1. The Neuroscience of Glaucoma
1.1 Glaucoma and Ocular Pressure
Glaucoma is an etiologically complex collection of optic neuropathies. For the most prevalent primary forms, stressors related to age and intraocular pressure (IOP) lead to progressive degeneration of the retinal projection to the brain (Calkins and Horner, 2012; Nickells et al., 2012). This definition has evolved from many traditional viewpoints in two important ways. In terms of etiology, rather than linking exclusively to elevated IOP (ocular hypertension) for primary glaucoma, which remains a prominent risk factor, the definition generalizes to include IOP of any magnitude including the nominally normal range. IOP-related stress generally, but not always, takes place on a backdrop of other age-related stressors that influence susceptibility for vision loss (Burgoyne, 2011). Indeed disease incidence increases with age even when accounting for elevated IOP (Leske et al., 2007). In terms of effects of the disease, rather than focusing on the apoptotic death of retinal ganglion cells (RGCs), whose axons comprise the optic projection to the brain (Figure 1), the definition emphasizes degenerative events along the entire optic projection (Nickells et al., 2012; Howell et al., 2012a; Levin, 1999). In doing so, the tacit implication is that events distal to the RGC cell body are fundamental for understanding both the progression of neurodegeneration and ultimate vision loss (Whitmore et al., 2005). The argument is made that RGC apoptosis, while doubtlessly relevant in progression, may not be the most meaningful feature of pathogenesis in terms of seeking novel interventional targets (Chang and Goldberg, 2012; Nickells, 2007).
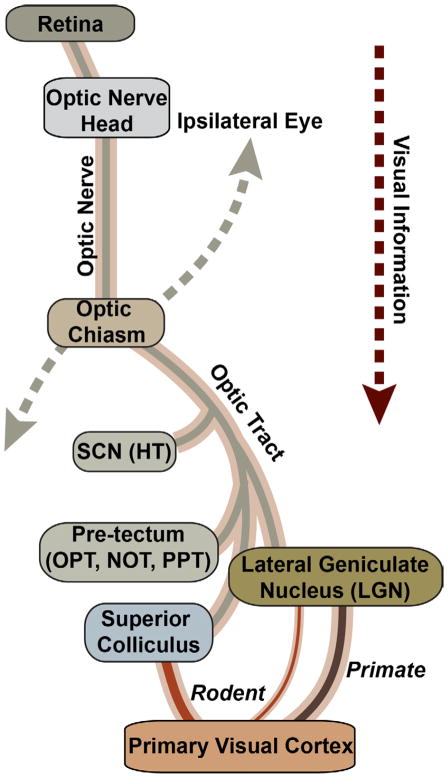
Figure 1. Retinal Projection in the Mammalian Brain.
Schematic of contralateral optic projection, which dominates in rodents. The axons of retinal ganglion cells (RGCs) exit the retina through the optic nerve head to form the nerve proper. The nerves from the two eyes meet at the optic chiasm, which parses axons to either the ipsilateral or contralateral optic tract in the brain. Central targets for RGC axons include the suprachiasmatic nucleus (SCN) of the hypothalamus (HT) and several subcortical midbrain nuclei lying distal to it. These include the olivary pretectal nucleus (OPN), the nucleus of the optic tract (NOT), and the posterior pretectal (PPT) nucleus. In primates, the lateral geniculate nucleus (LGN) of the thalamus is the dominant RGC relay to the primary visual cortex, while in rodents the more distal superior colliculus of the midbrain dominates with a smaller LGN projection to the cortex.
Glaucoma is typically (but not exclusively) associated with IOP, which remains the only treatable risk factor and therefore the predominant independent variable in animal models (McKinnon et al., 2009; Morrison et al., 2011; Pang and Clark, 2007; Sappington et al., 2010). Glaucoma’s association with IOP is a defining, yet complex feature of the disease. A substantial fraction of patients never present elevated IOP at all, but nevertheless demonstrate clinical progression similar to those who do (Heijl et al., 2002; Iester et al., 2012; Shields, 2008). Some with ocular hypertension never proceed to glaucomatous vision loss. From a clinical standpoint, many patients continue to progress despite regimens to manage IOP (Heijl et al., 2011; Pascale et al., 2012). For them, there is no clinical practice to prevent further loss of vision or to restore lost function. Once pathogenesis has proceeded past a critical point, irreversible loss of function is (for now) inevitable. Thus, emerging views of glaucoma with a nod towards clinical translation tend to deemphasize the apoptotic loss of RGCs, which we know now occurs later in animal models of progression, and focus more on the axonal projection, which is affected quite early.
1.2 Glaucoma and the Central Nervous System
Neuroscience-based approaches to understanding pathogenesis in glaucoma have surged, not only from the standpoint of preserving vision, but also because studies of pathogenesis in glaucoma have evolved as tools to inform and interrogate other degenerative disorders of the central nervous system (CNS). This surge brings with it the hope of better understanding the mechanisms of glaucomatous neurodegeneration and of identifying neuroprotective therapies independent of IOP management. The broader view expects that some of these may translate to interventions for other diseases, beginning with other optic neuropathies. For example, the potent and highly selective α2-adrenergic agonist brimonidine tartrate has direct neuroprotective efficacy for a variety of insults in animal models (Saylor et al., 2009), including axonopathy induced by elevated IOP (Lambert et al., 2011).
Even so, we must caution against any tendency to view glaucoma as a dichotomy, as either a disease of the eye involving ocular hypertension or as one of the CNS in cases where elevations in IOP are absent. Emphatically, this is an unnecessary and misleading distinction. It is a misinterpretation of the disease to conclude that glaucoma in the absence of ocular hypertension taken as a whole must evolve from a separate etiology rooted in the CNS and independent of IOP altogether. Certainly other stressors, some systemic, are known to induce glaucomatous neurodegeneration without elevated IOP (Wax et al., 2008). Etiologically, these may contribute to degeneration in a subset of those patients considered to have normal tension glaucoma. But just as glaucoma spans a broad IOP range, so too do those patients who respond with improved outcome to IOP-lowering therapies, including many with normal IOP (Leske et al., 2003). Thus, the absence of ocular hypertension does not translate a priori to categorical independence from an IOP-based etiology. The strongest hypothesis that we can pose, and therefore the most easily refuted, is that glaucoma is not about elevated IOP but rather sensitivity to IOP itself.
This hypothesis has enormous ramifications for how we approach neurodegeneration in the disease. We must consider states of the neural system in which IOP-related stressors could be injurious. Such states must be analyzed in the context of mechanisms that could mediate or transduce sensitivity to pressure. This also involves understanding how variability within these mechanisms might underlie individual susceptibility to injury, i.e., why IOP is stressful for some eyes but not for others (Burgoyne, 2011). It is also necessary to understand how neurodegeneration progresses from the transduction of IOP-related stress to vision loss in the disease. This understanding is predicated upon careful dissection of neuronal, glial and vascular mechanisms that promote progression (Almasieh et al., 2012). Finally, we must challenge ourselves to avoid a unidirectional viewpoint of glaucoma and consider how the neural system might respond to IOP-related stress to minimize injury or isolate damage already incurred. This response is of particular importance for identifying intrinsic factors that could be exploited and developed as novel neuroprotective therapies independent of IOP management.
2. Primary RGC Vulnerability in Glaucoma
2.1 The RGC Circuit
The human retina contains roughly1.5 million RGCs distributed among several types defined by a unique combination of morphologically, neurochemical and physiological parameters (Hendry and Calkins, 1998). Specialized tuning of each RGC type emerges from the complex summation of signals derived from distinct presynaptic circuits. The canonical feed-forward circuit in the retina is comprised of three classes of excitatory, glutamatergic neurons: photoreceptors, bipolar cells, and of course RGCs. Synaptic transmission occurs at two levels of connectivity: photoreceptor → bipolar cell in the outer retina and bipolar cell → RGC in the inner retina (Figure 2). Excitatory signaling at both levels is modulated by two major classes of interneurons: horizontal cells in the outer retina, which are GABAergic, and amacrine cells in the inner retina, which are mostly (but not entirely) GABAergic and glycinergic (Oesch et al., 2011; Wassle and Boycott, 1991). Three major classes of glia (astrocytes, microglia and Müller cells) in the retina contribute to the homeostatic environment of the RGC and its response to disease-relevant stressors through a variety of signaling cascades (Johnson and Morrison, 2009; Tezel, 2008).
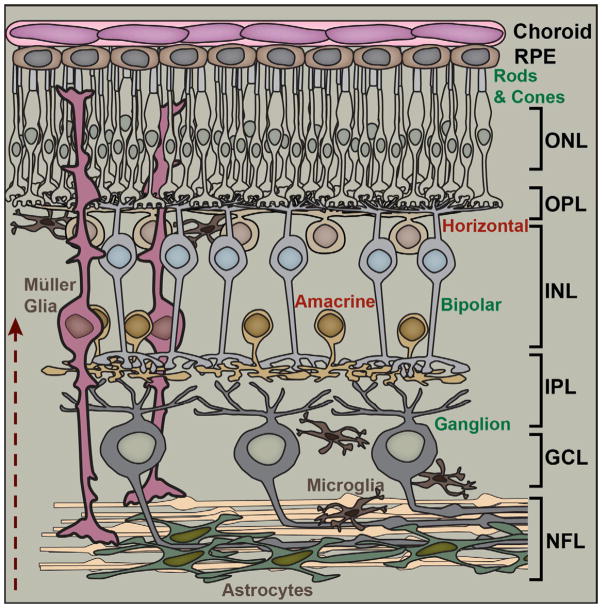
Figure 2. Fundamental Retinal Circuitry.
Basic retinal circuit includes three classes of excitatory (glutamatergic) neurons: rod and cone photoreceptors, bipolar cells, and retinal ganglion cells (RGCs). Synaptic transmission between photoreceptors and bipolar cells is modulated by inhibitory (GABAergic) horizontal cells in the outer plexiform layer (OPL). Amacrine cells (mostly GABAergic and glycinergic) modulate transmission between bipolar cells and RGCs in the inner plexiform layer (IPL). Müller glia have cell bodies in the inner nuclear layer (INL) along with bipolar, amacrine and horizontal cells and extend processes radially throughout the retina. Astrocyte glia form a dense plexus over RGC axons in the nerve fiber layer (NFL), while microglia distribute broadly. Arrow indicates the path of light. Other abbreviations: RPE (retinal pigment epithelium), ONL (outer nuclear layer), and GCL (ganglion cell layer).
2.2 RGCs as Direct Targets in Glaucoma
Like all optic neuropathies, the defining pathophysiological event in glaucoma is primary degeneration of the RGC and its axonal projection. The term “primary” is used in this context to mean that pathogenic mechanisms work against normal function of the RGC directly, though other cells and structures may suffer indirectly or secondarily to RGC degeneration. This is an important distinction, and one that should not be confused with indirect degeneration of additional RGCs secondary to a focal lesion, as in partial nerve transection (Levkovitch-Verbin et al., 2001). It is often assumed that primary injury to RGCs translates to selective injury, and many testimonials begin exactly with that definition of the disease. This assumption underscores the importance of asking whether other cells of the retina or optic projection are also susceptible as primary targets in parallel with RGCs or, what we presume would be more likely, undergo secondary degeneration once RGC pathogenesis begins. This subject has been equivocal and, at times, highly controversial (Quigley, 2001). As might be expected, studies of either human patients using imaging approaches or of human tissue post-mortem are accented by a large degree of unpredictability due to differences in progression, age and type of glaucoma. Thus, the variability between studies is immense (e.g., (Kendell et al., 1995) vs. (Fan et al., 2011)), as has been the range of conclusions from them.
2.3 Susceptibility of Other Retinal Neurons
2.3.1 Photoreceptors
A particular emphasis has been placed on photoreceptor survival in glaucoma, since this factor could most obviously influence the interpretation of visual field measurements in patients. An early histological study of human donor tissue found no association between photoreceptor number and disease severity in glaucomatous patients (Kendell et al., 1995), which was mirrored by results in a non-human primate model (Wygnanski et al., 1995). Another monkey study demonstrated decreased expression of cone opsin mRNA in the mid-peripheral retina; similar changes occurred in human glaucomatous retina at the same location, but without confirmed photoreceptor loss (Pelzel et al., 2006). However, a more recent analysis described swelling and patchy loss of cone photoreceptors in the macular region of some human glaucomatous retina and similar swelling of cones (but without loss) in monkeys with laser-induced hypertension (Nork et al., 2000). Increased thickness of the foveal photoreceptor layer in mildly glaucomatous human retina is also consistent with swelling (Fan et al., 2011).
Photoreceptor pathology appears to be spatially correlated with damage to the inner retina. A recent multi-modal imaging study of 10 patients across various ages and glaucoma subtypes found patchy compromise of cone photoreceptor density at retinal locations where visual field sensitivity and inner retinal structure also reflected damage (Choi et al., 2011). Similarly, a careful comparison of neuronal cell body populations in human glaucomatous retina found that compared to age-matched controls, loss of RGCs in the perifoveal region was spatially correlated with thinning of both the inner and outer nuclear layers (Lei et al., 2008). Loss of RGCs in the region of the retina quantified was about 45%, roughly four-fold greater than either the loss of inner nuclear cell bodies or of photoreceptors in the outer retina (Lei et al., 2008). Incidentally, the same approach (applied by the same group) found in aged human retina that thinning of the RGC population is spatially-correlated with modest loss of inner nuclear layer neurons and more extensive loss of photoreceptors (Lei et al., 2011). In that study, RGC loss over the lifetime was about 25% and photoreceptor loss about 15% compared to young retina (Lei et al., 2011); see also (Curcio and Allen, 1990). Taken as a whole, these studies indicate that RGC degeneration is accompanied at least by morphological indications of photoreceptor pathology that, as pathogenesis progresses, is likely to result in outright loss of cells. This conclusion is supported by a limited number of physiological studies of outer retina dysfunction in human patients (Vaegan et al., 1995; Velten et al., 2001) and in inducible monkey, mouse and rat models (Cuenca et al., 2010; Mittag et al., 2000; Raz et al., 2003).
The DBA (“dilute brown non-agouti”) mouse is one of the oldest known inbred strains and perhaps the best-characterized chronic model of glaucoma, presenting age-dependent (but variable) elevations in IOP due to iris atrophy and pigment dispersion (Anderson et al., 2002; Chang et al., 1999; Howell et al., 2007b; John et al., 1998). Early studies of the DBA/2J did not report photoreceptor or other non-RGC neuronal loss, the implication being that pathology in the retina was RGC-specific (Jakobs et al., 2005; John et al., 1998; Libby et al., 2005a; Schuettauf et al., 2004). However, a comparison of electroretinogram (ERG) measurements with retinal histology reported attenuation of scotopic a- and b-wave amplitude concurrent in progression with thinning of the outer retina and of the inner synaptic layer, respectively (Bayer et al., 2001). This reduction in a- and b-wave response has been corroborated more recently by independent groups (Harazny et al., 2009; Heiduschka et al., 2010), and stands in contrast to the photopic flash ERG, which remains largely intact in the DBA until late in progression (Saleh et al., 2007).
2.3.2 Retinal Interneurons
Other cells in the DBA inner retina may also be susceptible. For samples in which about half of the RGCs were lost compared to age-matched C57 mice, GABAergic types of amacrine cell were reduced by roughly the same fraction; the cholinergic subtype was particularly vulnerable (Moon et al., 2005). These results cannot be explained simply by lower starting numbers of neurons in the DBA retina. The young DBA retina has comparable numbers of cell bodies in each of the three nuclear layers compared to C57; these diminish with progression (Bayer et al., 2001). Interestingly, in older DBA retina, as RGCs degenerate, both glycinergic amacrine cells and horizontal cells (GABAergic) remain comparable in number to the C57 retina (Moon et al., 2005). Similarly, in a rat glaucoma model, even as RGCs diminish in number both GABAergic and glycinergic amacrine cells remain intact; this is not so with nerve transection (Kielczewski et al., 2005). While glial hypertrophy is an early hallmark of progression in the DBA retina, the numbers of Müller glia do not change (Inman and Horner, 2007). This does not preclude their contributing physiologically to changes in RGC function early on (Saleh et al., 2007).
2.3.3 Mechanism of Secondary Degeneration
Based on the available literature, it is reasonable to conclude that during progression other retinal neurons besides RGCs may also be vulnerable, at least eventually. However, there is little to suggest they represent primary targets independent of RGC degeneration. Importantly, glaucomatous pathology in other retinal layers appears to be spatially correlated with and depends upon the extent of RGC degeneration (Choi et al., 2011; Lei et al., 2008). This correlation is the best evidence that secondary degeneration is trans-neuronal from the RGC and does not simply represent diffuse, residual damage. In the most carefully quantified human glaucomatous retina, there was a gradient of correlated cell loss from RGCs to the inner nuclear layer to the outer nuclear layer (Lei et al., 2008). This stands in contrast to the normal aged retina in which significant RGC and photoreceptor loss sandwiches a less severe (but still spatially correlated) reduction in cells of the inner nuclear layer (Lei et al., 2011). This pattern suggests that age-related loss of RGCs and photoreceptors are dependent not upon one another, but rather conjointly upon a spatially-specific pathway that influences both sets of neuron.
Given this correlation, it is tempting to envision a degenerative cascade in glaucoma in which substantial reduction in RGCs leads to eventual trans-synaptic loss of bipolar cells and finally photoreceptors in retrograde progression. There is precedence for this kind of secondary degeneration elsewhere in the RGC pathway. For example, ablation of the primary visual cortex (V1) in non-human primates induces apoptotic degeneration of cortical-projecting neurons in the lateral geniculate nucleus (LGN) followed by retrograde degeneration of the optic projection and of RGCs sometime later (Cowey et al., 2011; Johnson and Cowey, 2000; Mihailovic et al., 1971; Niida T, 1990; Van Buren, 1963). A similar pattern of degeneration occurs in human hemianope patients, albeit with greater individual variability, and does so whether the condition is congenital or due to traumatic injury (Cowey et al., 2011; Jindahra et al., 2009). These examples illustrate how an anatomically distal stressor (e.g., a cortical lesion) can affect survival of more proximal neurons (e.g., RGCs) in retrograde order. For glaucoma and its experimental models, we presume that RGC injury represents the distal stressor that affects retrogradely the survival of upstream retinal neurons.
However, the analogy with cortical injury only goes so far. First, in terms of thinning of the inner nuclear layer, there is little evidence to support the idea that bipolar cells are more susceptible than either amacrine neurons or Müller glia. In fact, in the DBA retina two of the most common bipolar cell types are unaffected during progression, with the available data suggesting far greater loss of GABAergic amacrine cells (Moon et al., 2005), which are presynaptic to both RGCs and bipolar cells (Calkins and Sterling, 1996). In direct comparisons, RGC degeneration is always overwhelmingly greater in amplitude and more wide-spread retinotopically. For example, in aged DBA retina with few or no remaining RGCs and very little residual visually-evoked potential in the brain, a- and b-wave ERG signals were reduced by only 40% compared to C57 mice (Heiduschka et al., 2010). In the study of human tissue by Nork et al. (Nork et al., 2000), of those retinas with severe RGC dropout (>90%), photoreceptor pathology was observed in only 25% of the retina, with 35% having no loss.
2.4 Why are RGC Primarily Susceptible in Glaucoma?
In the retina, glutamatergic signaling from photoreceptor to bipolar neuron to RGC is accomplished without myelination of axons. What distinguishes the RGC axon is that it exits the eye and penetrates the laminar region of the nerve head where, in doing so, it becomes myelinated and continues through the nerve proper on its way to central targets in the brain (Figure 3). Thus, it has been appreciated for decades that RGC susceptibility in glaucoma must be at least in part axogenic – derived from the properties of the axon and its local milieu in the nerve (Levin, 1999). Indeed some of the earliest mechanistic work addressing RGC degeneration focused on axogenic mechanisms, particularly due to extrinsic factors at the optic nerve head. This is covered amply in earlier reviews (Burgoyne et al., 2005; Hernandez, 2000; Osborne et al., 2001; Quigley, 1999). It is worth considering whether additional factors intrinsic to the RGC axon itself could render it particularly vulnerable to insult.
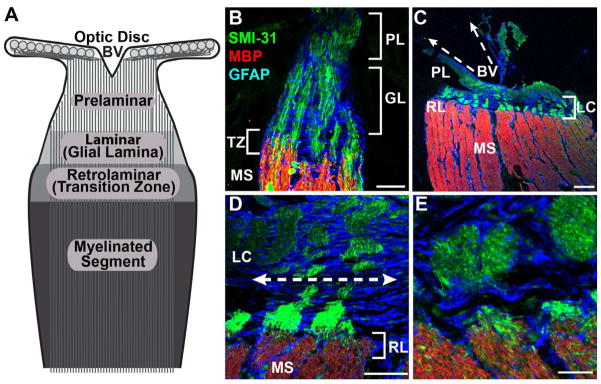
Figure 3. Structure of the Optic Nerve Head.
A: Primary zones of the mammalian optic nerve head. Optic disc marks exit of unmyelinated RGC axons from the retina to the prelaminar zone of the nerve head and the conduit for blood vessels (BV). The laminar region (glial lamina in rodents) contains a concentration of astrocytes that separate bundles of axons. The retrolaminar zone marks the transition to oligodendrocyte-derived myelination of axons. B: Immuno-fluorescent confocal micrograph through C57 mouse nerve head labeled for phosphorylated neurofilaments (SMI31) in axons, glial fibrillary acidid protein (GFAP) for astrocytes, and myelin basic protein (MBP). Prelaminar region (PL), glial lamina (GL), transition zone (TZ) and myelinated segment of nerve are indicated. C: optic nerve of squirrel monkey shows well-defined lamina cribrosa (LC) and retrolaminar (RL) region. D: higher magnification of monkey nerve head demonstrates lateral tiling of GFAP-labeled astrocyte processes and fascicles of RGC axons. E: higher magnification of retrolaminar zone in monkey shows transition to myelination. Scale = 50 μm (B, D); 100 μm (C); 20 μm (E).
2.4.1. Most RGC Axons are Thin
Axons are particularly metabolically demanding and require a consistent source of adenosine-5′-triphosphate (ATP) available for hydrolyzation. This source must be sufficient to maintain anterograde transport from the retina to the brain, retrograde transport from the brain to retina, and saltatory conduction between the cell body and axonal terminals (Carelli et al., 2004; Hollenbeck, 1996). Physiological studies of parallel visual pathways in primates highlight the faster axonal conduction velocity of large, magnocellular-projecting RGCs (mostly parasol cells) compared to that of the smaller and more numerous parvocellular-projecting midget-type RGCs (Callaway, 2005; Wassle and Boycott, 1991). Given the difference in conduction velocities, it is tempting to conceive of a bimodal distribution of small and large axon diameters. In point of fact, the distribution of axon sizes in the optic nerve is continuous, very narrow and heavily skewed towards small axons, as it is in other white-matter tracts (Reeves et al., 2012; Wang et al., 2008a). Most RGC axons are 0.2–0.7 μm in diameter; this is so from primates to rodents despite large differences in total length of the optic nerve (Perge et al., 2009).
While the largest (and rarest) RGC axons are 1.5–2.0 μm in diameter, auditory axons range from 1–4 μm and vestibular axons from 0.2–9 μm (Perge et al., 2012). The relatively small size of RGC axons appears to be optimized for both information rate (action potential production) and energy consumption, so that firing rate is set to the minimal level to maintain communication with central targets (Niven and Laughlin, 2008; Perge et al., 2009; Perge et al., 2012; Wang et al., 2008a). In other CNS regions, there is also a large difference in axon diameter between unmyelinated and myelinated axons even when the thickness of the myelin sheath is taken into account (Reeves et al., 2012; Wang et al., 2008a). Importantly, this is not true of the retina and optic nerve, where the distributions of RGC axon diameters are very similar (Perge et al., 2009; Perge et al., 2012), with continuity in their inner diameter as they pass through the laminar region of the nerve head (Minckler et al., 1976; Yu Wai Man et al., 2005). This could have important implications for glaucoma, as discussed below.
2.4.2. RGC Axons and Mitochondria
One purpose of myelination is to increase axonal efficiency by reducing energy consumption. Unmyelinated axons, in contrast, are relatively inefficient, requiring far greater energy to maintain axonal conduction owing to the absence of saltatory conduction (Perge et al., 2009; Waxman, 1978). This is reflected in the greater abundance of mitochondria in the unmyelinated portion of the RGC axon, creating a sharp gradient between the two compartments (Bristow et al., 2002; Morgan, 2004; Yu Wai Man et al., 2005). In the nerve fiber layer of the retina, RGC axons contain intermittent varicosities that are packed with mitochondria and mark points of inter-axonal and axonal-astrocyte contact (Wang et al., 2003). Most of the mitochondria in this region are contained in RGCs and their axons, unlike the optic nerve where an estimated 70% of the mitochondria are in astrocytes (Perge et al., 2009). In terms of energy usage, the unmyelinated axon is roughly 10-fold less efficient than the myelinated axon in conveying an action potential for the most common of axon sizes (Perge et al., 2009). This segment of the nerve head also has a higher density of voltage-gated Na+ channels, reflecting the higher energy demand for conduction (Barron et al., 2004).
Mitochondria are trafficked in both anterograde and retrograde directions along microtubule and actin molecular tracks in response to physiological signals (Hollenbeck, 1996; Hollenbeck and Saxton, 2005). While these signals are not entirely understood, they include local ATP demand and increased Ca2+ influx, which is countered in part by mitochondrial buffering (Hollenbeck and Saxton, 2005; Nicholls and Budd, 2000; Rintoul and Reynolds, 2010). Any condition that inhibits mitochondrial motility, such as diminished kinesin-mediated anterograde transport, can lead to the accumulation of toxic free radicals, amplified mitochondrial permeability, increased cytochrome c and oxidative stress (Lee et al., 2010; Rintoul and Reynolds, 2010; Wallace, 1999). Many of these elements are in fact observed in various models of glaucoma (Almasieh et al., 2012). In the retrograde direction, diminished motility can prevent the retrieval of dysfunctional mitochondria to the cell body for autophagic degradation (Rintoul et al., 2003). Early observations of the sharp gradient in mitochondrial concentration between the unmyelinated pre-laminar segment and the myelinated post-laminar region were construed as constricted motility and accumulation, rather than reflective of the intrinsic energy demand (Hollander et al., 1995; Minckler et al., 1976). These conclusions are likely unwarranted (Barron et al., 2004; Morgan, 2004), and any consideration of mitochondrial accumulation at the transition zone must consider the normal gradient there (Munemasa et al., 2010; Yu Wai Man et al., 2005).
A variety of intracellular conditions can lead to diminished mitochondrial transport within axons (Rintoul and Reynolds, 2010), including an overload of glutamate that is associated with a transformation of mitochondria from an elongated morphology to round (Rintoul et al., 2003). Yu Wai Man et al. (2005) have argued that such conditions could underlie a variety of mitochondrial-related hereditary optic neuropathies. In terms of the RGC, one can imagine scenarios in which either increased ATP demand along the distal axon or accumulation of stressed mitochondria in the cell body or unmyelinated axon would require rapid transport from the region of high mitochondrial concentration (unmyelinated segment) to the low (myelinated segment). Alternatively, disease states are often associated with increased mitochondrial volume of many orders of magnitude, which can greatly reduce motility (Chang and Reynolds, 2006). In either case, the RGC axon is at a disadvantage without the benefit of enlargement of the axon at the transition between unmyelinated to myelinated segment to minimize a bottleneck.
A uniformly small axon is also at a disadvantage should available ATP diminish rapidly, namely at distal sites along the myelinated axon and pre-synaptic active zones in central targets. An obvious consequence of reduced ATP is diminished capacity for action potential signaling along the axon due to inhibition of Na+/K+ ion pumps (Ames, 2000). However, failure of the Na+/K+ ion exchange also induces accumulation of axoplasmic Na+ which in turn promotes reverse operation of the Na+/Ca2+ exchanger leading to intra-axonal Ca2+ accumulation (Stys, 2004). Because axons are cylindrical, a smaller inner diameter translates to a higher surface-to-volume ratio. This implies less available cytoplasmic volume to buffer each local influx of Ca2+ (Reeves et al., 2012; Stys, 2004). We have reviewed elsewhere how excessive cytosolic Ca2+ activates Ca2+-dependent enzymes such as calpains and phospholipases that can induce irreversible injury by degradation of axonal cytoskeleton (Crish and Calkins, 2011). More generally, small axons are preferentially vulnerable in many conditions, particularly those in which anoxia is a factor (Bechtold et al., 2005).
The unmyelinated axons of the photoreceptor and bipolar cell, unlike the RGC axon, are fairly short. The longest of the photoreceptor axons serving foveal cones are 200–400 μm in length with very few mitochondria (Hsu et al., 1998). Most of the mitochondria are in the inner segment, reflecting a higher metabolic demand than the rest of the photoreceptor (Perge et al., 2012). Furthermore, though the unmyelinated RGC axon in the retina can be 50-fold longer than the cone axon, its mean diameter is half as thick (Perge et al., 2012). For unmyelinated axons, the consequences of a small inner diameter are even more devastating. The membrane-to-axoplasm ratio is even higher, which promotes a robust differential between available sites for Ca2+ influx and available cytosolic volume to buffer Ca2+ accumulation (Stys, 2004). This difference may underlie increased vulnerability of thin unmyelinated fibers in both traumatic injury and Parkinson’s disease (Braak et al., 2004; Reeves et al., 2007; Reeves et al., 2012). The vulnerability of the RGC axon to Ca2+ injury is underscored by the compartmentalized localization of Ca2+-binding proteins to the unmyelinated axon in the nerve fiber layer, but not to the initial axon segment where voltage-gated Na+ channels predominate (Mojumder et al., 2008).
2.4.3. Unmyelinated Axons and Light-Induced Damage
The design of the retina is such that light passing through the optical media of the eye transverses the inner limiting membrane, nerve fiber layer, and the rest of the neural retina before impinging upon photoreceptor outer segments. Photoreceptors themselves have a high concentration of mitochondria, primarily for driving protein synthesis necessary for outer segment renewal, but are shielded from high-energy short wavelength light where it is most concentrated in the central retina by the macular pigment. While RGC axons are not photosensitive per se, mitochondria are susceptible to light-induced damage through the formation of free radicals and damage to mitochondrial DNA (Godley et al., 2005).
Mitochondria produce ATP via oxidative phosphorylation, which produces toxic reactive oxygen species (ROS). Normally, mitochondrial machinery is exquisitely adapted to counter ROS formation within the organelle by endogenous antioxidant and repair systems. However, many mitochondrial components that include the enzyme cyclooxygenase (COX) and certain flavin protein nucleotides are highly absorptive of light, particularly the shorter wavelengths within the visible spectrum (reviewed by (Osborne, 2010). Over time, photointeractions with these components can cause breakdown of the endogenous mitochondrial pathways that counter oxidative stress (Godley et al., 2005). As the nervous system ages, mitochondria become less efficient at balancing ROS formation with metabolic demand (Navarro and Boveris, 2007). Some evidence suggests that RGC exposure to light over time accelerates this process, leading to increased susceptibility to disease processes in optic neuropathies (Osborne, 2010; Osborne et al., 2010).
2.4.4. ATP and Axonal Neurofilaments
The primary etiology of many optic neuropathies involves genetically-entrained mitochondrial dysfunction (Lee et al., 2010; Nguyen et al., 2011a; Yu Wai Man et al., 2005). In glaucoma, it is not clear to what extent the primary stressors (age and IOP) initiate early mitochondrial-related pathogenic events or whether such events are downstream of remodeling of the nerve head architecture or other factors (Burgoyne, 2011). Mitochondrial motility and maintenance of ATP concentration throughout the axoplasm are critical for normal axonal function. The broad implications of mitochondrial dysfunction for glaucoma have been reviewed extensively elsewhere (Almasieh et al., 2012; Carelli et al., 2002; Lee et al., 2011). Briefly, wherever mitochondrial density is typically high, reflective of higher metabolic demand, susceptibility to mitochondrial- and ATP-related stress will be higher, in particular as it relates to intracellular Ca2+ buffering (Sheng and Cai, 2012). Thus, the unmyelinated segment of the RGC axon is particularly vulnerable to stress due to accumulated ATP (Franke et al., 2006; Lee et al., 2011).
Normal aging involves a general reduction in neuronal ATP throughout the brain and other changes associated with mitochondrial stress (Chrysostomou et al., 2010; Lee et al., 2011; Navarro and Boveris, 2007). Mitochondrial transport within the axon, like all transport, relies heavily on available ATP. Diminished mitochondrial transport to distal neuronal processes has been implicated in Alzheimer’s and other age-related diseases (Chang and Reynolds, 2006; Ebneth et al., 1998; Rintoul and Reynolds, 2010). Recently, we have shown that in DBA, ATP diminishes in the optic nerve with age; this reduction is exacerbated by elevated IOP, leading to a decline in the compound action potential (Baltan et al., 2010).
Recent computational modeling indicates that stress due to elevated IOP could challenge axoplasmic flow, thereby inducing focal shortages in ATP along the axon (Band et al., 2009). This could challenge axon function earliest at distal sites along the nerve, which rely upon ATP-fueled mitochondrial transport from the cell body. Though mitochondrial transport is mediated by microtubule and actin filaments (Hollenbeck, 1996), their anchoring at local sites depends upon side-arm interactions with phosphorylated neurofilaments (Sheng and Cai, 2012; Wagner et al., 2003). Cytoskeletal transport of neurofilaments is fastest in the unmyelinated segment of the RGC axon, with a velocity gradient that slows with distance from the retina, probably due to increased phosphorylation (Li et al., 2012). An IOP-induced shortage of ATP could slow neurofilament transport even further, causing not only a shortage of stationary mitochondria at distal sites, but also accumulation of phosphorylated neurofilaments at the myelination transition zone. Indeed, immunolabeling in animal models indicates just such a pattern (Howell et al., 2007b; Soto et al., 2008). Finally, we have shown that axon transport in multiple animal models is challenged first at the most distal sites where RGC axons terminate (Crish et al., 2010). This and related topics are taken up in the next section.
3. Axonopathy in Glaucoma
3.1 Axonal Transport Deficits
Several intrinsic characteristics of the RGC axon are likely render it susceptible to injury in glaucoma, independent of the many external factors in the axon’s milieu that influence pathogenesis. Indeed, many of the earliest studies focused on the optic nerve head as a nexus of axon pathology, particularly in regard to depleted axoplasmic transport. These have been reviewed extensively before (Almasieh et al., 2012; Burgoyne, 2011; Knox et al., 2007; Whitmore et al., 2005). However, key issues are the timing of axonal transport failure relative to other degenerative events, whether loss of anterograde (retina to brain) and retrograde (brain to retina) transport are concurrent or occur separately, and whether the pattern of transport deficits tell us anything about mechanisms of progression.
3.1.1. Retrograde Transport and Cell Body Loss
The ultimate result of glaucomatous degeneration is caspase-dependent apoptotic loss of ganglion cell bodies in the retina, for both rodent and non-human primate models and human tissue (Cordeiro et al., 2004; Garcia-Valenzuela et al., 1995; Kerrigan-Baumrind et al., 2000; Kerrigan et al., 1997; Quigley, 1999; Quigley et al., 1995). The mechanisms that lead to apoptotic cascades in glaucoma are myriad with a large degree of overlap in terms of intracellular cascades (Almasieh et al., 2012; Nickells, 2007).
For RGCs, even acute damage to the axon through axotomy is followed by a period of several days of cell body survival followed by rapid apoptosis (Berkelaar et al., 1994; Levin, 1999; Magharious et al., 2011). Damage to the RGC axon via inflammation in the myelinated segment of the nerve during optic neuritis is often reversible, and vision loss is not permanent (Hickman et al., 2002). Such rescue of function would be impossible should RGC bodies disappear altogether. Similarly, deletion of the pro-apoptotic gene Bax in the DBA mouse does not inhibit RGC axon loss in the nerve, but does rescue RGC bodies (Libby et al., 2005b). Conversely, insertion of the Wallerian degeneration slow allele (Wlds), which produces a chimeric protein that protects axons against degenerative injury, slows axonal loss in both the DBA and an inducible rat model (Beirowski et al., 2008; Howell et al., 2007a). RGC bodies with intact axons remain in these models, but showed signs of early apoptotic injury (Howell et al., 2007a). These results suggest a certain degree of compartmentalization between axonal vs. somatic injury (Whitmore et al., 2005), though not necessarily complete independence of the two processes (Howell et al., 2012a).
Experiments using fluorogold to label RGCs following retrograde transport from central injection sites have been a basic staple of glaucoma research in recent years (Danias et al., 2003; Filippopoulos et al., 2006; Mittag et al., 2000; Vidal-Sanz et al., 2001). However, direct comparisons between transported tracers and immune-labeled RGCs underscore that axonal transport is challenged prior to loss of cell bodies. Thus, RGC counts based on retrograde labeling conducted at the end of the experimental period better reflect the degree of axonopathy than actual soma survival. For example, we and others have shown in the DBA tremendous persistence of the cell body following severe depletion of retrograde axonal transport of fluorogold from the superior colliculus (Buckingham et al., 2008; Jakobs et al., 2005), the primary and most distal site in the rodent RGC projection (Crish et al., 2010). Similarly, in a laser-induced mouse model, RGC bodies visualized by selective markers by far outnumber the fraction of cells labeled retrogradely from the colliculus (Salinas-Navarro et al., 2009; Vidal-Sanz et al., 2012). In this model, IOP is elevated rapidly (6 hrs post-surgery) and transiently (5–6 days; (Salinas-Navarro et al., 2009). While the population of immune-labeled RGCs diminishes slowly over the course of weeks, the number of retrogradely-labeled RGCs is reduced sharply by 1 week (Salinas-Navarro et al., 2009; Vidal-Sanz et al., 2012). A similar progression holds for the same model used in rats, though IOP is elevated a bit longer (Vidal-Sanz et al., 2012). These results contrast with the extremely rapid and severe loss of RGC bodies after NMDA-induced excitotoxic insult (Mittag et al., 2000).
3.1.2. Anterograde Transport Loss is an Early Reporter of Pathogenesis
The results from inducible rodent models just described would seem to indicate that depletion of retrograde transport occurs rapidly in glaucoma (Vidal-Sanz et al., 2012). However, inducible models often incorporate acute elevations in IOP are often extreme compared to IOP in human glaucoma. For example, in the mouse and rat studies just described IOP was increased by over 110% during the short experimental period (Salinas-Navarro et al., 2009; Vidal-Sanz et al., 2012). Discerning small differences in the progression of axonal transport deficits may be difficult with extreme elevations in IOP, since degenerative events may be too compressed to separate distinct outcomes.
In the DBA mouse, progression is spread over several months with modest IOP elevations for many animals as age increases (Inman et al., 2006; Libby et al., 2005a). We and others have found that in the DBA, retrograde transport of fluorogold from the SC to the retina is challenged at intermediate stages but persists quite late into progression, up to 18 months (Buckingham et al., 2008; Danias et al., 2003). In contrast, active anterograde transport of cholera toxin b (CTB) from the retina to the SC and other central targets is challenged much earlier, even by 3 months of age (Crish et al., 2010). Depletion of CTB signal in the SC depends primarily on age, with IOP as an additional influence, and is nearly eradicated by 11–12 months (Crish et al., 2010; Howell et al., 2012b). At these ages, a substantial number of animals have intact optic nerves (Buckingham et al., 2008; Inman et al., 2006), though this is highly variable between colonies (Howell et al., 2012b; Libby et al., 2005a; Libby et al., 2005b). Similarly, for rats with modest IOP elevations (40–45%) induced by microbead occlusion of the anterior chamber, anterograde transport is diminished for aged but not younger animals after only 2 weeks (Crish et al., 2010). This is well before overt pathology in the retina or nerve occurs in this model (Chen et al., 2010; Cone et al., 2010; Sappington et al., 2010).
Based on these comparisons, deficits in anterograde transport are an earlier reporter of RGC pathogenesis than retrograde transport, with the caveat that the two are usually probed using different tracers. Even so, there may be mechanistic reasons why anterograde transport would be challenged earliest. Axon transport is mediated by two molecular motors that bind microtubules, the largest of the protein filaments comprising the cytoskeleton. Anterograde transport towards the axon terminal is mediated by kinesin motors, while retrograde transport is mediated by dynein; both obtain energy by hydrolyzing ATP diffusing from mitochondria (De Vos et al., 2008). However, while kinesin hydrolyzes ATP at a single binding site and is confined to a single step size of 8 nm, dynein has four ATP hydrolyzing sites and can take step sizes up to 32 nm (Allan, 2011; Schnitzer and Block, 1997; Takahashi et al., 2004). As a result, the architecture of dynein minimizes ATP consumption to provide force appropriate for load capacity and renders the molecule four times more efficient at ATP consumption than kinesin (Mallik et al., 2004). Thus, in glaucoma, anterograde transport is more vulnerable to failure should available pools of axonal ATP diminish.
3.1.3. The Progression and Retinotopy of Distal Transport Loss
Crish et al. (Crish et al., 2010) found that for the DBA, transport of CTB fails first at the most distal site in the projection, where RGC axons terminate in the superficial SC. Subsequent failure of transport follows a distal-to-proximal progression, with deficits next appearing in the LGN followed by the optic tract, nerve and finally the retina. Indeed, in animals with no transported CTB anywhere in the brain, a robust CTB signal exits the eye, penetrates the nerve head, and extends into the nerve itself (see Figure 5 of Crish et al., 2010). In animals in which CTB signal is absent even in the nerve, a substantial number of RGCs demonstrate intact CTB uptake. Even with complete failure of transport at distal sites, active uptake of CTB remains largely intact; this indicates that failure at distal sites cannot be attributed to a simple blockade in more proximal structures (Crish et al., 2010). A similar distal-to-proximal progression was observed in C57 mice with microbead-induced elevations in IOP over the course of several weeks (Crish et al., 2010).
The superficial SC contains a complete topographical representation of the retina (Drager and Hubel, 1976). We found in the DBA that loss of anterograde transport in the SC follows a sectorial progression, in which deficits progress either caudally or rostrally to adjoin depletion near the optic disk (Crish et al., 2010). An entire caudal or rostral sector is lost before the opposing sector is affected, with the magnitude of sectorial loss increasing with age. We have also described a similar sectorial pattern of transport depletion for mice and rats with microbead-induced elevations in IOP and for rats with laser-induced hypertension (Crish et al., 2010; Lambert et al., 2011). Interestingly, a similar pattern of transport loss in the SC was described recently in rodents with diabetes induced by systemic streptozotocin (Fernandez et al., 2011). Thus, the sectorial nature of transport depletion in distal RGC targets may reflect a general mechanistic feature of how the RGC projection responds to stress regardless of the particular insult.
3.2. Degeneration of the RGC Projection
A host of studies using a variety of animal models examine how elevations in IOP influence RGC survival, as assessed either by axonal injury in the optic nerve or by cell body counts in the retina (Morrison et al., 2005). The cumulative results are as diverse as the models themselves, largely due to variability in the magnitude and duration of IOP elevations but also to the nature of the experimental insult itself (Morrison et al., 2005). Interestingly, very few compare somatic and axonal outcomes directly or track progression over time compared to a functional outcome, such as axonal transport. Those that do make such a comparison indicate that axonal degeneration, i.e., actual loss of axons in the nerve, is sandwiched in between transport dysfunction and final somatic drop-out in the retina. For example, in the study by Lambert et al. (Lambert et al., 2011), eight weeks of IOP elevation in rats induced a 70% reduction in anterograde transport to the colliculus, an average 33% loss of axons in the nerve, and an average16% reduction in RGC bodies assessed by immune-labeling that varied by retinal location (Figure 4). Also in rats, histological indications of axonal dysfunction (e.g., accumulation of phosphorylated neurofilaments and axonal enlargement) occur prior to actual axon loss (Johnson and Cowey, 2000). Studies using non-human primates also indicate a functional deficit followed by axonal degeneration and eventual RGC body loss (Quigley and Addicks, 1980). Finally, in an inducible C57 mouse model, 3 weeks of a 1.7-fold increase in IOP led to a 21% loss of Brn3-labeled RGC bodies, but a 35% loss of axons in the nerve (Zhu et al., 2012). This lends support to the axon-before-cell body progression with modest elevations in IOP.
Figure 4. Distal to Proximal Progression in an Inducible Rat Model.
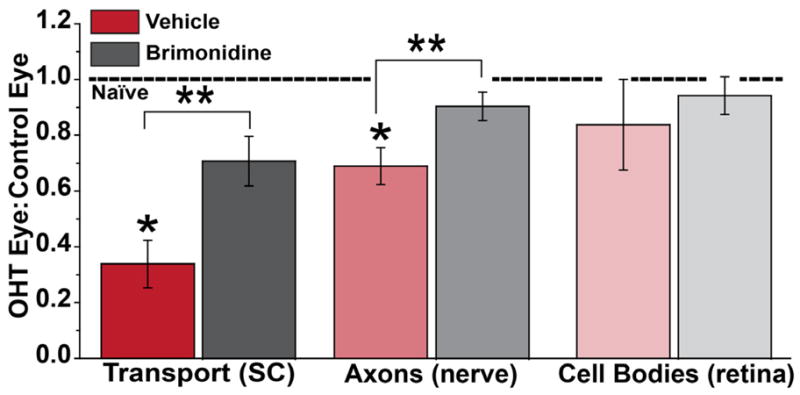
Three degenerative outcomes after 8 weeks of ocular hypertension (OHT) induced unilaterally in rats by laser cauterization of the episcleral veins. With elevated IOP in the OHT eye, anterograde transport to the SC was affected the worst, with a decrease of 70% compared to the SC from the control eye, followed by a 33% loss of axons in the nerve. RGC body loss was sporadic across the retina, with only a 16% decrease averaged across locations. Protection afforded by systemic treatment with brimonidine was proportionally efficacious. Ratio of unity for the two eyes from naïve animals indicated by dotted line. * and ** indicates significance compared to naïve ratio or vehicle treatment, respectively (p ≤ 0.02). Data reproduced from Lambert et al. (2011).
Results from the DBA mouse are consistent with this pattern of nerve-before-retina as well (Schlamp et al., 2006). Typical phenotypic penetrance of the iris defects underlying elevated IOP in the DBA is about 50% by 10–11 months (John et al., 1998; Libby et al., 2005a; Libby et al., 2005b; Scholz et al., 2008; Sheldon et al., 1995). We have shown at least two indices of axonal dysfunction occur earlier: depletion of anterograde transport to the SC beginning at 3–5 months and diminished compound action potential in the optic nerve by 6 months (Baltan et al., 2010; Crish et al., 2010). The DBA is intrinsically variable, with many animals showing little or no axon degeneration in the nerve as late as 10–11 months (Inman et al., 2006; Schlamp et al., 2006; Scholz et al., 2008). Somatic drop-out in retina is generally later (Buckingham et al., 2008; Zhong et al., 2007). Direct comparisons between axon counts in the nerve and RGC bodies show the former progressing approximately 2-fold faster (Howell et al., 2012b). On the other hand, functional deficits that influence RGC physiology may be detected prior to any axonal or somatic indications of degeneration (Saleh et al., 2007). This may reflect changes in pre-synaptic circuitry or retinal glial function, as discussed above (see section 2.3).
3.2.1. Axon Degeneration and IOP
Axon loss in the DBA is related to the magnitude of IOP elevation (Inman et al., 2006; Libby et al., 2005a; Libby et al., 2005b), though other studies have found a weaker relationship, probably due to the large variability in the model (Scholz et al., 2008). In fact, dependency on IOP appears to be a general feature of axonal drop-out in the nerve regardless of how IOP becomes elevated. An elegant series of studies using photocoagulation of the trabecular meshwork in rats described a strong correlation between the degree of axonal degeneration in the nerve and both the cumulative exposure to elevated IOP and the peak IOP (Levkovitch-Verbin et al., 2002). An inducible model in Swiss Black mice also supports a strong correlation between axon loss and cumulative IOP exposure (Mabuchi et al., 2003). The same is so in a non-human primate model (Yucel et al., 2003).
The dependency on IOP holds for a variety of rat models and across magnitudes and duration of exposure. Figure 5 shows how relative axon degeneration rises with cumulative exposure to IOP, using the thorough synopsis of various rat models provided by Morrison et al. (Morrison et al., 2005) and augmented with more recent studies. Even for short durations of very high elevations in IOP, axonal degeneration increases linearly with increasing exposure (Chauhan et al., 2002; Chidlow et al., 2011; Dai et al., 2012).
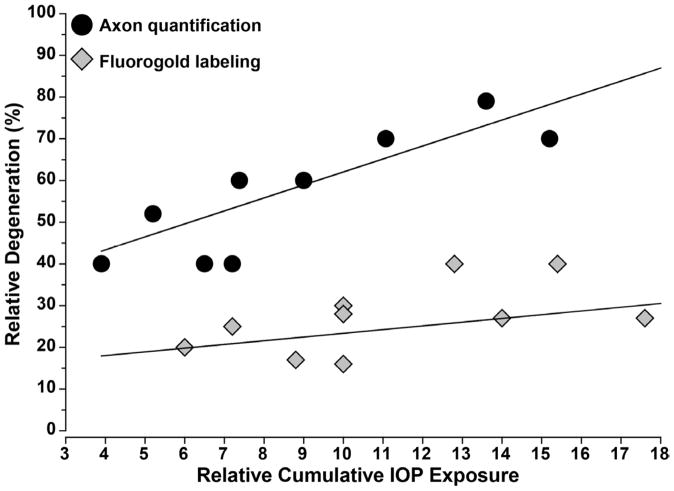
Figure 5. IOP-Induced Loss of Axons is Independent of Method.
Graph shows relative degeneration assessed by either quantification of surviving RGC axons in the nerve or of RGC bodies in the retina as a function of cumulative exposure to elevated IOP. Data obtained from three independent rat models of ocular hypertension as summarized in Morrison et al. (2005) and supplemented with additional quantification (Jiang et al., 2007; Doh et al., 2010; Munemasa et al., 2010). Relative cumulative IOP exposure calculated as product of fold-increase in IOP above baseline and period of elevation. Axon loss in the nerve increases linearly with IOP exposure independent of model used (r2 = 0.77, p=0.004). Back-filling of RGCs is less informative, with little dependency on IOP exposure (r2 = 0.04, p=0.59).
Figure 5 demonstrates another intriguing trend. When retrograde fluorogold labeling of RGCs is used to asses survival, the dependency on IOP exposure is far less robust. In fact, across the entire 6-fold range of cumulative IOP, the fraction of RGC somatic loss varies only between10–30%, with very little dependence on IOP exposure. This speaks to several points. The comparison suggests that nerve pathology is always greater than that based on RGC counts by a factor of 2–3 regardless of IOP exposure and that nature of the model. This underscores the axogenic nature of IOP-induced injury and the persistence of a large number of RGC bodies throughout progression. By the same token, the same comparison could be interpreted to suggest that for a given IOP exposure, axon degeneration significantly precedes loss of retrograde transport. However, most of the fluorogold studies referenced in Figure 5 applied the tracer either prior to or concurrent with IOP elevation as a means to pre-label RGCs (see Table 1 in Morrison et al., 2005). The tacit assumption in such experiments was that drop-out of fluorogold-labeled cell bodies would be the most telling outcome measure, and so the idea was to label them beforehand. This stands in contrast to its use in the chronic DBA model, in which the degree of retrograde labeling is a measure of intact axonal function in the animal’s history prior to injection (Buckingham et al., 2008; Danias et al., 2003; Jakobs et al., 2005; Zhong et al., 2007). Indeed, the number of successfully-filled RGCs based on retrograde transport of fluorogold diminishes proportionally as the number of overtly degenerating axon profiles in the nerve increases (Zhong et al., 2007).
The compound action potential (CAP) of the DBA optic nerve also depends strongly on IOP for young animals (6 months), with recovery of the CAP after oxygen-glucose deprivation diminishing linearly with increasing IOP above a certain threshold (Baltan et al., 2010). For older animals (10 months), IOP has little influence over CAP recovery, possibly due to already diminished ATP in the older nerves. However, CAP amplitude, which indicates the fraction of functional axons, in older animals is significantly diminished compared to young; elevated IOP for both groups exacerbates the loss (Baltan et al., 2010). This is interesting, given that depletion of anterograde transport in the DBA also depends primarily on age with IOP as an additional stressor (Crish et al., 2010).
3.2.2. RGC Synaptic Terminals and Central Projection Sites
Axon degeneration in the nerve is marked by reduction in both axon sampling density (number of axons per unit cross-sectional area) and total number of axons in the nerve. This is so for inducible rodent models (Dai et al., 2012; Morrison et al., 2005), DBA mice (Howell et al., 2012b; Inman et al., 2006; Libby et al., 2005a; Scholz et al., 2008), monkeys (Levkovitch-Verbin, 2004; Quigley et al., 1981; Quigley et al., 1987), and of course human patients (reviewed in (Quigley, 1999). However, functional studies utilizing active transport markers, whole nerve physiology, and ERG measurements of RGC response indicate an interval between functional impairment and outright degeneration of the optic projection (Baltan et al., 2010; Buckingham et al., 2008; Holcombe et al., 2008; Saleh et al., 2007). Such an interval is also implied by the reversibility of both axonal transport deficits induced acutely and diminishment of RGC signals in the ERG (Johansson, 1988; Minckler et al., 1977; Nagaraju et al., 2007; Porciatti and Nagaraju, 2010; Quigley and Addicks, 1980). A similar phenomenon has been described in human glaucoma, with reversal of physiological deficits subsequent to IOP-lowering surgery (Sehi et al., 2010).
How long is this interval? In the DBA mouse, anterograde transport in the SC is depleted by 11–12 months (Crish et al., 2010; Howell et al., 2012b), but protein markers for RGC presynaptic terminals and synapses persist up to 18–22 months (Crish et al., 2010). This is also consistent with the longer viability of retrograde transport from the SC (18 months: see section 3.1.2), which requires intact RGC axon terminals for uptake. We also found that SC volume persists for the same period, indicating little or no loss of post-synaptic relay neurons (Crish et al., 2010). Interestingly, King et al (King et al., 2006) showed an increase in receptive field size for glaucomatous SC relay neurons in an inducible rat model, supporting the possibility of physiological plasticity prior to degeneration of the projection.
A number of studies in non-human primates show significant shrinkage and loss of RGC relay cells in the LGN as well as reduced cytochrome oxidase activity after several months of induced ocular hypertension (Harwerth et al., 2002; Weber et al., 2000; Yucel et al., 2006; Yucel et al., 2003). A similar result was demonstrated in post-mortem tissue for patients with significant (50%) visual field deficits (Gupta et al., 2006). Presumably pathological changes in RGC projection sites ensue from a form of anterograde transsynaptic degeneration (Yucel and Gupta, 2008). In support of this, loss of LGN neurons increases as RGC axon degeneration in the nerve progresses, but generally lags by 20–30% (Yucel et al., 2003). For example, even with near 100% axonal degeneration, 30–40% of LGN relay neurons persist (Yucel et al., 2003).
3.2.3. Progression and Pattern of Degeneration in the Nerve
Our studies of the DBA indicate a distal-to-proximal progression in the depletion of anterograde transport from the retina to the SC (section 3.1.3 above). We also found that axon degeneration in the DBA optic nerve (myelinated) is always worse at sites nearer the optic chiasm than the orbit, as assessed by counts of degenerating profiles (Crish et al., 2010). Similarly, Schlamp et al. (Schlamp et al., 2006) showed a distal-to-proximal depletion of tracer transport in the DBA optic nerve, while axonal dystrophies decrease in severity from the SC towards the optic nerve head (Howell et al., 2007a). As discussed by Howell et al. (Howell et al., 2012a), this sort of pattern suggests a “dying back” progression of axonal degeneration, which involves the slow advancement of pathology from distal processes towards the cell body (Coleman, 2005). With a dying back program, one might expect substantially more loss of RGC axon terminals with tell-tale formation of end-bulbs at distal projection sites than axonal drop-out even in the distal nerve. However, a full complement of RGC axon terminals persists in the SC long after axon degeneration in the nerve begins (Crish et al., 2010; Inman et al., 2006; Scholz et al., 2008). Furthermore, axonal dystrophies in the SC are not as severe as one might expect from full-blown dying back (Howell et al., 2007a).
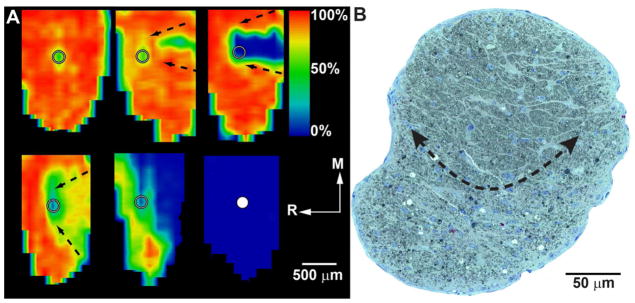
The pattern of axonopathy in the DBA nerve when viewed in cross-section mimics the sectorial retinotopy of transport deficits in the SC (Figure 6). This has been demonstrated by histological staining of axons, which shows degenerating axons forming clusters of affected fascicles or bundles separated by hypertrophic astroglial processes (Schlamp et al., 2006; Scholz et al., 2008). This sectorial progression is also evident in human glautomatous post-mortem tissue (Knox et al., 2007; Quigley, 1999). Similar patterns in the nerve emerge from immune-labeling for axonal components such as phosphorylated neurofilaments in conjunction with cytoskeletal markers for astrocytes (Howell et al., 2007a; Soto et al., 2008). These experiments suggest that as axons drop out of the nerve, hypertrophic astrocyte processes expand to preserve overall nerve volume by forming a glial scar. In inducible models for rodents, the progression of axon loss is often rapid, depending on the insult (Chidlow et al., 2011; Fu and Sretavan, 2010). This is discussed in detail below (see section 4.1). Nevertheless, histological staining of axons in these models also reveals a sectorial pattern of degeneration (Morrison et al., 1997; Soto et al., 2011), as does immune-labeling for axonal components (Vidal-Sanz et al., 2012).
Figure 6. Sectorial Progression in the DBA Mouse.
A: Retinotopic maps of SC from DBA mice reconstructed from serial coronal sections showing levels of cholera toxin B transported from the retina after intravitreal injection. Degree of intact signal density varies from 100% (red) to 50% (green) to 0% (blue). Maps show the representation of optic disk gap (circle) and sectorial progression of deficits in transport increasing in severity from the caudal edge to the optic disk gap (arrows). Ages are (left to right, top to bottom): 3, 3, 8, 10, 10 and 12 months. R: rostral; M: medial. Maps reproduced from Crish et al. (2010). B: Cross-section through optic nerve of DBA mouse. Arrows outline a sector with advanced progression containing numerous degenerating profiles and extensive gliosis. The unaffected region of the nerve contains fascicles of intact axons separated by astrocyte processes.
3.2.4 The Proximal Axonal Segment
RGC somatic degeneration, like axonal in the nerve, is also sectorial across animal models including DBA (Danias et al., 2003; Jakobs et al., 2005; Schlamp et al., 2006; Soto et al., 2008), inducible mouse and rat (Fu and Sretavan, 2010; Soto et al., 2011; Vidal-Sanz et al., 2012), and monkey (Harwerth et al., 2002). In the proximal portion of the optic nerve head, retinotopy is conserved such that specific retinal regions are represented by neighboring axonal bundles in the nerve. Using a DBA strain with an RGC reporter, Schlamp et al. (Schlamp et al., 2006) demonstrated that regions of axonal degeneration in the nerve map fairly well to regions of the retina lacking RGCs, though pathology in the nerve was always worse. Similarly, using another RGC reporter crossed into the DBA, Howell et al. (Howell et al., 2007a) showed that sectors of surviving RGCs in the retina mapped to intact axonal bundles in the nerve head. These results indicate that somatic degeneration follows axonal degeneration not only temporally, but topographically as well.
That somatic and axonal progressions are coupled might, at first blush, appear to contradict the evidence supporting compartmentalized degeneration (see section 3.1.1.). However, even in the DBA Wlds mouse, those eyes that eventually demonstrate severe axon degeneration in the nerve also show a correlative loss of RGC bodies (Howell et al., 2007a). Thus, loss of the axon even if delayed must, at some point, necessitate loss of the cell body. In the DBA Bax knockout, with disease progression the RGC unmyelinated axon survives along with the protected cell body and extends through the level of the glial lamina of the optic nerve head (Howell et al., 2007a). Label for axons in degenerating DBA nerve also extends well past the lamina region (Soto et al., 2008), reminiscent of transported fluorescent markers even late in progression (Crish et al., 2010; Schlamp et al., 2006). Were the unmyelinated segment to retract early in chronic glaucoma, one would expect a dominant pattern of dystrophic axons and end-bulbs in the nerve head and optic disk of the pre-laminar region indicative of acute degeneration; this is not the case (Howell et al., 2007a). Even with more acute (3-fold increase) and transient (days) elevations in IOP, tracers applied directly to the proximal nerve posterior to the laminar region label considerably more RGCs than fluorogold retrogradely transported from the SC (Salinas-Navarro et al., 2010; Vidal-Sanz et al., 2012). Thus, the unmyelinated axonal segment, while doubtlessly involved in transducing IOP-related stress at the nerve head, ironically appears to persist along with the cell body after distal axonopathy has progressed considerably.
4. Progression and the Nerve Head
4.1 Just Passing Through: The Optic Nerve Head
A major goal has been to understand how factors extrinsic to the RGC axon as it passes through the nerve head contribute to a pathogenic environment and initiate and modulate progression (Hernandez, 2000; Moore and Goldberg, 2010; Osborne et al., 2001; Venkataraman et al., 2010; Vrabec and Levin, 2007). The extra-axonal milieu includes the vascular and glial architecture of the retina and optic nerve head, the scleral-laminar interface, and of course the lamina cribrosa or glial lamina as it is known in rodents (Howell et al., 2007a; Jakobs et al., 2005; Morgan, 2000). Of the extrinsic modulators of survival that arise from these architectural substrates, it is useful to distinguish those that could transduce directly age- and IOP-related stress to the axon – which we might call primary transducers – from those that influence RGC survival but are themselves downstream of IOP- or age-dependent factors. This distinction is addressed in detail within the rubric recently formalized as the “biomechanical paradigm” (Burgoyne, 2011). This elegant paradigm seeks to understand how age- and IOP-related stressors are conveyed to the RGC axon through biomechanical, inflammatory and metabolic signals from the extra-axonal milieu in the nerve head. It links the cytoarchitecture of the nerve head to direct transduction of IOP-related stress on a backdrop of age-related changes in structure and neurochemistry. The paradigm can be no better articulated than it has been (Burgoyne, 2011; Burgoyne et al., 2005), and so will not be discussed further here.
4.2 Acute Injury and the Axonal “Choke Point”
The optic nerve head defines the transition between the unmyelinated RGC axon and the point at which it becomes myelinated by oligodendrocyte glia; this is also an important zone for astrocyte-axon interactions (Dai et al., 2012; Hernandez, 2000; Minckler et al., 1976; Nguyen et al., 2011b; Son et al., 2010). This zone has often been interpreted as an axonal “choke point” at which transport is acutely stressed for induced elevations in IOP exceeding what is typical in chronic models or human glaucoma (reviewed in (Knox et al., 2007). Acute stress in such models is typically transient (hours to days) and generally accompanied by rapid axonal degeneration in the nerve and compression of the interval between axon loss and somatic degeneration in the retina (Chidlow et al., 2011; Fu and Sretavan, 2010; Zhang et al., 2009).
The earliest studies describing the laminar region of the optic nerve head as a bottle-neck for antero- and retrograde transport in monkeys incorporated pressure increases of 100–1000% (Minckler et al., 1977; Quigley and Addicks, 1980). For monkeys with less IOP exposure, transport blockade was absent (Quigley and Addicks, 1980). In some cases, transport blockade was reversible (Minckler et al., 1977; Quigley and Addicks, 1980), suggesting that neither RGC bodies nor axons had undergone significant degeneration aside from the transport deficit. Axons subjected to very high pressure demonstrate clear morphological indications of mechanical damage (Quigley and Addicks, 1980; Quigley et al., 1980). Interestingly, like the chronic rodent models, the unmyelinated axonal segment and cell body often persist after overt degeneration of the retrobulbar nerve (Quigley and Addicks, 1980). Transport blockade with acute elevations in the monkey accompany rapid optic disc deformation, accumulation of cytoskeletal proteins in the pre-laminar region, and axonal dystrophies in the proximal nerve and nerve head (Balaratnasingam et al., 2007; Hayreh et al., 1999; Pease et al., 2000). With even higher elevations for several hours, anterograde transport is blocked at the laminar region with accumulation of cytoskeletal organelles and clear axonal dystrophies (Anderson and Hendrickson, 1974). Similar characteristics have been described in human post-mortem nerve from eyes with secondary acute glaucoma (Knox et al., 2007).
In rodents with acute elevations, the story is very much the same, with sharp, temporary increases eliciting reversible mechanical blockage of axoplasmic transport (Johansson, 1988). In rats with IOP acutely raised 3–5 times above normal, retrograde transport of radiolabeled BDNF (brain-derived neurotrophic factor) from the SC to the retina is diminished by 50% and accompanied by axonal dystrophies proximal to the myelination transition zone (Pease et al., 2000). For IOP exposures less than a week that elicit 25–50% axon loss in the rat proximal nerve, protein markers for axonal transport accumulate on either side of the transition zone (Chidlow et al., 2011), suggestive of a locus of acute insult. In contrast to chronic and more modest IOP elevations, axon degeneration with acute elevations can appear worse proximal to the laminar region of the nerve head than it is at distal sites further along the nerve (Beirowski et al., 2008).
4.3 Two Forms of Progression … or More?
The distinction just made between acute elevations in inducible models and IOP in human glaucoma is not intended to detract from the importance of such experiments, which have been seminal in establishing the link between IOP-related biomechanical force at the nerve head and pathogenesis. Taken as a whole, the acute studies support two important conclusions about axonopathy and the optic nerve head. First, for extreme elevations in IOP which lead to dramatic diminishment of ocular perfusion pressure (Anderson and Hendrickson, 1974; Knox et al., 2007; Quigley and Addicks, 1980), mechanical compression of the unmyelinated axon as it transverses the laminar region is the likely mechanism of focal depletion of axoplasmic transport (Quigley and Addicks, 1980; Quigley et al., 1980). Such a scenario could be considered the strong form of the biomechanical paradigm, for it certainly follows from the architecture of the scleral-nerve interface, especially in the primate with its well-defined lamina cribrosa (Burgoyne, 2011; Burgoyne et al., 2005). Second, in contrast to the slower distal-to-proximal progression in the DBA nerve (Crish et al., 2010; Schlamp et al., 2006), acute elevations may induce Wallerian-like degeneration distal to the point of injury. This sort of injury is characterized by early axonal dystrophies followed by rapid disassembly of the axon distal to the injury (Coleman, 2005; Howell et al., 2012a; Whitmore et al., 2005). Interestingly, even in the chronic DBA, axonal dystrophies along the optic nerve are highly suggestive of Wallerian degeneration (Howell et al., 2007a).
Wallerian degeneration has also been linked to another mode of axonopathy termed “acute axon degeneration”, which occurs both distal and proximal to the point of stress (Coleman, 2005). This may explain in some inducible models the presence of dystrophies on either side of the myelination transition zone (Chidlow et al., 2011), as well as early axonal loss in the proximal nerve compared to distal (Beirowski et al., 2008). Aberrant unmyelinated axonal processes observed late in progression in the retina of both the DBA and an acute model are also suggestive of a separation on the proximal side of the nerve head (Fu and Sretavan, 2010; Soto et al., 2008), although they could represent anomalous sprouting as seen in white matter tracts in Parkinson’s disease (Braak et al., 2004).
We are left with a model in which progression in glaucoma likely involves both early and progressive distal axonopathy with components of more acuteWallerian degeneration (Nickells et al., 2012; Howell et al., 2012a; Whitmore et al., 2005), as summarized below (Figure 7). It is possible that the balance between the two programs depends on the degree of stress placed on a lynchpin defined by the unmyelinated axon as it passes through the laminar region of the optic nerve head. The transduction of stress directly to the axon is presumably mediated by the constellation of architectural, inflammatory, vascular and other signaling factors included in the biomechanical paradigm poised by Burgoyne and colleagues (Burgoyne, 2011). This model would mandate that the more acute the stress, the greater the pressure on the system towards Wallerian degeneration (Howell et al., 2012a). In human patients IOP-related stress is subject to tremendous variability, as in the DBA model (Inman et al., 2006; Schlamp et al., 2006). Thus, Wallerian forces may move in and out of the fore as distal axonopathy progresses.
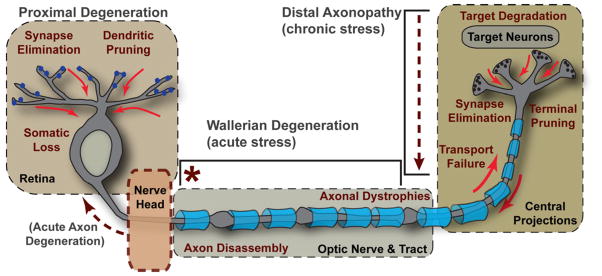
Figure 7. Key Events in Progression.
The events defining RGC degeneration in glaucoma span four critical regions: retina, optic nerve head, optic nerve and tract, and the central projection to the brain. Disease-relevant stress originating in the nerve head (marked by *) induces a program of distal axonopathy with several characteristics of chronic progression. These include failure of intra-axonal transport and subsequent loss of RGC pre-synaptic active zones and axon terminals in central projection sites, followed by degeneration of target neurons. Pathogenic features follow a distal-to-proximal progression, as in “dying back” neuropathies. In the optic nerve and tract, degeneration of the myelinated RGC axon (indicated by blue oligodendrocyte sheaths) includes features of both distal axonopathy and Wallerian degeneration, including axonal dystrophies and more acute axon disassembly beginning distal to the nerve head. Proximal degeneration is marked by elimination of synapses to RGCs, dendritic pruning and eventual somatic drop-out in the retina. In some models, retraction of the unmyelinated segment from the nerve head could arise from acute axon degeneration (see Coleman, 2005).
4.4 Relation to Dendritic Pruning
A well-known aspect of neuronal degeneration in a variety of diseases is morphological changes in the dendritic arbor, in the form of both dendritic thinning and dendritic loss (Liu et al., 2011). Dendritic stress and the loss of synapses associated with it are potential targets for therapeutic intervention, and so it is important to establish their relation to axonal degeneration (Morquette and Di Polo, 2008). A study using severely diseased human post-mortem tissue found that the few surviving midget and parasol ganglion cells demonstrated a reduction in both dendritic complexity (branching) and dendritic field size (Pavlidis et al., 2003). That both were observed indicates that RGC dendritic arbors do not simply shrink down during pathogenesis while conserving complexity, but actually undergo pruning at distal branch points. Interestingly, in the same study, cell body size was unchanged compared to normal tissue (Pavlidis et al., 2003). This could be interpreted as an indication that dendritic pruning occurs prior to apoptotic changes in the cell body.
A key question is how early dendritic changes occur. The answer based on animal models is not straightforward. In an inducible cat model, substantial dendritic pruning was concurrent with RGC body drop-out in the retina after a month of hypertension (Shou et al., 2003). While average IOP was only about twice that of normal (18 mmHG), the peak IOP was often 20–25 mmHG higher. Similarly, in glaucomatous monkey retina, shrinkage of dendritic arbors for parasol cells occurred over the course of 2–4 weeks along with shrinkage of cell bodies (Weber et al., 1998). Again, IOP in this study involved elevation 5–7 times normal with peaks near 90 mmHG, Later studies in monkeys examined dendritic changes months and even years after injury (Weber and Harman, 2005). Importantly, in the monkey model most affected RGCs retained at least the proximal axon segment.
In the DBA retina, severe progression is marked by sectors containing far fewer identifiable RGC bodies (Jakobs et al., 2005). Surviving RGCs in these sectors have fewer dendritic branch points, smaller cell bodies, and thinner axons, although whether these features always occur together is not established. Some cells have tortuous dendritic arbors suggestive of remodeling (Jakobs et al., 2005). The same study showed that RGCs with dramatic dendritic or somatic shrinkage are never filled by retrograde label from the superior colliculus. That these cells retain partial dendritic arbors indicates that dendritic pruning in the DBA occurs either after or concurrent with failure of retrograde transport but before final apoptotic elimination of the cells. Also in the DBA, moderate progression is characterized by upregulation of C1q, the initiating protein in the classical complement cascade, with localization proximal to synapses in the inner plexiform layer (Stevens et al., 2007). The implication of this finding is that synapses to RGC dendrites may be targeted in progression for microglial-mediated elimination. In the DBA, microglia activation is early, and suppression of this response is protective of RGCs (Bosco et al., 2008; Bosco et al., 2011).
Thus, it is likely that acute elevations in IOP (as in the monkey model) induce a Wallerian-like rapid degeneration of dendrites concurrent with somal shrinkage and subsequent apoptosis. There is precedence for such a program in other neurons (Tao and Rolls, 2011). However, chronic stress as in the human disease and the DBA appears to lead to a slower pruning of the dendritic tree that seems to precede changes in the cell body that would be indicative of apoptotic processes. This raises the intriguing possibility that like axonal degeneration, dendritic degeneration is to some degree compartmentalized in glaucoma.
5. Epilogue: Gaps in our Knowledge and Future Directions
5.1 Transduction of IOP Stress
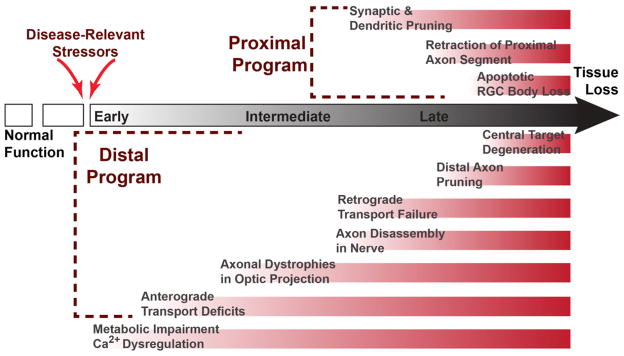
Our view of glaucoma has evolved considerably in recent years, from a disease involving mechanical injury due to elevated IOP to a disease involving a confluence of age- and IOP-related stressors acting upon a neural substrate. Glaucoma is largely axogenic, like other CNS diseases (Coleman, 2005; Whitmore et al., 2005), with axonal dysfunction in the optic projecting occurring prior to outright degeneration and loss of tissue (Figure 8). This progression could explain why, in non-human primates, significant loss of visual function occurs with only minor changes in the numbers of RGC bodies in the retina (Harwerth et al., 2002). This interval defines an obvious window of opportunity for neuroprotective therapeutic intervention, especially for those patients who do not respond to hypotensive regimens or other treatments, especially for secondary glaucomas. It is therefore critical to focus our energy on understanding the molecular mechanisms through which glaucomatous stressors induce loss of function early in progression.
Figure 8. Proposed Timeline of Key Events in RGC Degeneration.
Normal function in the optic projection is interrupted by disease-relevant stressors, inducing RGC metabolic impairment and dysregulation of intracellular Ca2+. An early consequence is disruption of anterograde axonal transport at distal sites in the optic projection followed by formation of axonal dystrophies. Retrograde transport persists longer in progression and is likely to fail concurrent with axon disassembly. Later in progression, proximal degeneration affects RGC processes in the retina, though the exact timing of synaptic and dendritic pruning is unknown. In this proposed model, the last events are RGC somatic loss proximally and degeneration of neurons in central targets distally, resulting in irreversible tissue loss.
The unmyelinated segment of the axon both in the retina and in the nerve head is especially vulnerable to disease-related stressors from a metabolic standpoint. This vulnerability is likely to affect distal axonal processes. Additionally, the extracellular milieu of the axon as it passes through the nerve head is likely to become pathogenic. This is so for two primary reasons. First, a confluence of age-related factors diminishes the architectural capacity of the nerve head for IOP-related stress (Burgoyne, 2011). Second, glial and vascular components create an environment that is increasingly inhospitable for the axon through biochemical, metabolic and inflammatory signaling (Almasieh et al., 2012; Hernandez, 2000). Thus, ironically, the very segment of the RGC axon that is likely most intrinsically vulnerable to stress is also poised to directly transduce stress from the extra-axonal environment.
We understand very well possible ramifications of this transduction. For example, as discussed above (section 4.2), one consequence of disrupted axonal transport is deprivation of BDNF-mediated trophic support to RGCs from the SC (Pease et al., 2000; Quigley et al., 2000). Though strong evidence for this hypothesis arises from models employing extreme elevations in IOP, early reduced transport is a fact – and so it is reasonable to conclude that reduced trophic support is as well. Reduced axonal transport is likely to affect a host of other factors as well, including mitochondrial motility and ATP availability (section 2.4.4).
However, setting aside mechanical compression, we do not understand how IOP-related stress is transduced from cellular structures in the nerve head to the axon to cause early transport deficits. Astrocytes are likely to be important mediators in this transduction pathway (Hernandez, 2000; Morgan, 2000; Son et al., 2010). One possibility (of many) is astrocyte release of the dually neuroactive and vasoactive peptide endothelin-1 (Rosenthal and Fromm, 2011). Cultured optic nerve astrocytes release endothelin-1 in response to tumor necrosis factor α (TNF α: (Desai et al., 2004), which is also upregulated in the glaucomatous nerve head and contributes to axonal degeneration (Nakazawa et al., 2006; Tezel, 2008). Endothelin-1 is known to impede anterograde and retrograde transport in RGC axons, possibly by increasing intra-axonal Ca2+ (Stokely et al., 2002; Wang et al., 2008b), which in turn may disrupt the axonal cytoskeleton (Crish and Calkins, 2011). Another possibility suggested by morphological changes in the nerve head is withdrawal of astrocyte-derived metabolic support of the axon (Dai et al., 2012).
An intriguing possibility is that RGCs themselves also sense stress-related changes in their environment. We have shown that RGCs express the transient receptor potential vanilloid-1 subunit (TRPV1) in both the dendritic arbor and the unmyelinated axonal segment and that this channel gates a potent Ca2+ conductance in response to stress (Sappington et al., 2009). The TRPV1 channel responds to a variety of insults in the CNS and may mediate pathways that counter reduced function (Ho, 2012). RGCs also express purinergic receptors that respond to autocrine release of ATP following mechanical stress (Xia et al., 2012). Such a system could counter diminished availability of ATP in the local milieu during progression.
5.2. Mediation of Sectorial Progression
We have discussed herein how aspects of RGC degeneration in both the distal projection and in proximal structures follow a sectorial progression, filling in from one retinotopic region to the next. This progression is analogous to the pattern of visual field deficits in both human glaucoma and in non-human primate models (Harwerth et al., 2002). While it is tempting to consider this pattern etiologically-specific to glaucoma, other common optic neuropathies demonstrate sectorial deficits as well (Bernstein et al., 2007a; Bernstein et al., 2007b; Kedar et al., 2011), as does an animal model of streptozotocin-induced diabetes (Fernandez et al., 2011). The latter is especially interesting since the insult is systemic. Thus, retinotopically-defined progression seems to be a general feature of RGC degeneration that transforms a diverse array of disease-specific stressors into a common pathogenic arm.
What such an arm might comprise is unknown. Axons in the optic nerve cluster into well-defined fascicles separated by astrocyte processes. In the proximal nerve, these form a loose retinotopic map, supporting the idea that sectorial progression is axogenic, involving both inter-axonal and axon-astrocyte signaling. In glaucoma, early field loss is modest and first appears as a discrete scotoma before progressing throughout a retinotopic quadrant (Iester et al., 2012). A key question is whether a pathogenic sector begins as a single locus that expands or as several discrete foci within a local region that eventually coalesce as a solid lesion. Once a lesion begins, it is important to understand the processes that confine the injury to a particular sector. Thus, new investigations must focus not only on the mechanisms of progression within a sector, but on possible mechanisms that sequester the injury to delay spreading between sectors.
5.3 Self-Repair Pathways
Other retinal neurons are only moderately susceptible in glaucoma to trans-synaptic degeneration. Whatever loss that does occur does so in varying degrees and with high variability between studies and models. This loss is also retinotopically coincident with regions of high RGC damage (section 2.3.3). However, given that RGCs are so robustly targeted in the disease through processes that include early functional deficits, it is most puzzling that trans-synaptic degeneration of their pre-synaptic circuits is not far greater than it is.
One conjecture to explain this paucity is that even late in progression, RGCs maintain a certain degree, however minimal, of residual physiological activity that drives the maintenance of pre-synaptic connectivity. We found using the Ca2+ surrogate manganese coupled with magnetic resonance imaging very little change in cationic activity in the DBA inner retina for ages associated with axonopathy (Calkins et al., 2008). This would seem to speak against early loss of synaptic activity that would be required by dendritic pruning. Similar, a rarely cited study using rats found that modest IOP elevations were associated with expanded RGC physiological receptive fields, possibly attributable to enlargement of their dendritic arbors (King et al., 2006).
Perhaps pruning of RGC synapses and dendrites in the inner retina is far slower than predicted by acute models, which likely do not recapitulate the more measured progression in the human disease. Another possibility is that despite ongoing targeting of RGC synapses and dendrites by disease processes, excitatory RGC activity persists because of intrinsic mechanisms for self-repair that counter the degenerative cascade to maintain connectivity with presynaptic circuits. The potential for such a self-repair pathway is evident in experiments demonstrating the utility of exogenous electrical stimulation for promoting RGC survival (Chang and Goldberg, 2012). An exciting possibility is that self-repair pathways may also operate distally, promoting the persistence of RGC axon terminals at their central projection sites even well-after the depletion of anterograde signaling from the retina (Crish et al., 2010).
Acknowledgments
The author wishes to dedicate this article to Thomas Brunner, for his unwavering and innovative support of the Glaucoma Research Foundation and its Catalyst for a Cure program. The author also expresses his sincerest gratitude to many gifted and dedicated collaborators over the years, in particular Catalyst for a Cure members Rebecca Sappington, Philip Horner, Samuel Crish, Denise Inman, Monica Vetter, Alejandra Bosco, Martin Wax, and Nicholas Marsh-Armstrong. Special thanks are due Wendi Lambert for her expert proof-reading, commentary and critique. This work would not have been possible without the generous financial support of the Melza M. and Frank Theodore Barr Foundation through the Glaucoma Research Foundation, the American Health Assistance Foundation, and Research to Prevent Blindness, Inc. Funding also provided through NIH EY017427 (DJC) and NIH P30 core grant EY008126 to the Vanderbilt Vision Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Allan VJ. Cytoplasmic dynein. Biochem Soc Trans. 2011;39:1169–1178. doi: 10.1042/BST0391169. [DOI] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Morgan WH, Bass L, Matich G, Cringle SJ, Yu DY. Axonal transport and cytoskeletal changes in the laminar regions after elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2007;48:3632–3644. doi: 10.1167/iovs.06-1002. [DOI] [PubMed] [Google Scholar]
- Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci. 2010;30:5644–5652. doi: 10.1523/JNEUROSCI.5956-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band LR, Hall CL, Richardson G, Jensen OE, Siggers JH, Foss AJ. Intracellular flow in optic nerve axons: a mechanism for cell death in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:3750–3758. doi: 10.1167/iovs.08-2396. [DOI] [PubMed] [Google Scholar]
- Barron MJ, Griffiths P, Turnbull DM, Bates D, Nichols P. The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. Br J Ophthalmol. 2004;88:286–290. doi: 10.1136/bjo.2003.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AU, Neuhardt T, May AC, Martus P, Maag KP, Brodie S, Lutjen-Drecoll E, Podos SM, Mittag T. Retinal morphology and ERG response in the DBA/2NNia mouse model of angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2001;42:1258–1265. [PubMed] [Google Scholar]
- Bechtold DA, Yue X, Evans RM, Davies M, Gregson NA, Smith KJ. Axonal protection in experimental autoimmune neuritis by the sodium channel blocking agent flecainide. Brain. 2005;128:18–28. doi: 10.1093/brain/awh328. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Coleman MP, Martin KR. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SL, Guo Y, Slater BJ, Puche A, Kelman SE. Neuron stress and loss following rodent anterior ischemic optic neuropathy in double-reporter transgenic mice. Invest Ophthalmol Vis Sci. 2007a;48:2304–2310. doi: 10.1167/iovs.06-0486. [DOI] [PubMed] [Google Scholar]
- Bernstein SL, Mehrabyan Z, Guo Y, Moianie N. Estrogen is not neuroprotective in a rodent model of optic nerve stroke. Mol Vis. 2007b;13:1920–1925. [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437–1446. doi: 10.1167/iovs.07-1337. [DOI] [PubMed] [Google Scholar]
- Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519:599–620. doi: 10.1002/cne.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM. The distribution of mitochondrial activity in relation to optic nerve structure. Arch Ophthalmol. 2002;120:791–796. doi: 10.1001/archopht.120.6.791. [DOI] [PubMed] [Google Scholar]
- Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;93:120–132. doi: 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Horner PJ. The cell and molecular biology of glaucoma: axonopathy and the brain. Invest Ophthalmol Vis Sci. 2012;53:2482–2484. doi: 10.1167/iovs.12-9483i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ, Horner PJ, Roberts R, Gradianu M, Berkowitz BA. Manganese-enhanced MRI of the DBA/2J mouse model of hereditary glaucoma. Invest Ophthalmol Vis Sci. 2008;49:5083–5088. doi: 10.1167/iovs.08-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Absence of spectrally specific lateral inputs to midget ganglion cells in primate retina. Nature. 1996;381:613–615. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Structure and function of parallel pathways in the primate early visual system. J Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V, Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem Int. 2002;40:573–584. doi: 10.1016/s0197-0186(01)00129-2. [DOI] [PubMed] [Google Scholar]
- Carelli V, Rugolo M, Sgarbi G, Ghelli A, Zanna C, Baracca A, Lenaz G, Napoli E, Martinuzzi A, Solaini G. Bioenergetics shapes cellular death pathways in Leber’s hereditary optic neuropathy: a model of mitochondrial neurodegeneration. Biochim Biophys Acta. 2004;1658:172–179. doi: 10.1016/j.bbabio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–986. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BC, Pan J, Archibald ML, LeVatte TL, Kelly ME, Tremblay F. Effect of intraocular pressure on optic disc topography, electroretinography, and axonal loss in a chronic pressure-induced rat model of optic nerve damage. Invest Ophthalmol Vis Sci. 2002;43:2969–2976. [PubMed] [Google Scholar]
- Chen H, Wei X, Cho KS, Chen G, Sappington R, Calkins DJ, Chen DF. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest Ophthalmol Vis Sci. 2010;52:36–44. doi: 10.1167/iovs.09-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, Ebneter A, Wood JP, Casson RJ. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 2011;121:737–751. doi: 10.1007/s00401-011-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Zawadzki RJ, Lim MC, Brandt JD, Keltner JL, Doble N, Werner JS. Evidence of outer retinal changes in glaucoma patients as revealed by ultrahigh-resolution in vivo retinal imaging. Br J Ophthalmol. 2011;95:131–141. doi: 10.1136/bjo.2010.183756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Trounce IA, Crowston JG. Mechanisms of retinal ganglion cell injury in aging and glaucoma. Ophthalmic Res. 2010;44:173–178. doi: 10.1159/000316478. [DOI] [PubMed] [Google Scholar]
- Coleman MP. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;11:889–98. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res. 2010;91:415–424. doi: 10.1016/j.exer.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MF, Guo L, Luong V, Harding G, Wang W, Jones HE, Moss SE, Sillito AM, Fitzke FW. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain. 2011;134:2149–2157. doi: 10.1093/brain/awr125. [DOI] [PubMed] [Google Scholar]
- Crish SD, Calkins DJ. Neurodegeneration in glaucoma: progression and calcium-dependent intracellular mechanisms. Neuroscience. 2011;176:1–11. doi: 10.1016/j.neuroscience.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010;107:5196–5201. doi: 10.1073/pnas.0913141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Pinilla I, Fernandez-Sanchez L, Salinas-Navarro M, Alarcon-Martinez L, Aviles-Trigueros M, de la Villa P, Miralles de Imperial J, Villegas-Perez MP, Vidal-Sanz M. Changes in the inner and outer retinal layers after acute increase of the intraocular pressure in adult albino Swiss mice. Exp Eye Res. 2010;91:273–285. doi: 10.1016/j.exer.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y. Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia. 2012;60:13–28. doi: 10.1002/glia.21242. [DOI] [PubMed] [Google Scholar]
- Danias J, Lee KC, Zamora MF, Chen B, Shen F, Filippopoulos T, Su Y, Goldblum D, Podos SM, Mittag T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–5162. doi: 10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- Desai D, He S, Yorio T, Krishnamoorthy RR, Prasanna G. Hypoxia augments TNF-alpha-mediated endothelin-1 release and cell proliferation in human optic nerve head astrocytes. Biochem Biophys Res Commun. 2004;318:642–648. doi: 10.1016/j.bbrc.2004.04.073. [DOI] [PubMed] [Google Scholar]
- Doh SH, Kim JH, Lee KM, Park HY, Park CK. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010;1308:158–166. doi: 10.1016/j.brainres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Topography of visual and somatosensory projections to mouse superior colliculus. J Neurophysiol. 1976;39:91–101. doi: 10.1152/jn.1976.39.1.91. [DOI] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan N, Huang N, Lam DS, Leung CK. Measurement of photoreceptor layer in glaucoma: a spectral-domain optical coherence tomography study. J Ophthalmol. 2011;2011:264803. doi: 10.1155/2011/264803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Pasquini LA, Dorfman D, Aldana Marcos HJ, Rosenstein RE. Early distal axonopathy of the visual pathway in experimental diabetes. Am J Pathol. 2011;180:303–313. doi: 10.1016/j.ajpath.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippopoulos T, Danias J, Chen B, Podos SM, Mittag TW. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006;47:1968–1974. doi: 10.1167/iovs.05-0955. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- Fu CT, Sretavan D. Laser-induced ocular hypertension in albino CD-1 mice. Invest Ophthalmol Vis Sci. 2010;51:980–990. doi: 10.1167/iovs.09-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674–678. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harazny J, Scholz M, Buder T, Lausen B, Kremers J. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc Ophthalmol. 2009;119:181–197. doi: 10.1007/s10633-009-9194-5. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL, 3rd, Carter-Dawson L. Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res. 2002;21:91–125. doi: 10.1016/s1350-9462(01)00022-2. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Pe’er J, Zimmerman MB. Morphologic changes in chronic high-pressure experimental glaucoma in rhesus monkeys. J Glaucoma. 1999;8:56–71. [PubMed] [Google Scholar]
- Heiduschka P, Julien S, Schuettauf F, Schnichels S. Loss of retinal function in aged DBA/2J mice - New insights into retinal neurodegeneration. Exp Eye Res. 2010;91:779–783. doi: 10.1016/j.exer.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Heijl A, Aspberg J, Bengtsson B. The effect of different criteria on the number of patients blind from open-angle glaucoma. BMC Ophthalmol. 2011;11:31. doi: 10.1186/1471-2415-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Calkins DJ. Neuronal chemistry and functional organization in the primate visual system. Trends Neurosci. 1998;21:344–349. doi: 10.1016/s0166-2236(98)01245-4. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Brierley CM, Brex PA, MacManus DG, Scolding NJ, Compston DA, Miller DH. Continuing optic nerve atrophy following optic neuritis: a serial MRI study. Mult Scler. 2002;8:339–342. doi: 10.1191/1352458502ms809oa. [DOI] [PubMed] [Google Scholar]
- Ho KW, Ward NJ, Calkins DJ. TRPV1: a stress response protein in the central nervous system. Am J Neurodegener Dis. 2012;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- Holcombe DJ, Lengefeld N, Gole GA, Barnett NL. Selective inner retinal dysfunction precedes ganglion cell loss in a mouse glaucoma model. Br J Ophthalmol. 2008;92:683–688. doi: 10.1136/bjo.2007.133223. [DOI] [PubMed] [Google Scholar]
- Hollander H, Makarov F, Stefani FH, Stone J. Evidence of constriction of optic nerve axons at the lamina cribrosa in the normotensive eye in humans and other mammals. Ophthalmic Res. 1995;27:296–309. doi: 10.1159/000267739. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:d91–102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007a;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Marchant JK, Wilson LA, Cosma IM, Smith RS, Anderson MG, John SW. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007b;8:45. doi: 10.1186/1471-2156-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Libby RT, John SW. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol. 2012a doi: 10.1016/j.expneurol.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, Macnicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT, John SW. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest. 2012b;122:1246–1261. doi: 10.1172/JCI61135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A, Tsukamoto Y, Smith RG, Sterling P. Functional architecture of primate cone and rod axons. Vision Res. 1998;38:2539–2549. doi: 10.1016/s0042-6989(97)00370-2. [DOI] [PubMed] [Google Scholar]
- Iester M, De Feo F, Douglas GR. Visual field loss morphology in high- and normal-tension glaucoma. J Ophthalmol. 2012;2012:327326. doi: 10.1155/2012/327326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–953. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- Inman DM, Sappington RM, Horner PJ, Calkins DJ. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:986–996. doi: 10.1167/iovs.05-0925. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007;13:1783–1792. [PubMed] [Google Scholar]
- Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132:628–634. doi: 10.1093/brain/awp001. [DOI] [PubMed] [Google Scholar]
- Johansson JO. Inhibition and recovery of retrograde axoplasmic transport in rat optic nerve during and after elevated IOP in vivo. Exp Eye Res. 1988;46:223–227. doi: 10.1016/s0014-4835(88)80079-4. [DOI] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- Johnson EC, Morrison JC. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma. 2009;18:341–353. doi: 10.1097/IJG.0b013e31818c6ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H, Cowey A. Transneuronal retrograde degeneration of retinal ganglion cells following restricted lesions of striate cortex in the monkey. Exp Brain Res. 2000;132:269–275. doi: 10.1007/s002210000384. [DOI] [PubMed] [Google Scholar]
- Kedar S, Ghate D, Corbett JJ. Visual fields in neuro-ophthalmology. Indian J Ophthalmol. 2011;59:103–109. doi: 10.4103/0301-4738.77013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendell KR, Quigley HA, Kerrigan LA, Pease ME, Quigley EN. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci. 1995;36:200–205. [PubMed] [Google Scholar]
- Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- Kielczewski JL, Pease ME, Quigley HA. The effect of experimental glaucoma and optic nerve transection on amacrine cells in the rat retina. Invest Ophthalmol Vis Sci. 2005;46:3188–3196. doi: 10.1167/iovs.05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WM, Sarup V, Sauve Y, Moreland CM, Carpenter DO, Sharma SC. Expansion of visual receptive fields in experimental glaucoma. Vis Neurosci. 2006;23:137–142. doi: 10.1017/S0952523806231122. [DOI] [PubMed] [Google Scholar]
- Knox DL, Eagle RC, Jr, Green WR. Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport: histopathologic features in human high-pressure secondary glaucoma. Arch Ophthalmol. 2007;125:347–353. doi: 10.1001/archopht.125.3.347. [DOI] [PubMed] [Google Scholar]
- Lambert WS, Ruiz L, Crish SD, Wheeler LA, Calkins DJ. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol Neurodegener. 2011;6:4. doi: 10.1186/1750-1326-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Van Bergen NJ, Kong GY, Chrysostomou V, Waugh HS, O’Neill EC, Crowston JG, Trounce IA. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2010;93:204–212. doi: 10.1016/j.exer.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Lei Y, Garrahan N, Hermann B, Becker DL, Hernandez MR, Boulton ME, Morgan JE. Quantification of retinal transneuronal degeneration in human glaucoma: a novel multiphoton-DAPI approach. Invest Ophthalmol Vis Sci. 2008;49:1940–1945. doi: 10.1167/iovs.07-0735. [DOI] [PubMed] [Google Scholar]
- Lei Y, Garrahan N, Hermann B, Fautsch MP, Johnson DH, Hernandez MR, Boulton M, Morgan JE. Transretinal degeneration in ageing human retina: a multiphoton microscopy analysis. Br J Ophthalmol. 2011;95:727–730. doi: 10.1136/bjo.2010.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Levin LA. Intrinsic survival mechanisms for retinal ganglion cells. Eur J Ophthalmol. 1999;9(Suppl 1):S12–16. doi: 10.1177/112067219900901S08. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H. Animal models of optic nerve diseases. Eye (Lond) 2004;18:1066–1074. doi: 10.1038/sj.eye.6701576. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D’Anna SA, Kerrigan D, Pease ME. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2001;42:975–982. [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002;43:402–410. [PubMed] [Google Scholar]
- Li Y, Jung P, Brown A. Axonal transport of neurofilaments: a single population of intermittently moving polymers. J Neurosci. 2012;32:746–758. doi: 10.1523/JNEUROSCI.4926-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005a;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005b;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Duggan J, Salt TE, Cordeiro MF. Dendritic changes in visual pathways in glaucoma and other neurodegenerative conditions. Exp Eye Res. 2011;92:244–250. doi: 10.1016/j.exer.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Mabuchi F, Aihara M, Mackey MR, Lindsey JD, Weinreb RN. Optic nerve damage in experimental mouse ocular hypertension. Invest Ophthalmol Vis Sci. 2003;44:4321–4330. doi: 10.1167/iovs.03-0138. [DOI] [PubMed] [Google Scholar]
- Magharious MM, D’Onofrio PM, Koeberle PD. Optic nerve transection: a model of adult neuron apoptosis in the central nervous system. J Vis Exp. 2011 doi: 10.3791/2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Exp Eye Res. 2009;88:816–824. doi: 10.1016/j.exer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailovic LT, Cupic D, Dekleva N. Changes in the numbers of neurons and glial cells in the lateral geniculate nucleus of the monkey during retrograde cell degeneration. J Comp Neurol. 1971;142:223–229. doi: 10.1002/cne.901420207. [DOI] [PubMed] [Google Scholar]
- Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977;16:426–441. [PubMed] [Google Scholar]
- Minckler DS, McLean IW, Tso MO. Distribution of axonal and glial elements in the rhesus optic nerve head studied by electron microscopy. Am J Ophthalmol. 1976;82:179–187. doi: 10.1016/0002-9394(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Mittag TW, Danias J, Pohorenec G, Yuan HM, Burakgazi E, Chalmers-Redman R, Podos SM, Tatton WG. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2000;41:3451–3459. [PubMed] [Google Scholar]
- Mojumder DK, Wensel TG, Frishman LJ. Subcellular compartmentalization of two calcium binding proteins, calretinin and calbindin-28 kDa, in ganglion and amacrine cells of the rat retina. Mol Vis. 2008;14:1600–1613. [PMC free article] [PubMed] [Google Scholar]
- Moon JI, Kim IB, Gwon JS, Park MH, Kang TH, Lim EJ, Choi KR, Chun MH. Changes in retinal neuronal populations in the DBA/2J mouse. Cell Tissue Res. 2005;320:51–59. doi: 10.1007/s00441-004-1062-8. [DOI] [PubMed] [Google Scholar]
- Moore DL, Goldberg JL. Four steps to optic nerve regeneration. J Neuroophthalmol. 2010;30:347–360. doi: 10.1097/WNO.0b013e3181e755af. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye (Lond) 2000;14 ( Pt 3B):437–444. doi: 10.1038/eye.2000.128. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Circulation and axonal transport in the optic nerve. Eye (Lond) 2004;18:1089–1095. doi: 10.1038/sj.eye.6701574. [DOI] [PubMed] [Google Scholar]
- Morquette JB, Di Polo A. Dendritic and synaptic protection: is it enough to save the retinal ganglion cell body and axon? J Neuroophthalmol. 2008;28:144–154. doi: 10.1097/WNO.0b013e318177edf0. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Cepurna Ying Guo WO, Johnson EC. Pathophysiology of human glaucomatous optic nerve damage: insights from rodent models of glaucoma. Exp Eye Res. 2011;93:156–164. doi: 10.1016/j.exer.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Johnson EC, Cepurna W, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog Retin Eye Res. 2005;24:217–240. doi: 10.1016/j.preteyeres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Kitaoka Y, Kuribayashi J, Ueno S. Modulation of mitochondria in the axon and soma of retinal ganglion cells in a rat glaucoma model. J Neurochem. 2010;115:1508–1519. doi: 10.1111/j.1471-4159.2010.07057.x. [DOI] [PubMed] [Google Scholar]
- Nagaraju M, Saleh M, Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Invest Ophthalmol Vis Sci. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Alavi MV, Kim KY, Kang T, Scott RT, Noh YH, Lindsey JD, Wissinger B, Ellisman MH, Weinreb RN, Perkins GA, Ju WK. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis. 2011a;2:e240. doi: 10.1038/cddis.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JV, Soto I, Kim KY, Bushong EA, Oglesby E, Valiente-Soriano FJ, Yang Z, Davis CH, Bedont JL, Son JL, Wei JO, Buchman VL, Zack DJ, Vidal-Sanz M, Ellisman MH, Marsh-Armstrong N. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A. 2011b;108:1176–1181. doi: 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–287. [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–79. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- Niida T, MK, Ishikawa S, Iwai E. Transneuronal retrograde degeneration in adult monkey retina following ablation of the striate cortex. In: Iwai E, MM, editors. Vision, memory, and the temporal lobe. Elsevier; New York: 1990. pp. 369–375. [Google Scholar]
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- Nork TM, Ver Hoeve JN, Poulsen GL, Nickells RW, Davis MD, Weber AJ, Vaegan, Sarks SH, Lemley HL, Millecchia LL. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol. 2000;118:235–245. doi: 10.1001/archopht.118.2.235. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Kothmann WW, Diamond JS. Illuminating synapses and circuitry in the retina. Curr Opin Neurobiol. 2011;21:238–244. doi: 10.1016/j.conb.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN. Mitochondria: Their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res. 2010;90:750–757. doi: 10.1016/j.exer.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Kamalden TA, Majid AS, del Olmo-Aguado S, Manso AG, Ji D. Light effects on mitochondrial photosensitizers in relation to retinal degeneration. Neurochem Res. 2010;35:2027–2034. doi: 10.1007/s11064-010-0273-5. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Melena J, Chidlow G, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 2001;85:1252–1259. doi: 10.1136/bjo.85.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IH, Clark AF. Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma. 2007;16:483–505. doi: 10.1097/IJG.0b013e3181405d4f. [DOI] [PubMed] [Google Scholar]
- Pascale A, Drago F, Govoni S. Protecting the retinal neurons from glaucoma: Lowering ocular pressure is not enough. Pharmacol Res. 2012 doi: 10.1016/j.phrs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Pavlidis M, Stupp T, Naskar R, Cengiz C, Thanos S. Retinal ganglion cells resistant to advanced glaucoma: a postmortem study of human retinas with the carbocyanine dye DiI. Invest Ophthalmol Vis Sci. 2003;44:5196–5205. doi: 10.1167/iovs.03-0614. [DOI] [PubMed] [Google Scholar]
- Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:764–774. [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, Poulsen GL, Ver Hoeve JA, Nork TM, Nickells RW. Decrease of cone opsin mRNA in experimental ocular hypertension. Mol Vis. 2006;12:1272–1282. [PubMed] [Google Scholar]
- Perge JA, Koch K, Miller R, Sterling P, Balasubramanian V. How the optic nerve allocates space, energy capacity, and information. J Neurosci. 2009;29:7917–7928. doi: 10.1523/JNEUROSCI.5200-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perge JA, Niven JE, Mugnaini E, Balasubramanian V, Sterling P. Why do axons differ in caliber? J Neurosci. 2012;32:626–638. doi: 10.1523/JNEUROSCI.4254-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Nagaraju M. Head-up tilt lowers IOP and improves RGC dysfunction in glaucomatous DBA/2J mice. Exp Eye Res. 2010;90:452–460. doi: 10.1016/j.exer.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Selective citation of evidence regarding photoreceptor loss in glaucoma. Arch Ophthalmol. 2001;119:1390–1391. doi: 10.1001/archopht.119.9.1390. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci. 1980;19:137–152. [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Flower RW, Addicks EM, McLeod DS. The mechanism of optic nerve damage in experimental acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 1980;19:505–517. [PubMed] [Google Scholar]
- Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- Quigley HA, Sanchez RM, Dunkelberger GR, L’Hernault NL, Baginski TA. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28:913–920. [PubMed] [Google Scholar]
- Raz D, Perlman I, Percicot CL, Lambrou GN, Ofri R. Functional damage to inner and outer retinal cells in experimental glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3675–3684. doi: 10.1167/iovs.02-1236. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Lee NN, Povlishock JT. Preferential neuroprotective effect of tacrolimus (FK506) on unmyelinated axons following traumatic brain injury. Brain Res. 2007;1154:225–236. doi: 10.1016/j.brainres.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves TM, Smith TL, Williamson JC, Phillips LL. Unmyelinated axons show selective rostrocaudal pathology in the corpus callosum after traumatic brain injury. J Neuropathol Exp Neurol. 2012;71:198–210. doi: 10.1097/NEN.0b013e3182482590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul GL, Reynolds IJ. Mitochondrial trafficking and morphology in neuronal injury. Biochim Biophys Acta. 2010;1802:143–150. doi: 10.1016/j.bbadis.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Fromm M. Endothelin antagonism as an active principle for glaucoma therapy. Br J Pharmacol. 2011;162:806–816. doi: 10.1111/j.1476-5381.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Nagaraju M, Porciatti V. Longitudinal evaluation of retinal ganglion cell function and IOP in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2007;48:4564–4572. doi: 10.1167/iovs.07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Navarro M, Alarcon-Martinez L, Valiente-Soriano FJ, Jimenez-Lopez M, Mayor-Torroglosa S, Aviles-Trigueros M, Villegas-Perez MP, Vidal-Sanz M. Ocular hypertension impairs optic nerve axonal transport leading to progressive retinal ganglion cell degeneration. Exp Eye Res. 2010;90:168–183. doi: 10.1016/j.exer.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salinas-Navarro M, Alarcon-Martinez L, Valiente-Soriano FJ, Ortin-Martinez A, Jimenez-Lopez M, Aviles-Trigueros M, Villegas-Perez MP, de la Villa P, Vidal-Sanz M. Functional and morphological effects of laser-induced ocular hypertension in retinas of adult albino Swiss mice. Mol Vis. 2009;15:2578–2598. [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010;51:207–216. doi: 10.1167/iovs.09-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Sidorova T, Long DJ, Calkins DJ. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest Ophthalmol Vis Sci. 2009;50:717–728. doi: 10.1167/iovs.08-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor M, McLoon LK, Harrison AR, Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: an evidence-based review. Arch Ophthalmol. 2009;127:402–406. doi: 10.1001/archophthalmol.2009.9. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer MJ, Block SM. Kinesin hydrolyses one ATP per 8-nm step. Nature. 1997;388:386–390. doi: 10.1038/41111. [DOI] [PubMed] [Google Scholar]
- Scholz M, Buder T, Seeber S, Adamek E, Becker CM, Lutjen-Drecoll E. Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J-Rj mice. Invest Ophthalmol Vis Sci. 2008;49:613–621. doi: 10.1167/iovs.07-0745. [DOI] [PubMed] [Google Scholar]
- Schuettauf F, Rejdak R, Walski M, Frontczak-Baniewicz M, Voelker M, Blatsios G, Shinoda K, Zagorski Z, Zrenner E, Grieb P. Retinal neurodegeneration in the DBA/2J mouse-a model for ocular hypertension. Acta Neuropathol. 2004;107:352–358. doi: 10.1007/s00401-003-0816-9. [DOI] [PubMed] [Google Scholar]
- Sehi M, Grewal DS, Goodkin ML, Greenfield DS. Reversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressure. Ophthalmology. 2010;117:2329–2336. doi: 10.1016/j.ophtha.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Sheldon WG, Warbritton AR, Bucci TJ, Turturro A. Glaucoma in food-restricted and ad libitum-fed DBA/2NNia mice. Lab Anim Sci. 1995;45:508–518. [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008;19:85–88. doi: 10.1097/ICU.0b013e3282f3919b. [DOI] [PubMed] [Google Scholar]
- Shou T, Liu J, Wang W, Zhou Y, Zhao K. Differential dendritic shrinkage of alpha and beta retinal ganglion cells in cats with chronic glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3005–3010. doi: 10.1167/iovs.02-0620. [DOI] [PubMed] [Google Scholar]
- Son JL, Soto I, Oglesby E, Lopez-Roca T, Pease ME, Quigley HA, Marsh-Armstrong N. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010;58:780–789. doi: 10.1002/glia.20962. [DOI] [PubMed] [Google Scholar]
- Soto I, Oglesby E, Buckingham BP, Son JL, Roberson ED, Steele MR, Inman DM, Vetter ML, Horner PJ, Marsh-Armstrong N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28:548–561. doi: 10.1523/JNEUROSCI.3714-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Pease ME, Son JL, Shi X, Quigley HA, Marsh-Armstrong N. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest Ophthalmol Vis Sci. 2011;52:434–441. doi: 10.1167/iovs.10-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stokely ME, Brady ST, Yorio T. Effects of endothelin-1 on components of anterograde axonal transport in optic nerve. Invest Ophthalmol Vis Sci. 2002;43:3223–3230. [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Edamatsu M, Toyoshima YY. Multiple ATP-hydrolyzing sites that potentially function in cytoplasmic dynein. Proc Natl Acad Sci U S A. 2004;101:12865–12869. doi: 10.1073/pnas.0403429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Rolls MM. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J Neurosci. 2011;31:5398–5405. doi: 10.1523/JNEUROSCI.3826-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res. 2008;173:409–421. doi: 10.1016/S0079-6123(08)01128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaegan, Graham SL, Goldberg I, Buckland L, Hollows FC. Flash and pattern electroretinogram changes with optic atrophy and glaucoma. Exp Eye Res. 1995;60:697–706. doi: 10.1016/s0014-4835(05)80011-9. [DOI] [PubMed] [Google Scholar]
- Van Buren JM. The abdominal aura. A study of abdominal sensations occurring in epilepsy and produced by depth stimulation. Electroencephalogr Clin Neurophysiol. 1963;15:1–19. doi: 10.1016/0013-4694(63)90035-x. [DOI] [PubMed] [Google Scholar]
- Velten IM, Korth M, Horn FK. The a-wave of the dark adapted electroretinogram in glaucomas: are photoreceptors affected? Br J Ophthalmol. 2001;85:397–402. doi: 10.1136/bjo.85.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman ST, Flanagan JG, Hudson C. Vascular reactivity of optic nerve head and retinal blood vessels in glaucoma--a review. Microcirculation. 2010;17:568–581. doi: 10.1111/j.1549-8719.2010.00045.x. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M, Lafuente MP, Mayor S, de Imperial JM, Villegas-Perez MP. Retinal ganglion cell death induced by retinal ischemia. neuroprotective effects of two alpha-2 agonists. Surv Ophthalmol. 2001;45(Suppl 3):S261–267. doi: 10.1016/s0039-6257(01)00205-3. discussion S273-266. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M, Salinas-Navarro M, Nadal-Nicolas FM, Alarcon-Martinez L, Valiente-Soriano FJ, de Imperial JM, Aviles-Trigueros M, Agudo-Barriuso M, Villegas-Perez MP. Understanding glaucomatous damage: anatomical and functional data from ocular hypertensive rodent retinas. Prog Retin Eye Res. 2012;31:1–27. doi: 10.1016/j.preteyeres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye (Lond) 2007;21(Suppl 1):S11–14. doi: 10.1038/sj.eye.6702880. [DOI] [PubMed] [Google Scholar]
- Wagner OI, Lifshitz J, Janmey PA, Linden M, McIntosh TK, Leterrier JF. Mechanisms of mitochondria-neurofilament interactions. J Neurosci. 2003;23:9046–9058. doi: 10.1523/JNEUROSCI.23-27-09046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- Wang L, Dong J, Cull G, Fortune B, Cioffi GA. Varicosities of intraretinal ganglion cell axons in human and nonhuman primates. Invest Ophthalmol Vis Sci. 2003;44:2–9. doi: 10.1167/iovs.02-0333. [DOI] [PubMed] [Google Scholar]
- Wang SS, Shultz JR, Burish MJ, Harrison KH, Hof PR, Towns LC, Wagers MW, Wyatt KD. Functional trade-offs in white matter axonal scaling. J Neurosci. 2008a;28:4047–4056. doi: 10.1523/JNEUROSCI.5559-05.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Baldridge WH, Chauhan BC. Acute endothelin-1 application induces reversible fast axonal transport blockade in adult rat optic nerve. Invest Ophthalmol Vis Sci. 2008b;49:961–967. doi: 10.1167/iovs.07-1243. [DOI] [PubMed] [Google Scholar]
- Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Yang J, Peng G, Patil RV, Agarwal N, Sappington RM, Calkins DJ. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 2008;28:12085–12096. doi: 10.1523/JNEUROSCI.3200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. Prerequisites for conduction in demyelinated fibers. Neurology. 1978;28:27–33. doi: 10.1212/wnl.28.9_part_2.27. [DOI] [PubMed] [Google Scholar]
- Weber AJ, Chen H, Hubbard WC, Kaufman PL. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Invest Ophthalmol Vis Sci. 2000;41:1370–1379. [PubMed] [Google Scholar]
- Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46:3197–3207. doi: 10.1167/iovs.04-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Wygnanski T, Desatnik H, Quigley HA, Glovinsky Y. Comparison of ganglion cell loss and cone loss in experimental glaucoma. Am J Ophthalmol. 1995;120:184–189. doi: 10.1016/s0002-9394(14)72606-6. [DOI] [PubMed] [Google Scholar]
- Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J Physiol. 2012;590:2285–2304. doi: 10.1113/jphysiol.2012.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Wai Man CY, Chinnery PF, Griffiths PG. Optic neuropathies--importance of spatial distribution of mitochondria as well as function. Med Hypotheses. 2005;65:1038–1042. doi: 10.1016/j.mehy.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Yucel Y, Gupta N. Glaucoma of the brain: a disease model for the study of transsynaptic neural degeneration. Prog Brain Res. 2008;173:465–478. doi: 10.1016/S0079-6123(08)01132-1. [DOI] [PubMed] [Google Scholar]
- Yucel YH, Gupta N, Zhang Q, Mizisin AP, Kalichman MW, Weinreb RN. Memantine protects neurons from shrinkage in the lateral geniculate nucleus in experimental glaucoma. Arch Ophthalmol. 2006;124:217–225. doi: 10.1001/archopht.124.2.217. [DOI] [PubMed] [Google Scholar]
- Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang H, Lu Q, Qing G, Wang N, Wang Y, Li S, Yang D, Yan F. Detection of early neuron degeneration and accompanying glial responses in the visual pathway in a rat model of acute intraocular hypertension. Brain Res. 2009;1303:131–143. doi: 10.1016/j.brainres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Zhong L, Bradley J, Schubert W, Ahmed E, Adamis AP, Shima DT, Robinson GS, Ng YS. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Schmidt JF, Gidday JM. Glaucoma-Induced Degeneration of Retinal Ganglion Cells Prevented by Hypoxic Preconditioning: A Model of “Glaucoma Tolerance”. Mol Med. 2012 Feb 29; doi: 10.2119/molmed.2012.00050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]