5 f9.1·10−4 |
| Fractional mesangial area (%) | 7.5±2 | 16±5 | 17±6 | 30±9 |
a1.1·10−4 d0.68 |
b7.2·10−6 e3.8·10−4 |
c1.1·10−5 f5.6·10−4 |
| Filtration surface area density (μm2/μm3) | 0.12±0.03 | 0.08±0.03 | 0.08±0.02 | 0.05±0.02 |
a1.5·10−3 d0.98 |
b4.3·10−4 e4.8·10−3 |
c1.1·10−5 f4.1·10−3 |
| Total surface area (× 105 μm2) | 3.8±1.4 | 4.4±1.2 | 5.1±1.9 | 3.4±1.4 |
a0.15 d0.25 |
b0.06 e0.03 |
c0.72 f8.7·10−3 |
| GBM width (nm) | 322±41 | 438±84 | 499±153 | 608±131 |
a2.1·10−3 d0.48 |
b7.2·10−6 e4.8·10−3 |
c1.1·10−5 f0.05 |
| Non-podocyte cell (number/glomerulus) | 691±500 | 3214±1004 | 3989±1807 | 5363±2482 |
a5.7·10−6 d0.39 |
b3.8·10−7 e0.02 |
c1.1·10−5 f0.04 |
| Podocyte structural variables | |||||||
| Podocyte cell number/glomerulus | 880±316 | 686±249 | 644±295 | 611±168 |
a0.22 d0.48 |
b7.3·10−3 e0.60 |
c0.02 f0.94 |
| Filtration slit frequency (slits/mm) | 1507±154 | 1429±179 | 1348±154 | 1172±204 |
a0.42 d0.23 |
b0.02 e0.01 |
c9.9·10−4 f0.04 |
| Foot process width (nm) | 429±49 | 441±61 | 484±57 | 599±174 |
a0.85 d0.09 |
b0.01 e2.1·10−3 |
c6.9·10−4 f0.05 |
| Podocyte detachment (%) | 0.03±0.05 | 0.41±0.56 | 0.39±0.57 | 1.48±1.56 |
a0.05 d0.81 |
b0.05 e0.04 |
c3.6·10−3 f0.02 |
| Endothelial fenestration variable | |||||||
| Fenestrated endothelium (%) | 43.5±5.1 | 27.4±5.5 | 27.2±5.7 | 19.3±6.5 |
a2.3·10−5 d0.98 |
b2.6·10−6 e0.01 |
c2.2·10−5 f5.0·10−3 |
P values are shown for
donors vs. normo-,
donors vs. micro-,
donors vs. macro-,
normo- vs. micro-,
normo- vs. macro-, and
micro- vs. macroalbuminuric groups.
P values less than 0.05 are shown in bold.
Data are means ± SD. GBM, glomerular basement membrane.
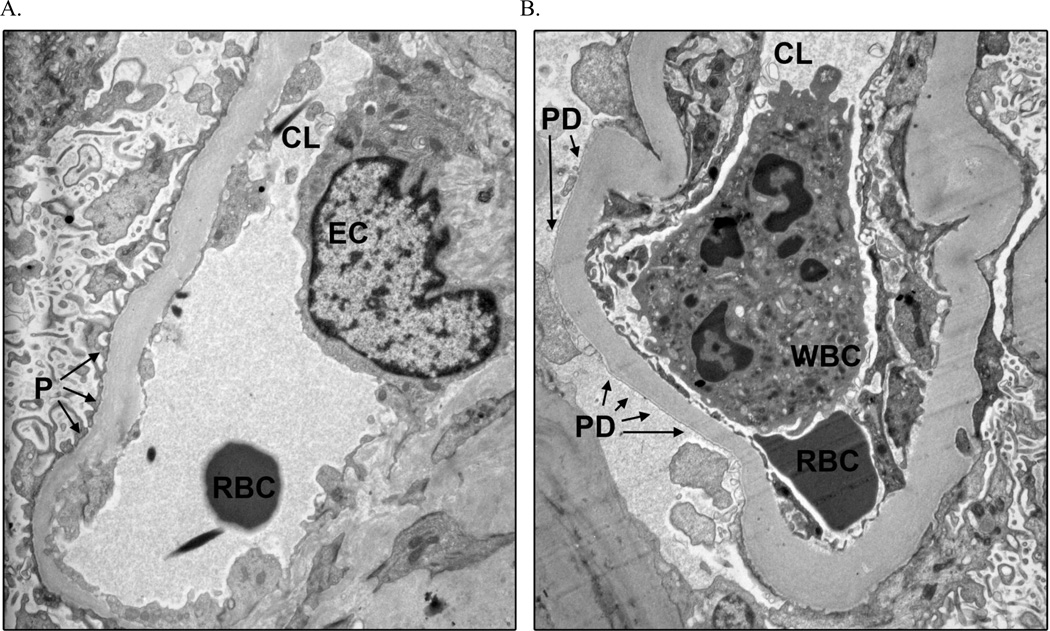
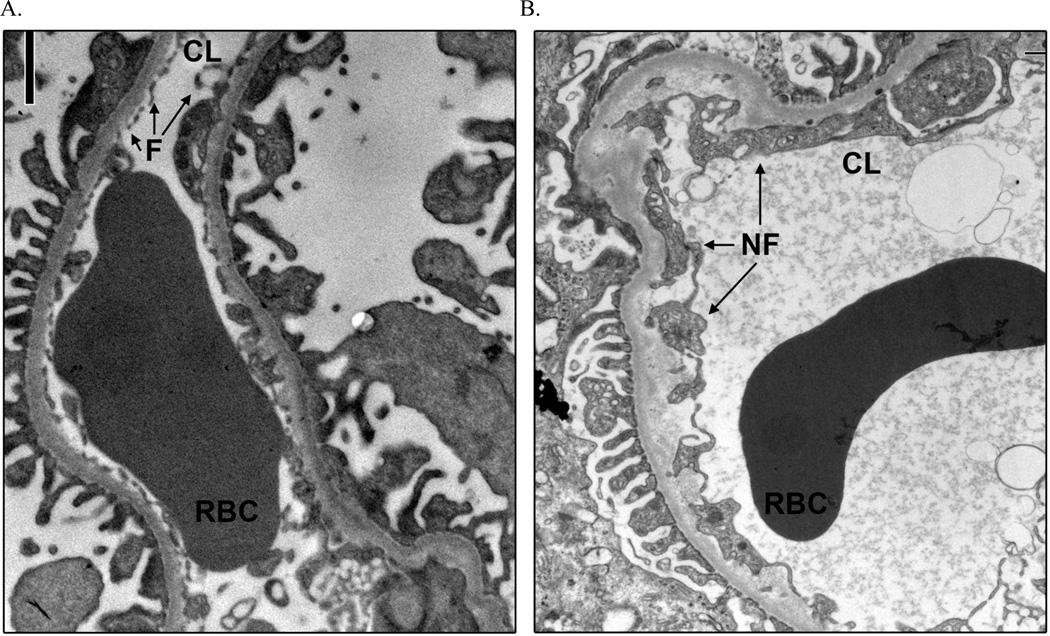
Mean percentage of PD was higher in participants with macroalbuminuria (1.48%) than in those with normo- (0.41%; p=0.04) or microalbuminuria (0.37%; p=0.02). Mean percentage of normal ECF was lower in the macroalbuminuric group (19.3%) than in those with normo- (27.4%; p=0.01) or microalbuminuria (27.2; p=5.0×10−3). PD was virtually nonexistent (0.03%) and ECF was 43.5% in normal kidney donors. Figures 1 and 2 illustrate PD and abnormal ECF in T2DM. Percentage of normal ECF directly across the GBM from detached podocytes was 37.0% in the diabetic subjects, and the overall percentage of normal ECF was 27.2% in the same subjects (p=0.09).
Figure 1.
Peripheral glomerular capillaries illustrating intact and detached podocyte foot processes from a macroalbuminuric Pima Indian with type 2 diabetes mellitus, trasnmission electron microscopy ×11,280. (a) Intact podocyte foot processes. (b) Podocyte foot process detachment. CL, capillary lumen; EC, endothelial cell; P, podocyte foot processes intact; PD, podocyte foot process detachment; RBC, red blood cell; WBC, white blood cell.
Figure 2.
Peripheral glomerular capillaries illustrating an absence of normal endothelial cell fenestration, transmission electron microscoy ×11,280. (a) From a normal kidney donor, with normal endothelial cell fenestration. (b) Froma normoalbuminuric Pima Indian with type 2 diabetes mellitus and nonfenestrated endothelium. CL, capillary lumen; F, fenestrated endothelial cell; NF, nonfenestrated endothelial cell; RBC, red blood cell.
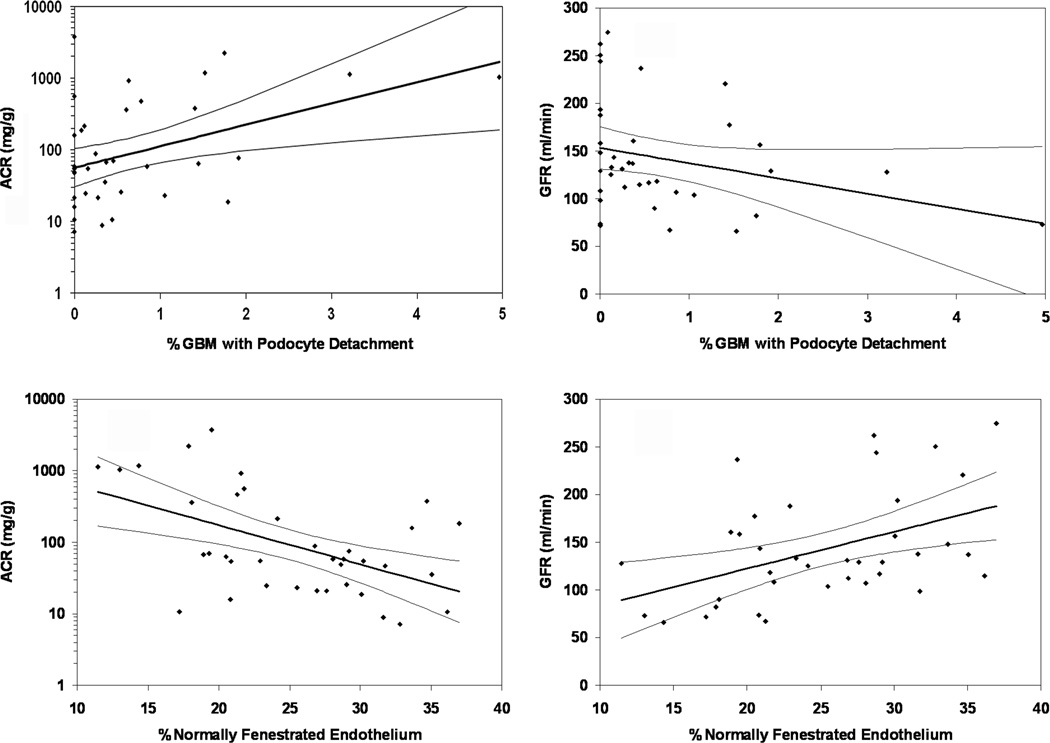
Spearman's correlations between structural and functional parameters for diabetic subjects are shown in Table 3; relationships between PD or ECF and functional characteristics are shown in Figure 3. Fractional mesangial area correlated positively with ACR (r=0.55, p<0.001) and negatively (r=−0.35, p=0.036) with GFR. Total surface area (SA) correlated positively with GFR (r=0.49, p=0.002). Foot process width (FPW) correlated positively with ACR (r=0.55, p<0.001), GBM width (r=0.38, p=0.020), and fractional mesangial area (r=0.59, p<0.001). The percentage of PD correlated positively with ACR (r=0.36, p=0.028) and negatively with podocyte number per glomerulus (r= −0.41, p=0.011). The percentage of fenestrated endothelium correlated negatively with GBM width (r=−0.38, p=0.019), FIA (r= −0.33, p=0.049), fractional mesangial area (r=−0.54, p<0.001) and ACR (r=−0.45, p=0.006) and positively with filtration surface area density (r=0.65, p<0.001) and GFR (r=0.44, p=0.006). The percentage of PD and of ECF were not correlated (r=−0.25, p=0.131).
Table 3.
Spearman's correlations (P-values) between physiologic and morphometric variables in 37 Pima Indians with type 2 diabetes mellitus.
| GFR | ACR | % GS | VG | FIA | AA | SV | SA | GBM | # E+M | # Podo | FSF | FPW | % Det | % Fen | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GFR | 1.0 | −0.12 (0.471) |
−0.25 (0.143) |
0.41 (0.012) |
−0.24 (0.151) |
−0.35 (0.036) |
0.24 (0.151) |
0.49 (0.002) |
−0.24 (0.154) |
0.01 (0.948) |
0.21 (0.207) |
−0.13 (0.457) |
0.15 (0.366) |
−0.30 (0.071) |
0.44 (0.006) |
| ACR | 1.0 | 0.31 (0.059) |
0.23 (0.174) |
0.48 (0.003) |
0.55 (<0.001) |
−0.47 (0.003) |
−0.27 (0.106) |
0.50 (0.002) |
0.41 (0.011) |
−0.14 (0.424) |
−0.50 (0.002) |
0.55 (<0.001) |
0.36 (0.028) |
−0.45 (0.006) |
|
| % GS | 1.0 | −0.09 (0.586) |
0.36 (0.028) |
0.25 (0.133) |
−0.35 (0.031) |
−0.28 (0.087) |
0.15 (0.360) |
0.17 (0.296) |
0.12 (0.476) |
−0.14 (0.406) |
0.14 (0.397) |
0.06 (0.730) |
−0.33 (0.044) |
||
| VG | 1.0 | −0.22 (0.192) |
0.04 (0.801) |
−0.26 (0.118) |
0.44 (0.006) |
0.15 (0.366) |
0.57 (<0.001) |
0.41 (0.012) |
−0.29 (0.082) |
0.35 (0.036) |
−0.05 (0.743) |
−0.01 (0.974) |
|||
| FIA | 1.0 |
0.38 (0.021) |
−0.26 (0.115) |
−0.37 (0.073) |
0.37 (0.026) |
0.16 (0.342) |
−0.22 (0.182) |
−0.23 (0.161) |
0.30 (0.074) |
0.25 (0.141) |
−0.33 (0.049) |
||||
| AA | 1.0 |
−0.73 (<0.001) |
−0.64 (<0.001) |
0.71 (<0.001) |
0.37 (0.024) |
−0.19 (0.250) |
−0.50 (0.002) |
0.59 (<0.001) |
0.19 (0.248) |
−0.54 (<0.001) |
|||||
| SV | 1.0 |
0.69 (<0.001) |
−0.50 (0.002) |
−0.43 (0.008) |
0.10 (0.537) |
0.52 (0.001) |
−0.51 (0.001) |
−0.40 (0.014) |
0.65 (<0.001) |
||||||
| SA | 1.0 |
−0.45 (0.005) |
0.05 (0.773) |
0.48 (0.002) |
0.22 (0.184) |
−0.168 (0.321) |
−0.38 (0.021) |
0.55 (0.056) |
|||||||
| GBM | 1.0 | 0.18 (0.285) |
−0.39 (0.017) |
−0.32 (0.051) |
0.38 (0.020) |
0.07 (0.672) |
−0.38 (0.019) |
||||||||
| # E+M | 1.0 |
0.49 (0.002) |
−0.35 (0.032) |
0.43 (0.009) |
−0.00 (0.999) |
−0.42 (0.010) |
|||||||||
| # Podo | 1.0 | 0.16 (0.361) |
−0.05 (0.756) |
−0.42 (0.011) |
0.06 (0.693) |
||||||||||
| FSF | 1.0 |
−0.93 (<0.001) |
−0.36 (0.027) |
0.27 (0.102) |
|||||||||||
| FPW | 1.0 | 0.28 (0.088) |
−0.28 (0.091) |
||||||||||||
| % Det | 1.0 | −0.25 (0.131) |
|||||||||||||
| % Fen | 1.0 |
P values less than 0.05 are shown in bold.
GFR, glomerular filtration rate (ml/min); ACR, albumin to creatinine ratio (mg/g); % GS, % global sclerosis; VG, glomerular volume (µm3); FIA, fractional interstitial area (%); AA, fractional mesangial area (%); SV, filtration surface area density (µm2/µm3); SA, total surface area (µm2); GBM, glomerular basement membrane width (nm); # E+M, number of endothelial + mesangial cells per glomerulus; # Podo, number of podocytes per glomerulus; FSF, filtration slit frequency (number/mm); FPW, foot process width (nm); % Det, % podocyte detachment along GBM; % Fen, % fenestrated endothelial cells.
Figure 3.
Correlations of albumin/creatinine ratio (ACR) or glomerular filtration rate (GFR) with percentage of podocyte detachment or percentage of normally fenestrated endothelium. (a, b) ACR (Pearson's r − 0.43, P = 0.0009) and GFR (Pearson's r = − 0.29, P = 0.084) as a function of percentage podocyte detachment in 37 Pima Indians with type 2 diabetes mellitus. The linear functions were not changed substantially by omission of the two individuals with the greatest amount of podocyte detachment. GBM, glomerular basement membrane. (c, d) ACR (Pearson's r = −0.51, P = 0.001) and GFR (Pearson's r = 0.45, P = 0.0005) as a function of the percentage of normally fenestrated endothelium in the same individuals. Regression lines and 95% confidence intervals are shown.
DISCUSSION
PD and reduced glomerular ECF are quantifiable structural features of nephropathy in T2DM that correlate positively with ACR and worsen in parallel with the classic structural features of diabetic kidney disease. Reduced glomerular ECF also correlates negatively with GFR. This is the first study to demonstrate an association between loss of normal ECF and declining GFR. We confirm that increasing PD correlates positively with increasing albuminuria, as shown in T1DM [9].
PD correlated negatively with podocyte number per glomerulus, a finding consistent with previous reports of reduced podocyte number and/or podocyte density in T1DM and T2DM [4–9, 13] and with the presence of podocyturia in patients with T2DM and diabetic nephropathy [14]. PD also correlated positively with albuminuria, whereas podocyte number did not, providing further evidence to implicate podocyte injury rather than podocyte loss as the primary structural factor responsible for decreased permselectivity of the glomerulus in diabetic nephropathy. Studies in Pima Indians with T2DM found that subjects with macroalbuminuria had increased filtration of high molecular weight dextrans through an excess of large pores that serve as macromolecular shunts, whereas microalbuminuric subjects had a shunt size no different from controls [15], suggesting that low-grade albuminuria may result from a different mechanism than nephrotic-range proteinuria. The significant increase in PD in the macroalbuminuric subjects suggests that these areas of detachment may represent the physical substrate of the excess shunt magnitude, and complete detachment of foot processes may occur along some portions of the GBM without loss of the entire podocyte. PD also correlated modestly, but not significantly, with declining GFR. As we suggested previously, when foot processes become detached, the remaining podocytes may spread their foot processes more broadly to cover what would otherwise be denuded basement membrane [10]. This broadening of the foot processes reduces filtration slit frequency, which in turn should lower hydraulic permeability and probably GFR [15, 16]. The negative correlation we found between PD and filtration slit frequency is consistent with this scenario.
ECF was reduced significantly in normoalbuminuric subjects compared with healthy kidney donors, and among those with T2DM, loss of ECF correlated significantly with albuminuria and GFR (Figure 3). Diminution of the endothelial glycocalyx occurs with exposure to high glucose concentrations. We speculate that the loss of this negatively charged portion of the filtration barrier could then result in the enhanced filtration of polyanionic albumin that has been reported both in vitro and in vivo [17, 18]. Loss of ECF may represent another pathological response of endothelial cells to hyperglycemia, but our fixation methods do not allow investigation of whether the degree of ECF correlates with the thickness of the glycocalyx. Lowered glomerular hydraulic permeability due, in part, to a reduction in the density and size of endothelial fenestrations is believed to be responsible for the decline in GFR in pre-eclampsia [19], and may also contribute to the decline in GFR in diabetes. The only other morphometric variable to correlate significantly with both albuminuria and GFR was fractional mesangial area, a finding reported previously in T1DM [20] and T2DM [10].
Participants with diabetes had significantly fewer endothelial fenestrae than nondiabetic kidney donors, yet the GFR in those with normo- or microalbuminuria was higher (Table 2). This seeming paradox is explained, in part, by diabetes-related glomerulomegaly increasing the total filtration surface area, thereby offsetting declines in glomerular hydraulic permeability caused by the reduced fenestration and the diminished filtration surface area density (Table 2).
The percentage of PD was determined by measuring the fraction of the entire peripheral capillary GBM length completely devoid of podocyte foot processes. Although we did not measure PD along the peri-mesangial GBM, PD was observed there as well. We defined PD as complete absence of foot processes because we wanted to avoid characterizing electron-lucent foot processes (which may represent sublethal cell injury) as areas of detachment, as filtration slit diaphragms between foot processes are still potentially intact in these areas, and the functional characteristics of the glomerular membrane potentially remain unchanged [21]. Moreover, we did not want to include areas with a mixture of intact and detached foot processes in our definition of detachment, because of uncertainties about their effect on permselectivity. In comparison, Toyoda et al. [9] counted areas including a mixture of intact and detached foot processes (“mixed” foot processes) in their measurements of detachment. These authors did not mention how electron-lucent foot processes were characterized according to their definitions of ‘attached,’ ‘detached,’ and ‘mixed.’ Accordingly, they reported 22% PD, whereas our different definition results in just over 1% PD. Regardless of these quantitative differences, the two studies demonstrate a process common to diabetic glomerulopathy in both T1DM and T2DM, but absent in normal kidneys [22]. Moreover, the two studies demonstrate strong similarities in the relationship of PD with the classic lesions of diabetic nephropathy and its associated functional abnormalities.
Podocyte production of vascular endothelial growth factor (VEGF) is important for the maintenance of normal ECF. Blocking production of VEGF or its effector pathways decreases ECF, capillary permeability, and GFR, and increases proteinuria [19, 23–25]. However, when we specifically examined endothelium abutting sections of basement membrane that were devoid of overlying foot processes we found no decrease in the mean proportion of normal ECF compared with the overall proportion of normal ECF, suggesting that loss of foot processes does not affect ECF locally and that loss of ECF does not affect local foot process attachment. One potential explanation is that impairment in production and release of VEGF by podocytes may be independent of foot process detachment. Indeed, PD was localized and limited, whereas reduction in ECF was relatively widespread, especially in macroalbuminuric subjects. Despite demonstrated podocyte-endothelial cross-talk in some circumstances, these cellular compartments appear to be differentially regulated (and perturbed).
The present cross-sectional study was designed to assess the functional and structural correlates of PD and loss of ECF at various stages of diabetic kidney disease defined by level of albuminuria. This comparison took place within an ongoing clinical trial, and treatment with angiotensin blockade may have affected the level of albuminuria. Moreover, although albuminuria and GFR strongly predict progressive kidney disease in diabetic Pima Indians [26] and the combination of these functional variables is a better predictor of ESRD and death than either measure alone [27], some investigators suggest that microalbuminuria is an unreliable predictor of diabetic nephropathy because it may regress spontaneously [28], and albuminuria may never increase in some patients despite worsening GFR [29]. Accordingly, some caution should be exercised in interpreting the relationship between these structural and functional measures.
Pima kidney transplant donors were not available to serve as controls. Instead, morphometric measurements from healthy non-diabetic Caucasian, African American, and Asian kidney donors were used for comparison. There were striking structural differences between the normal kidney donors and noromalbuminuric diabetic subjects in many stereological and morphometric variables. However, in the absence of information from non-diabetic Pima controls, conclusions regarding genetic, environmental, or disease-based differences cannot be inferred with certainty.
The results of this study are likely to be applicable to other populations. Previous studies in Pima Indians have provided valuable, generalizable insights into the pathophysiology of diabetic nephropathy. Aside from the larger size of glomeruli in both diabetic and non-diabetic Pima Indians [30], the observed structural changes of diabetic nephropathy are indistinguishable from those in other populations. Moreover, because details of the onset and duration of diabetes are known with much greater precision than in other populations, and because nearly all kidney disease is attributable to diabetes [31], findings in the Pima Indians may in fact offer a clearer picture of the effects of T2DM on the kidney than can be found in other populations.
In conclusion, we document PD and loss of ECF in humans with T2DM even prior to the development of overt nephropathy, and correlate these quantitative structural variables with other glomerular structural and functional characteristics of diabetic nephropathy. Our findings are consistent with those reported previously in T1DM [9], and confirm the important role that these distinct cellular injuries may play in the development and progression of diabetic glomerular disease in both T1DM and T2DM.
METHODS
Participants included 37 Pima Indians enrolled in a randomized, placebo-controlled, clinical trial to evaluate the renoprotective efficacy of losartan in T2DM (ClinicalTrials.gov number, NCT00340678). This study was approved by the review board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant gave informed consent. Subjects were ≥18 years old at enrollment in the trial. In addition to the randomized study treatment, diabetes and other anti-hypertensive therapies (including angiotensin converting enzyme inhibitors) were individualized for each patient by his or her primary care provider. Subjects underwent annual measurements of kidney function and a percutaneous kidney biopsy at the end of the randomized study treatment (~ 6 years). Kidney biopsies were performed under ultrasound guidance with a 15-gauge Boston Scientific Easy Core® biopsy needle. One or two cores of tissue were fixed in buffered 4% glutaraldehyde and shipped to the Beckman Center for Electron Microscopy at Stanford University where they were processed. Tissue was dehydrated through a series of graded ethanols, and the fixed tissues were embedded in Epon 812® before preparation for light and electron microscopy.
Ten healthy, non-Native American living kidney donors (not American Indians, who consented to participate in a longitudinal study of kidney function after donation, served as controls. After negative screening for diabetes, hypertension, and proteinuria, GFR from the urinary clearance of non-radioactive iothalamate was measured immediately before donation [32].
Measurements were made using strict stereologic and morphometric methods to account for two-dimensional sampling of three-dimensional objects, as described previously [10, 11]. To ensure randomized sampling and the use of only one cross-sectional profile per glomerulus, 0.5 µm sections taken at least 200 µm (average glomerular diameter) apart were cut, mounted on glass slides, and stained with toluidine blue. For light microscopy, photomicrographs were created at ×273 and ×2191 magnification. Those at ×273 were used to count the number of patent and globally-sclerotic glomeruli in sections of cortical tissue. Only glomeruli contained entirely within the core were studied. An average of 16 (range 5–79) of these complete glomeruli per subject were included in the measurements. An equation that takes into account the smaller diameter of sclerotic glomeruli, and the consequent difference in the probability of encountering a sclerotic or non-sclerotic glomerulus in a random cross-section, was used to calculate the percentage of sclerotic glomeruli [32]. Photomicrographs at ×273 were also used to estimate glomerular volume (VG) from the cross-sectional area of at least 5 non-sclerotic tufts using the Weibel-Gomez method. Average glomerular tuft area for a group stabilizes after measurement of ~5 profiles per individual [33]. Fifteen photomicrographs selected randomly from the renal cortex at ×2191 magnification were used to determine the FIA expressed as a percentage of total cortical area by intercept counting, using a 15 µm counting grid containing 225 cells. A total of 3375 intercepts were counted per subject. Interstitial area was defined as any tissue, including interstitial cells, outside of tubular and vascular structures larger than peritubular capillaries. Glomerular profiles, large vessels, and tubules plus interstitium were included in the reference cortical area.
For transmission electron microscopy, 0.5 µm sections stained with toluidine blue were surveyed to identify ≥3 open glomerular profiles. Further sections (70 nm thick) were cut and collected on 3 mm copper grids, stained with lead citrate and uranyl acetate, and examined in a Hitachi H-600 transmission electron microscope (Hitachi High-Tech, Pleasanton, CA). Low-power electron photomicrographs (×2820) were obtained to complete a montage of each glomerulus. Fractional mesangial area (AA), composed of both cells and matrix and expressed as a percent of total tuft area, was determined by intercept counting on the ×2820 montage micrographs. Intercept counting using lines 20µ in length on the ×2820 montage was used to determine the average peripheral capillary filtration surface area density (SV), expressed as µm2/µm3 (=1/µm). Total surface area (SA) was calculated as the product of SV × VG. Numerical volume density of podocytes per glomerulus was determined using the Weibel-Gomez formula (β=1.45 and κ=1.1) [16]. The absolute number of podocyte nuclei per glomerulus was then determined by multiplying the average glomerular tuft volume by the numerical volume density of the podocytes. Numbers of endothelial and mesangial cells per glomerulus were determined by the same method (β = 1.5). Endothelial and mesangial cell numbers were combined in the analysis because distinctions between these cell types were not always possible. Twenty high-power photomicrographs (×11,280) were also obtained from each glomerular profile. Harmonic mean GBM width was determined from the high-power (×11,280) electron photomicrographs [34]. Filtration slit frequency (FSF), expressed as the number of slits per mm of GBM, was determined by counting the total number of slits and dividing it by the total length of peripheral capillary wall captured on the high-power electron micrographs [16, 35–37]. Average FPW for a section of peripheral capillary wall was determined by dividing the total length of the peripheral capillary wall by the number of slits covering the wall.
Podocyte Detachment
The ×11,280 images were used to determine the percentage of GBM denuded of foot processes (PD) [21]. PD was defined as an absence of foot processes along the abluminal aspect of the GBMs. This determination was limited to the podocytes covering the peripheral capillary filtration surface of the GBM, and did not include foot processes covering the peri-mesangial GBMs. The majority of these denuded areas were free of any podocyte membrane or cytoplasm. An area of GBM stet was not considered denuded if podocyte foot processes were distributed normally along the GBM, but their cytoplasm appeared abnormal (i.e. relatively electron-lucent) [21]. Similarly, if only alternate foot processes were electron-lucent, that portion of the capillary was not considered denuded. The denuded length was divided by the total measured length of the GBM stet along the filtration surface to calculate the percentage of GBM length from which podocytes were detached [21].
Endothelial Fenestration
Percentage of ECF was calculated using an unbiased counting grid (6.7 × 6.7 µm) with fine parallel lines 120 nm apart and one thick line for every 7 fine lines superimposed randomly onto the ×11,280 images. Sections of endothelium intersected by thick grid lines were studied. If the intersected segment of endothelium had no fenestrae for more than 3 fine lines (360 nm) on either side of the intersection with the thick grid line, it was considered unfenestrated. Percentage of normal ECF was calculated for each individual by dividing the number of intersections that were normally fenestrated by the total number of endothelial intersections counted [9].
Endothelial Fenestration Opposite Detached Podocytes
Every ×11,280 image (n=74) showing a length of complete PD was examined to determine the percentage of ECF directly across the basement membrane denuded of podocytes using the same counting grid and method used to determine the total percent ECR. As PD was a rare event, the grid was placed non-randomly to ensure measurement of ECF directly opposite denuded areas. The percentage of normal ECF opposite basement membranes denuded of podocytes was compared with the percentage of normal ECF in the same subjects.
Physiologic Measures
Kidney function was measured annually in all diabetic subjects, and the physiologic measures were obtained from the study closest to the date of the kidney biopsy. Urinary albumin was measured by nephelometric immunoassay [38]. Urinary and serum creatinine were measured by a modification of the Jaffe reaction [39]. Albumin excretion was determined by computing the urinary ACR in an untimed urine specimen. GFR was measured by the urinary clearance of non-radioactive iothalamate and expressed in ml/min, that is, not normalized to body surface area, for reasons discussed previously [40].
Statistical Analysis
In the morphometric analysis, glomerular variables for each individual were calculated as the mean of all glomeruli evaluated for that individual. Final data for each study group were expressed as means ±SD, unless otherwise specified. The exact Wilcoxon two-sample test was used uniformly for comparisons of continuous variables between the study groups, as several variables were heavily skewed; the exact Fisher’s test was used for dichotomous variables. The Wilcoxon signed rank sum test was used to compare paired differences. Spearman's correlations were calculated between structural and functional variables. Pearson's correlations were calculated for selected linear functions. The analyses were carried out using SAS® version 9.1, Cary, NC.
ACKNOWLEDGMENTS
We are indebted to the participants in this investigation, and to the doctors, nurses, and support staff involved in collecting and processing the data. This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and by the American Diabetes Association (Clinical Science Award 1-08-CR-42).
Footnotes
DISCLOSURE
The authors declared no competing interests.
REFERENCES
- 1.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 2.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296:F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 4.Steffes MW, Schmidt D, McCrery R, Basgen JM. International Diabetic Nephropathy Study Group. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 5.White KE, Bilous RW Diabiopsies Study Group. Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant. 2004;19:1437–1440. doi: 10.1093/ndt/gfh129. [DOI] [PubMed] [Google Scholar]
- 6.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 7.Miyauchi M, Toyoda M, Kobayashi K, et al. Hypertrophy and loss of podocytes in diabetic nephropathy. Intern Med. 2009;48:1615–1620. doi: 10.2169/internalmedicine.48.2137. [DOI] [PubMed] [Google Scholar]
- 8.Lindenmeyer MT, Kretzler M, Boucherot A, et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 9.Toyoda M, Najafian B, Kim Y, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 10.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 12.Lemley KV, Abdullah I, Myers BD, et al. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney Int. 2000;58:1228–1237. doi: 10.1046/j.1523-1755.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 13.Dalla Vestra M, Masiero A, Roiter AM, et al. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031–1035. doi: 10.2337/diabetes.52.4.1031. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Ushiyama C, Suzuki S, et al. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 15.Lemley KV, Blouch K, Abdullah I, et al. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J Am Soc Nephrol. 2000;11:2095–2105. doi: 10.1681/ASN.V11112095. [DOI] [PubMed] [Google Scholar]
- 16.Drumond MC, Deen WM. Structural determinants of glomerular capillary hydraulic permeability. Am J Physiol (Fluid, Renal, Electrolyte) 1994;35:F1–F12. doi: 10.1152/ajprenal.1994.266.1.F1. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Fridén V, Dasgupta I, et al. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol. 2011;300:F40–F48. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieuwdorp M, van Haeften TW, Gouverneur MC, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 19.Lafayette RA, Druzin M, Sibley R, et al. Nature of glomerular dysfunction in pre-eclampsia. Kidney Int. 1998;54:1240–1249. doi: 10.1046/j.1523-1755.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 20.Mauer SM, Steffes MW, Ellis EN, et al. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weil EJ, Lemley KV, Yee B, et al. Podocyte detachment in type 2 diabetic nephropathy. Am J Nephrol. 2011;33(suppl 1):21–24. doi: 10.1159/000327047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen AH, Mampaso F, Zamboni L. Glomerular podocyte degeneration in human renal disease: an ultrastructural study. Lab Invest. 1977;37:30–42. [PubMed] [Google Scholar]
- 23.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baelde HJ, Eikmans M, Lappin DW, et al. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007;71:637–645. doi: 10.1038/sj.ki.5002101. [DOI] [PubMed] [Google Scholar]
- 26.Nelson RG, Meyer TW, Myers BD, Bennett PH. Course of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Kidney Int Suppl. 1997;63:S45–S48. [PubMed] [Google Scholar]
- 27.Berhane AM, Weil EJ, Knowler WC, et al. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. 2011;6:2444–2451. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkins BA, Ficociello LH, Silva KH, et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 29.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K, Pesce C, Liu Q, et al. Large glomerular size in Pima Indians: lack of change with diabetic nephropathy. J Am Soc Nephrol. 1992;3:229–223. doi: 10.1681/ASN.V32229. [DOI] [PubMed] [Google Scholar]
- 31.Nelson RG, Newman JM, Knowler WC, et al. Incidence of end-stage renal disease in type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia. 1988;31:730–736. doi: 10.1007/BF00274774. [DOI] [PubMed] [Google Scholar]
- 32.Tan JC, Busque S, Workeneh B, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010;78:686–692. doi: 10.1038/ki.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoy WE, Samuel T, Hughson MD, et al. How many glomerular profiles must be measured to obtain reliable estimates of mean glomerular areas in human renal biopsies? J Am Soc Nephrol. 2006;17:556–563. doi: 10.1681/ASN.2005070772. [DOI] [PubMed] [Google Scholar]
- 34.Jensen EB, Gundersen HJG, Osterby R. Determination of membrane thickness of distribution form orthogonal intercepts. J Microscopy. 1979;115:19–33. doi: 10.1111/j.1365-2818.1979.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 35.Weibel ER. Stereological Methods. New York: Academic Press; 1979. Volume 1: Practical Methods for Biological Morphometry. [Google Scholar]
- 36.Guasch A, Sibley RK, Huie P, Myers BD. Extent and course of glomerular injury in human membranous glomerulopathy. Am J Physiol. 1992;264:F1034–F1043. doi: 10.1152/ajprenal.1992.263.6.F1034. [DOI] [PubMed] [Google Scholar]
- 37.Ting BD, Kristal B, Myers BD. The biophysical basis of hypofiltration in nephrotic humans with membranous nephropathy. Kidney Int. 1994;40:390–397. doi: 10.1038/ki.1994.50. [DOI] [PubMed] [Google Scholar]
- 38.Vasquez B, Flock EV, Savage PJ, et al. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycemia. Diabetologia. 1984;26:127–133. doi: 10.1007/BF00281119. [DOI] [PubMed] [Google Scholar]
- 39.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol. 1960;30:207–212. [PubMed] [Google Scholar]
- 40.Delanaye P, Radermecker RP, Rorive M, et al. Indexing glomerular filtration rate for body surface areas in obese patients is misleading: Concepts and example. Nephrol Dial Transplant. 2005;20:2024–2028. doi: 10.1093/ndt/gfh983. [DOI] [PubMed] [Google Scholar]