Abstract
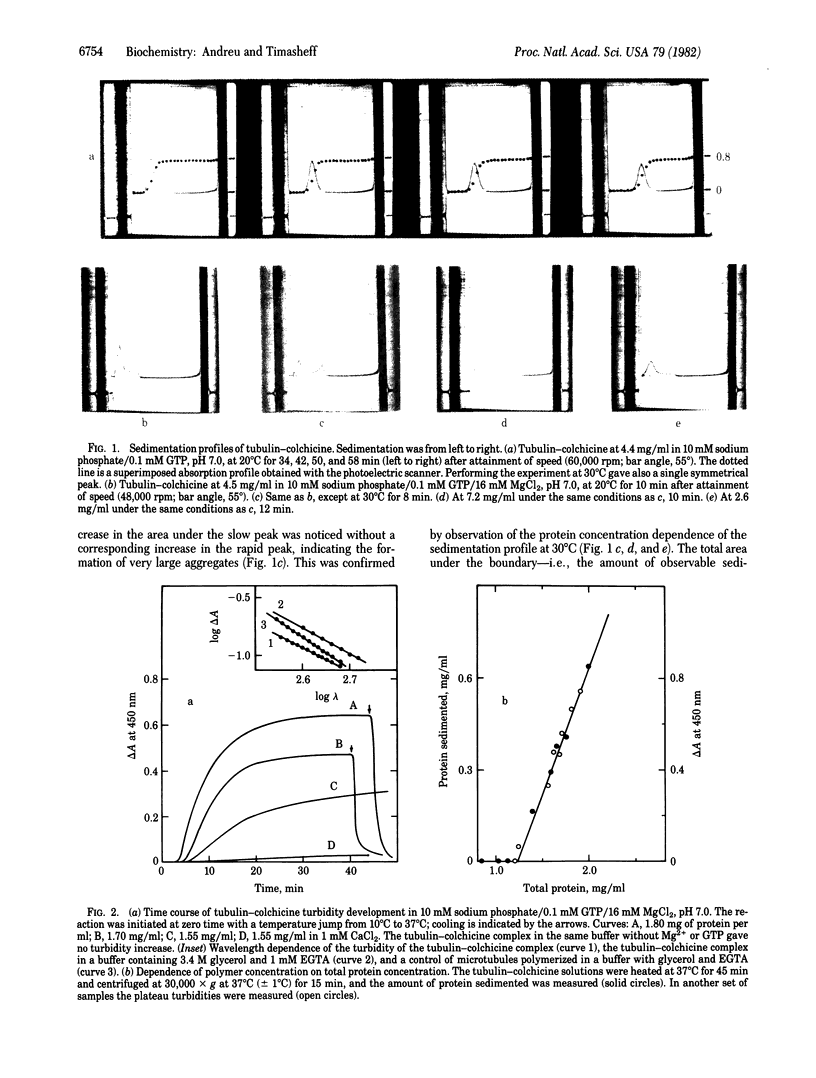
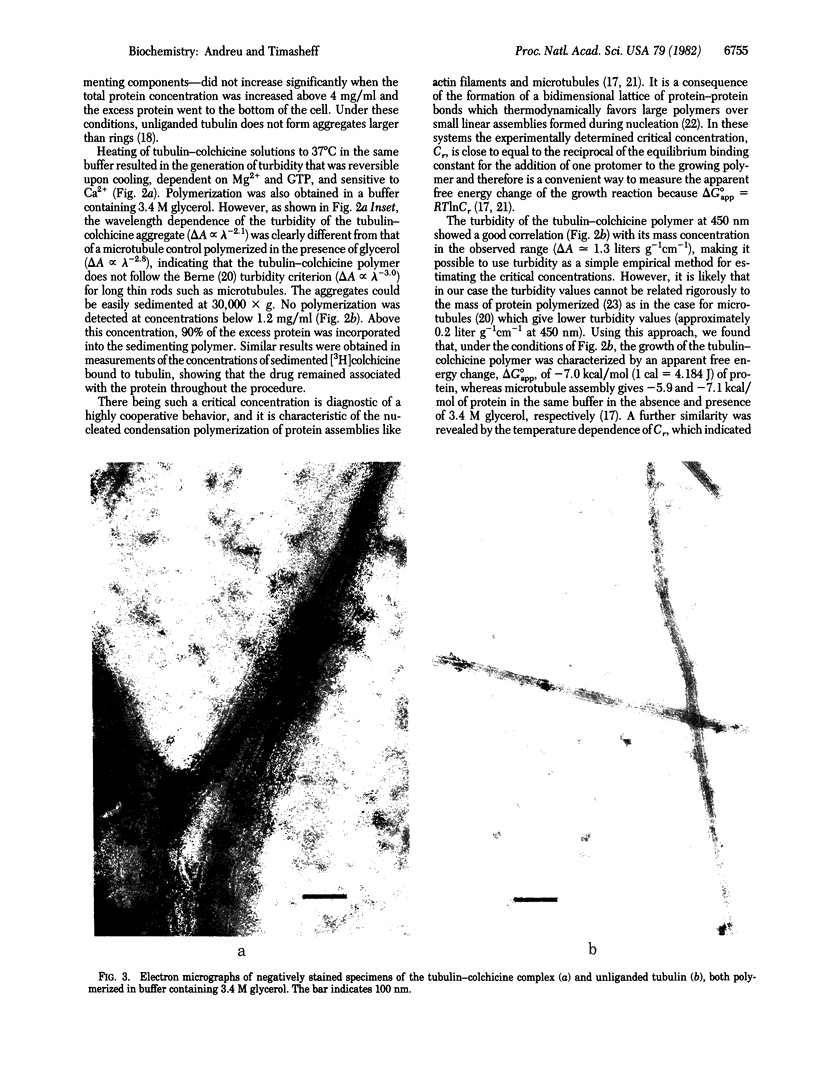
The purified tubulin-colchicine complex undergoes in vitro polymerization under the same conditions that promote the assembly of microtubules from purified tubulin. The need for a critical concentration, the apparent free energy change of the reaction, and the effects of divalent cations and nucleotide binding indicate interactions similar to those involved in microtubule formation. The large polymers formed are not microtubules, suggesting that the mode of action of the antimitotic drug may be the production of an incorrect bonding geometry between tubulin molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Timasheff S. N. Interaction of tubulin with single ring analogues of colchicine. Biochemistry. 1982 Feb 2;21(3):534–543. doi: 10.1021/bi00532a019. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Timasheff S. N. The ligand- and microtubule assembly-induced GTPase activity of purified calf brain tubulin. Arch Biochem Biophys. 1981 Oct 1;211(1):151–157. doi: 10.1016/0003-9861(81)90440-9. [DOI] [PubMed] [Google Scholar]
- Asnes C. F., Wilson L. Isolation of bovine brain microtubule protein without glycerol: polymerization kinetics change during purification cycles. Anal Biochem. 1979 Sep 15;98(1):64–73. doi: 10.1016/0003-2697(79)90706-1. [DOI] [PubMed] [Google Scholar]
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T., Simon C., Pantaloni D. Effect of antimitotic drugs on tubulin GTPase activity and self-assembly. J Biol Chem. 1979 Nov 25;254(22):11696–11702. [PubMed] [Google Scholar]
- Erickson H. P., Pantaloni D. The role of subunit entropy in cooperative assembly. Nucleation of microtubules and other two-dimensional polymers. Biophys J. 1981 May;34(2):293–309. doi: 10.1016/S0006-3495(81)84850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. I. Stoichiometry. Biochemistry. 1975 Oct 21;14(21):4559–4566. doi: 10.1021/bi00692a001. [DOI] [PubMed] [Google Scholar]
- Garland D. L. Kinetics and mechanism of colchicine binding to tubulin: evidence for ligand-induced conformational change. Biochemistry. 1978 Oct 3;17(20):4266–4272. doi: 10.1021/bi00613a024. [DOI] [PubMed] [Google Scholar]
- Keates R. A., Mason G. B. Inhibition of microtubule polymerization by the tubulin-colchicine complex: inhibition of spontaneous assembly. Can J Biochem. 1981 May;59(5):361–370. doi: 10.1139/o81-050. [DOI] [PubMed] [Google Scholar]
- Lambeir A., Engelborghs Y. A fluorescence stopped flow study of colchicine binding to tubulin. J Biol Chem. 1981 Apr 10;256(7):3279–3282. [PubMed] [Google Scholar]
- Lambier A., Engelborghs Y. A quantitative analysis of tubulin-colchicine binding to microtubules. Eur J Biochem. 1980 Aug;109(2):619–624. doi: 10.1111/j.1432-1033.1980.tb04835.x. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Frigon R. P., Timasheff S. N. The chemical characterization of calf brain microtubule protein subunits. J Biol Chem. 1973 Oct 25;248(20):7253–7262. [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. In vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry. 1977 Apr 19;16(8):1754–1764. doi: 10.1021/bi00627a037. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Rauch C. T., Wilson L. Mechanism of colchicine-dimer addition to microtubule ends: implications for the microtubule polymerization mechanism. Biochemistry. 1980 Nov 25;19(24):5550–5557. doi: 10.1021/bi00565a014. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Addition of colchicine--tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3466–3470. doi: 10.1073/pnas.74.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Hayashi M. Polymorphism of tubulin assembly. In vitro formation of sheet, twisted ribbon and microtubule. Biochim Biophys Acta. 1976 Nov 26;453(1):162–175. doi: 10.1016/0005-2795(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., Weber K. Polymerization of tubulin in the presence of colchicine or podophyllotoxin. Formation of a ribbon structure induced by guanylyl-5'-methylene diphosphonate. J Mol Biol. 1979 Oct 15;134(1):159–172. doi: 10.1016/0022-2836(79)90418-2. [DOI] [PubMed] [Google Scholar]
- Sternlicht H., Ringel I. Colchicine inhibition of microtubule assembly via copolymer formation. J Biol Chem. 1979 Oct 25;254(20):10540–10550. [PubMed] [Google Scholar]
- TAYLOR E. W. THE MECHANISM OF COLCHICINE INHIBITION OF MITOSIS. I. KINETICS OF INHIBITION AND THE BINDING OF H3-COLCHICINE. J Cell Biol. 1965 Apr;25:SUPPL–SUPPL:160. doi: 10.1083/jcb.25.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]