Summary
Proteins of unknown function comprise a significant fraction of sequenced genomes. Defining the roles of these proteins is vital to understanding cellular processes. Here, we describe a method to determine a protein function based on the identification of its natural ligand(s) by the crystallographic screening of the binding of a metabolite library, followed by a focused search in the metabolic space. The method was applied to two protein families with unknown function, PF01256 and YjeF_N. The PF01256 proteins, represented by YxkO from Bacillus subtilis and the C-terminal domain of Tm0922 from Thermotoga maritima, were shown to catalyze ADP/ATP-dependent NAD(P)H-hydrate dehydratation, a previously described orphan activity. The YjeF_N proteins, represented by mouse apolipoprotein A-I binding protein and the N-terminal domain of Tm0922, were found to interact with an adenosine diphosphoribose-related substrate and likely serve as ADP-ribosyltransferases. Crystallographic screening of metabolites serves as an efficient tool in functional analyses of uncharacterized proteins.
Introduction
The molecular function of an uncharacterized protein is commonly assigned on the basis of its sequence or structural similarity to proteins of characterized function. Several experimental and computational methods have been proposed to assign function in the absence of functionally characterized homologs, or to refine a general function often inferred by a similarity assignment. However, the functions of proteins encoded by an estimated 30-40% of bacterial genes, and even higher fractions of the genomes of archaea and eukaryotes, remain uncharacterized and await novel functional assignment methods (Galperin and Koonin, 2004).
The identification of a natural small molecule ligand (substrate, effector, etc.) is one starting point for the determination of a protein's molecular function. However, comprehensive screening of all compounds in a metabolome, estimated at 5,000 – 25,000 per individual species (Nicholson et al., 2007), is a formidable task. In order to identify natural ligands, we employed a two-phase approach that took advantage of structural relationships among metabolites. In phase I, the binding of compounds in a diverse metabolic library to a target protein was studied to identify metabolites that were structurally similar to a natural ligand and possessed its protein-binding determinants. X-ray diffraction analysis of protein crystals soaked with multi-compound cocktails was used due to its ability to detect low affinity binding of ligand analogs. In phase II, the binding of metabolites structurally related to the hits from phase I was evaluated to identify those that bound to the protein with high affinity. Isothermal calorimetry (ITC) and crystallographic analysis were used as detection methods because both high and low affinity interactions were expected.
This functional assignment strategy was applied to proteins from two families of unknown function, YjeF_N and PF01256. In most prokaryotes, the domains representing these two families exist as a fusion protein with the YjeF_N-PF01256 architecture. Fused domains are commonly related in function and often interact with structurally-related ligands (Suhre, 2007). In order to optimize the odds of detecting a ligand, three proteins were included in the study: two single domain proteins (one from each family) and a fusion protein (Fig. 1A). The single domain proteins are mouse apolipoprotein A-I binding protein (AI-BP) and YxkO from Bacillus subtilis, representing the YjeF_N and PF01256 families, respectively. The YjeF_N-PF01256 fusion protein included in the study is Tm0922 from Thermotoga maritima.
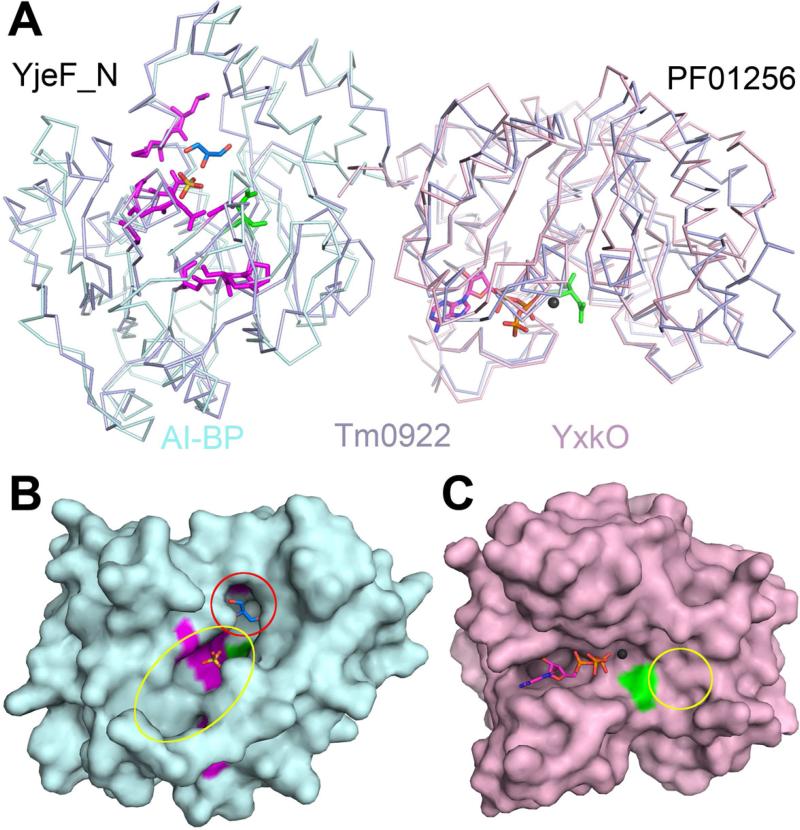
Figure 1.
Subunit structures and putative active sites of the proteins from the YjeF_N and PF01256 families. (A) The superposition of Tm0922, a YjeF_N-PF01256 fusion (blue) with AI-BP, a single YjeF_N domain (cyan) and YxkO, a single PF01256 domain (pink). In the YjeF_N domain, the invariant D188AI-BP is green and highly conserved residues (Jha et al., 2008) are purple. The sulfate bound in the AI-BP structure is yellow/red and glycerol bound in the Tm0922 structure is navy/red. In the PF01256 domain, the invariant D216YxkO is green and the modeled ATP and Mg2+ (Zhang et al., 2002) are shown. (B) Surface of the AI-BP subunit colored as in Fig. 1A. The pocket compartment of the putative YjeF_N active site is marked by the red circle and its trench compartment is marked by the yellow oval. (C) Surface of the YxkO subunit colored as in Fig. 1A. The predicted kinase substrate binding site is marked by the yellow circle. The side chains of F162AI-BP and H149YxkO that block active sites in the apo-structures are removed.
YjeF_N family
The family of YjeF_N proteins represented a particularly challenging case for functional assignment, and was included in a top 10 list of highly attractive targets for functional characterization (Galperin and Koonin, 2004). Currently, there are 2411 sequences of the YjeF_N family members from 2009 bacterial, archaeal and eukaryotic species in Pfam (Finn et al., 2010). A single YjeF_N protein is typically present in bacteria and archaea, but eukaryotes possess up to three paralogs from the family. The human genome encodes for three YjeF_N paralogs: the single domain proteins AI-BP and YjeF_N3, as well as Edc3, which consists of an N-terminal Lsm domain, a central FDF domain, and a C-terminal YjeF_N domain (Ling et al., 2008; Rudolph et al., 2007).
Despite their widespread distribution, no biochemical function has been assigned to any member of the YjeF_N family, and no information was available regarding their ligand binding properties. They were suggested to be involved in a conserved enzymatic process (Anantharaman and Aravind, 2004; Galperin and Koonin, 2004; Jha et al., 2008).
Edc3 is the only member of the YjeF_N family involved in a defined cellular process, and serves as an activator of mRNA decapping. However, its mechanistic role in decapping has not been established. Edc3 binds to the multiprotein decapping complex via its Lsm and FDF domains (Decker et al., 2007; Harigaya et al., 2010; Tritschler et al., 2009) while the YjeF_N domain was posited to possess an unknown enzymatic activity potentially including dephosphorylation, demethylation, phosphoester or glycosyl bond hydrolysis (Anantharaman and Aravind, 2004). The only demonstrated binding interaction for a YjeF_N protein is the binding of AI-BP to apolipoprotein A-I, but its significance is not understood (Ritter et al., 2002).
The YjeF_N proteins are apparently not essential for cell survival. Saccharomyces cerevisiae strains with a deletion of either YNL200C or Edc3 of the YjeF_N family continued to be viable (Cherry et al., 2012). The single YjeF_N gene in E. coli, which encodes a PF01256-YjeF_N fusion protein, was also characterized as non-essential (Gerdes et al., 2003; Kato and Hashimoto, 2007).
Analysis of the genomic context of Yje_N domains revealed that they commonly fuse with other domains. Single domain YjeF_N proteins represent only 14% of the sequences available in Pfam. The dominant fusion partner of the YjeF_N domain in prokaryotes, constituting 80% of the Pfam sequences, is a PF01256 domain, annotated as a carbohydrate kinase (see below). Eukaryotes contain Edc3 orthologs with a (Lsm)-FDF-YjeF_N architecture (4% of Pfam sequences) which are likely to be involved in mRNA processing. In some plants, Yje_N is fused to a putative pyridoxamine 5′-phosphate oxidase (1% of Pfram sequences). In bacteria and archaea, YjeF_N genes belong to operons that encode enzymes of diverse functions: pyridoxal phosphate biosynthetic protein PdxJ; phosphopantetheine-protein transferase; ATP/GTP hydrolase; and pyruvate-formate lyase 1-activating enzyme. Thus, in genomes YjeF_N domains are commonly associated with a diverse set of enzymes and are likely to serve as enzymes; however, attempts to assign a metabolic context to this putative activity were unproductive.
Crystal structures available for several members of the YjeF_N family provided another hint that YjeF_N proteins are enzymes and suggested the location of a putative active site. The YjeF_N domains form a modified Rossmann fold (Chance et al., 2002; Jha et al., 2008; Ling et al., 2008) which is common for enzymes involved in metabolic functions (Wolf et al., 1999). Two invariant (G86AI-BP/G48Tm0922 and D188AI-BP/D147Tm0922) and eleven highly conserved residues in the YjeF_N family are clustered within or near the cavity on the protein surface (Fig.1A, B) (Jha et al., 2008). This cavity, observed in every available YjeF_N structure, is positioned at the typical location of the active site in Rossmann-fold enzymes. The cavity is comprised of two compartments, a trench that runs along the protein surface and a perpendicular pocket (Chance et al., 2002; Jha et al., 2008). In AI-BP, the trench is about 10Å long with a cross-section of ~4Å × 4Å while the pocket is ~10Å deep with a ~3Å × 7Å opening. In the available apo-structures, the trench compartment binds anions present in crystallization media (sulfate in PDB:2O8N (Fig. 1A, B), phosphate in PDB:3K5W, or chloride in PDB:1JZT) while the pocket contained a glycerol molecule used as cryoprotectant in PDB:2AX3. The two-compartmental cavity is likely to serve as an active site in YjeF_N enzymes. However, attempts to detect residue patterns associated with a known activity did not produce any definitive results.
PF01256
The PF01256 family contains 2945 sequences from 2445 species representing every kingdom of life. A single PF01256 protein is present in most organisms. Gene deletion of PF01256 does not affect cell viability in either E. coli (Gerdes et al., 2003; Kato and Hashimoto, 2007) or S. cerevisiae (Cherry et al., 2012). 65% of PF01256 domains described in Pfam exist as YjeF_N-PF01256 fusions, 33% are present as single domain PF01256 proteins, and 0.4% form fusions with a 4'-phosphopantetheinyl transferase domain.
The PF01256 proteins were annotated with the general function of carbohydrate kinase, due to their sequence and structural similarity to small molecule kinases from the ribokinase clan (Zhang et al., 2002). Enzymes from this clan catalyze the transfer of a phosphate group from ATP or ADP to a hydroxyl group of various small molecule substrates. Based on high structural similarity among members of the ribokinase clan, ATP/Mg2+ binding in PF01256 has been modeled and a catalytic role was proposed for the invariant aspartate residue (D216YxkO/D431Tm0922) (Fig. 1A, C) (Zhang et al., 2002). However, the second substrate, and therefore the function of PF01256 proteins, remained unknown.
Results and discussion
Phase I: crystallographic screening of metabolite cocktails
Proteins have evolved to efficiently interact with their partner compounds in the cell, and to ignore unrelated metabolites present at physiological concentrations. This natural selection has not been driven by compounds foreign to the cell, such as drugs which may exhibit strong interactions with a protein. We suggest that 1) the high selectivity of a protein towards its natural ligand may not preclude low affinity binding of ligand analogs that bind in the same mode, and 2) a search for ligand analogs in the metabolome decreases the probability of non-specific binding in comparison to using non-physiological compounds.
The crystals of the apo-forms of AI-BP, Tm0922, and YxkO were soaked with 11 cocktails comprising 87 commercially available natural metabolites, each present at 10 mM concentration (Table 1), and the structures of these crystals were determined. Each cocktail included 5-11 metabolites. Structurally similar compounds were usually combined in the same cocktail in order to increase the efficient concentration of their common fragments and facilitate the detection of binding. The metabolite library was mostly composed of cofactors and compounds that contain common metabolic building blocks. Some groups of common metabolites such as amino acids and lipids were omitted from the initial library in order to be tested in the next round of screening if the first metabolite selection did not produce results.
Table 1.
Composition of metabolite cocktails and binding in the putative active sites of the AI-BP, Tm0922, and YxkO crystals.
| Cocktail | Cocktail composition | YjeF_N active sites | PF01256 active sites | ||
|---|---|---|---|---|---|
| AI-BP | N-Tm0922 | C-Tm0922 | YxkO | ||
| apo | - | sulfate1 | glycerol2 | - | - |
| A | adenine, adenosine, AMP, ADP, ATP, cAMP, dAMP, sucrose, L-arabinose | sulfate1 | glycerol2 | ADP/ATP | ADP/ATP |
| B | guanine, guanosine, 2’-deoxyguanosine, GDP, GTP, dGMP, dGDP, dGTP, D-sorbitol, D-fructose | sulfate1 | glycerol2 | - | - |
| C | cytosine, cytidine, CMP, CDP, dCMP, DL-carnitine, D-trehalose, xylitol | sulfate1 | glycerol2 | - | - |
| D | uracil, uridine, UMP, UDP, UTP, dUMP, UDP-glucose, D-mannitol, myo-inositol | sulfate1 | glycerol2 | - | - |
| E | thymine, thymidine, TMP, TDP, TTP, cTMP, TDP-glucose, 6-phosphogluconic acid | thymine/thymidine, sulfate1 | glycerol2 | - | - |
| F | pyridoxal phosphate, pyridoxal, pyridoxamine, phosphocholine, phosphoethanolamine, phosphoserine, phosphoglycerol | no diffraction | glycerol2 | - | - |
| G | ascorbic acid, NAD, NADP, FAD, betaine, 2’-dIMP, deoxycholic acid, CoQ10, ergocalciferol, α-tocopherol | no diffraction | NAD/NADP | NAD | NAD |
| H | SAM, SAH, CoA, NAAD, nicotinic acid, biotin, folic acid, thiamine, D-pantothenic acid, p-aminobenzoic acid, p-hydroxybenzoic acid | no diffraction | p-aminobenzoic acid, p-hydroxybenzoic acid | - | NAAD |
| I | FMN, riboflavin, uric acid, xanthine, pterin | no diffraction | glycerol2 | - | - |
| J | D-glucose, D-xylose, L-rhamnose, N-acetylgalactosamine, N-acetyl muramic acid | sulfate1 | glycerol2 | - | - |
| K | D-galactose, D-mannose, L-fucose, N-acetylglucosamine, N-acetyl neuraminic acid | sulfate1 | glycerol2 | - | - |
Sulfate, a component of crystallization media for AI-BP
Glycerol, a cryoprotectant for Tm0922 crystals, was bound in the pocket compartment of the YjeF_N site.
Indications of a ligand binding to a crystal include the presence of additional electron density in the structure or abolishment of diffraction, though non-specific disruption of crystal packing cannot be ruled out in the latter case. Determination of the structures of cocktail-soaked crystals revealed new electron density peaks in all four putative active sites.
YjeF_N sites
The putative YjeF_N active sites present in AI-BP and the N-terminal domain of Tm0922 (N-Tm0922) are composed of a trench and a pocket compartments as described above. In structures of apo-protein, the trench of the AI-BP active site is occupied by a sulfate present in the crystallization media while the pocket of N-Tm0922 contains a bound glycerol (which serves as a cryoprotectant).
Upon the exposure to metabolite cocktails, all Tm0922 crystals retained their diffraction power and two acquired new electron density peaks in their YjeF_N sites. The electron density acquired from crystals soaked in cocktail G (Table 1) was readily assigned to NADP which bound along the trench compartment (Fig. 2A-B, 3). This NADP binding site is likely to be partially occupied by NAD, as indicated by the higher B-factors of the 2’ phosphate group of the modeled NADP. The exposure of Tm0922 crystals to cocktail H gave rise to new electron density in the pocket compartment of the YjeF_N site. The compounds responsible for this electron density were not immediately recognized but subsequent soaks with individual components demonstrated binding of both p-aminobenzoate and p-hydroxybenzoate. All other cocktail-exposed crystals of Tm0922 contained a glycerol molecule bound in the YjeF_N site as in the structure of the apo-protein.
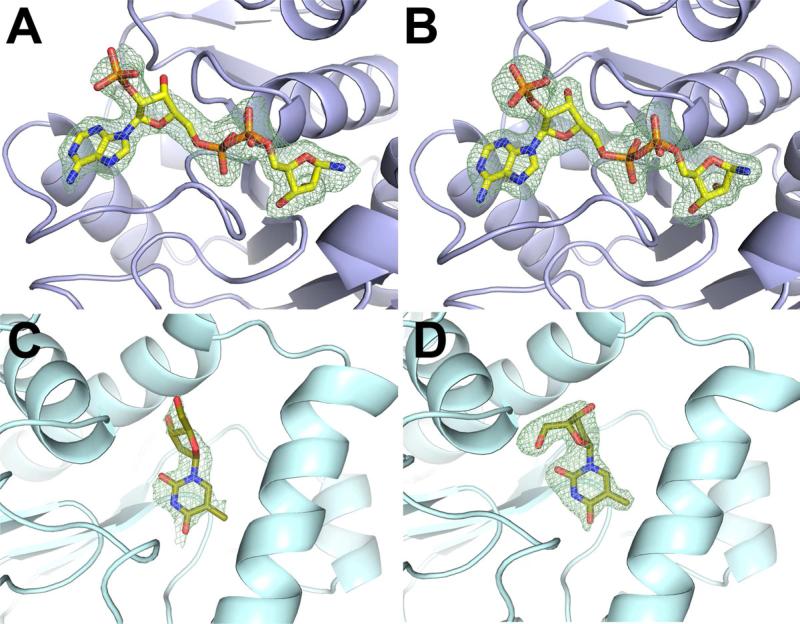
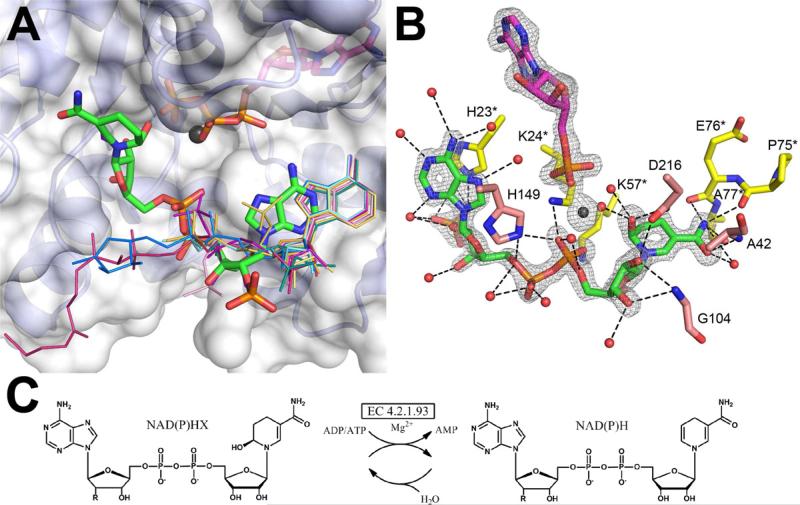
Figure 2.
Representative FO-FC omit electron density maps of the bound ligands in metabolite cocktail soaks and soaks with single ligands. (A) NADP is modeled in the NAD/NADP density in the Tm0922 crystal soaked with the cocktail G (resolution 2.05 Å, σ = 2.5). The disordered or hydrolyzed nicotinamide ring is not shown. (B) NADP soaked into the Tm0922 crystal (resolution 1.95 Å, σ = 2.5). (C). Thymidine is modeled in the thymine/thymidine density in the AI-BP crystal soaked with the cocktail E (resolution 2.5 Å, σ = 2.0). (D) Thymidine soaked into the AI-BP crystal (resolution 2.11 Å, σ = 2.5).
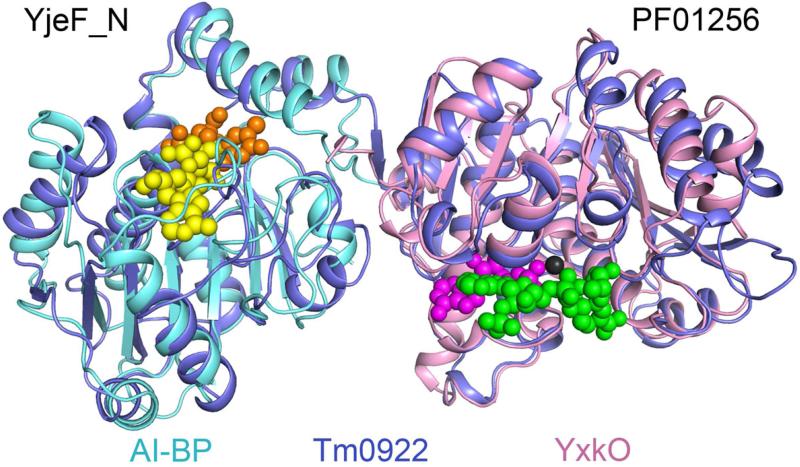
Figure 3.
The binding sites of metabolitic ligands in the YjeF_N and PF01256 active sites. In the YjeF_N domain, ADP-ribose (yellow) marks the binding site for the “NAD set” of ligands while thymidine (orange) is bound at the “thymine set” site. In the PF01256 domain, bound AMP, Mg2+, and NADPHX are shown in magenta, black, and green, respectively.
Residual electron density was observed in the pocket compartment of the putative active site in the AI-BP crystal exposed to cocktail E (Table 1, Fig. 2C, 3). This crystal diffracted to a relatively low resolution of 2.7 Å precluding reliable identification of the bound ligand(s). However, subsequent soaking with individual metabolites from cocktail E identified bound compounds as thymine and thymidine (Fig. 2D). AI-BP crystals soaked with cocktails retained a sulfate bound in the trench compartment of the YjeF_N site due to the presence of 1.5 M sulfate in the crystallization media.
In contrast to the Tm0922 crystals, the diffracting power of cocktail-exposed AI-BP crystals was diminished. Most of them diffracted to a resolution better than 3.0 Å, which did not preclude the detection of bound ligands. However, diffraction of the crystals soaked with cocktails F, G, H, and I was completely abolished. Soaks with individual components showed that the compounds responsible for the loss of diffraction were pyridoxal phosphate in cocktail F and both flavin mononucleotide (FMN) and riboflavin in cocktail I. It is likely that FMN and riboflavin affected the crystal packing of AI-BP, because the properties of the crystals soaked with either compound were clearly altered. In addition to acquiring a yellow color, the AI-BP crystals soaked with FMN became gel-like while crystals exposed to riboflavin acquired resistance to breaking uncharacteristic of the crystals of the apo-protein. Disruptions of the crystal packing of AI-BP may be related to binding of both FMN and riboflavin in the pocket compartment of the AI-BP active site. These compounds differ from each other only by the presence of a phosphate group and both contain a substructure similar to thymine (Fig. S2). Thus FMN and riboflavin may interact with AI-BP in a similar manner. The AI-BP crystals soaked with individual components within cocktails G and H showed neither abolished diffraction nor ligand binding. In this case, abolishment of AI-BP crystal diffraction was likely due to a change in solution composition rather than the influence of any component.
PF01256 sites
The diffracting power of YxkO and Tm0922 crystals was not significantly altered by soaking with metabolite cocktails. The putative PF01256 active sites present in YxkO and the C-terminal domain of Tm0922 (C-Tm0922) bound ATP and ADP in the predicted ATP-binding site of the crystals exposed to cocktail A. In addition, both YxkO and Tm0922 crystals soaked with cocktail G contained NAD in the predicted ATP-binding site. In YxkO crystals soaked in cocktail H, nicotinic acid adenine dinucleotide (NAAD) is bound in the same position. The nearly identical coordination of NAD and NAAD in the ATP-binding sites mimicked the mode of ATP/ADP binding and, in addition, involved the interaction of the nicotinamide/nicotinate ribose with Mg2+. In YxkO, the binding of these compounds caused the ordering of a binding site loop that was disordered in the apo-structure. No indication of binding to the kinase substrate site was obtained in cocktail soaks.
Phase II: elucidation of YjeF_N ligands and discovery of the PF01256 kinase substrate site
A protein is expected to bind its natural ligand(s) with significantly higher affinity than other compounds present in the cell. Following identification of the initial binding hits, studies of structurally related metabolites were undertaken with the assumption that compounds similar to the natural ligand bind with higher affinity while dissimilar compounds bind with lower affinity.
Two sets of metabolites were designed to elucidate a natural ligand(s) for the YjeF_N sites based on the binding hits discovered by the cocktail screening in N-Tm0922 and AI-BP. The “NAD set” contained metabolites structurally related to NAD and NADP that had been identified in the trench compartment of the N-Tm0922 site while the “thymine set” consisted of the compounds related to thymine and thymidine that had been noted in the pocket compartment of AI-BP. The “NAD set” included NAD, NADP, adenine, adenosine, AMP, cAMP, ADP, ADP-ribose (ADPR), NADH, NAAD, NADPH, NAADP, ADP-glucose (ADPG), ATP, P1,P3-di(adenosine-5′) triphosphate (AP3A), P1,P4-di(adenosine-5′) tetraphosphate (AP4A), P1,P5-di(adenosine-5′) pentaphosphate (AP5A), P1,P6-di(adenosine-5′) hexaphosphate (AP6A), FAD, CoA, acetyl CoA, adenosine 3′,5′-diphosphate, SAM, SAH, and β-nicotinamide mononucleotide (Fig. S1). The “thymine set” included thymine, thymidine, TMP, cTMP, thymidine 3′-monophosphate (T3’MP), TDP, TDP-glucose, TTP, uracil, uridine, UMP, dUMP, UDP, UDP-glucose, UTP, cytosine, cytidine, CMP, dCMP, CDP, riboflavin, FMN, lumazine, uric acid, xanthine, guanine, and pterin (Fig. S2).
Binding of metabolites from both sets to Tm0922 and AI-BP was characterized both by crystallographic and ITC analysis. We did not intend to characterize interactions of the listed metabolites with a PF01256 domain because the ligands observed in the PF01256 domains (the C-terminal domain of Tm0922 and YxkO) by cocktail screening occupied only the predicted ATP-binding site, and did not provide any leads in a search for the unknown second substrate. However, the presence of both domains in Tm0922 provided these data, leading to the discovery of a PF01256 function.
Binding of NAD-related metabolites
N-terminal (YjeF_N) domain of Tm0922 – crystallography
In Tm0922 crystals soaked with individual metabolites from the “NAD set”, fourteen of fifteen compounds containing an ADP moiety were found in the trench of the YjeF_N site (Fig. 3, 4, Table S1). The remaining ADP-containing ligand, AP6A, may have also bound to Tm0922 but the crystals soaked with AP6A did not diffract. The position of the ADP fragment and its coordination by the residues of the YjeF_N site was nearly identical for each of the bound compounds (Fig. 4). The binding was accompanied by a conformational change in the active site loop that created a specific binding site for the ADP fragment. This conformational change included flips of the T119-G120 and R122-G123 peptide planes that enabled coordination of the α-phosphate and adenine groups of the ligands, respectively. The coordination of NADP/NADPH/NAADP or CoA/acetyl CoA also involved the interaction between K78 and their 2’ or 3’ phosphate groups, respectively. The non-ADP tails of the bound ligands directed toward the pocket compartment of the YjeF_N site were partially disordered in most complexes (Fig. 4A). However, when the ligand contained a ribose attached to the β-phosphate of the ADP fragment (as in ADPR, NAD, NADH, NAAD, NADP, NADPH and NAADP), this ribose was well defined in the electron density and coordinated by several active site residues including the invariant D147 (Fig. 4B). In contrast, the nicotinamide rings of the NAD(P) analogs were not visible in the electron density.
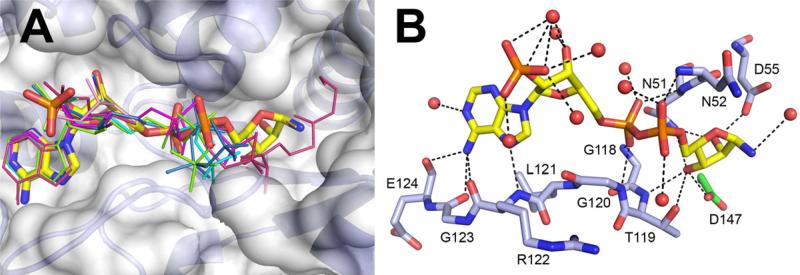
Figure 4.
Binding of the “NAD set” ligands in the YjeF_N site of Tm0922. (A) All ligands that contain an ADP moiety are bound in a similar mode along the trench compartment. NADP missing the nicotinamide ring is in a thick stick representation colored by the atom type. The other ligands (Table S1) are drawn as thin sticks and colored by the compound. Only the fragments visible in the electron density are shown. (B) The coordinating polar interactions of NADP. The invariant D147Tm0922 is green.
AI-BP – crystallography
The trench compartment of the YjeF_N site in AI-BP crystals bound NADP (Fig. 3, Table S2). ADPR, NADH and NADPH might also have bound but the diffraction of AI-BP crystals soaked with these compounds deteriorated below 5 Å, impeding the reliable modeling of ligands. The coordination of NADP in the YjeF_N site of AI-BP was similar to that in the N-Tm0922 site. Flips in two peptide planes enabled binding of the ADP fragment. As in the Tm0922 complex, the nicotinamide ribose of NADP was well-defined in the electron density due to extensive coordination, but the nicotinamide ring was not visible. The apparent higher selectivity of the YjeF_N site in AI-BP as compared to N-Tm0922 is likely to be related to the presence of sulfate in the AI-BP but not Tm0922 crystallization media. This sulfate replaced ligands with lower affinities in the trench compartment of AI-BP.
C-terminal (PF01256) domain of Tm0922 – crystallography
Interactions of test ligands with the PF01256 site of Tm0922 complexes were also assessed. The ATP binding site accommodated ligands that mimicked the binding of ATP/Mg2+ as was observed in the cocktail screening. In addition, several “NAD set” metabolites bound in the vicinity of the ATP-binding site at the interface of two C-Tm0922 domains. This second site was designated a kinase substrate binding site (Fig. 3, 5).
Figure 5.
Binding of the “NAD set” metabolites in the PF01256 active sites and the reaction catalyzed by PF01256 proteins. (A) Ligands in the NAD(P)H soaks of Tm0922 bind in its kinase substrate site differently from the other ligands. NADPHX is in a thick stick representation colored by the atom type. The other ligands (Table S1) are drawn as thin sticks and colored by the compound. Ligands bound at the ATP binding site in the upper half are not shown except for ATP and Mg2+ from the ATP soak. Only the fragments visible in the electron density are shown. The second subunit of Tm0922 contributing to the PF01256 active site is not shown for clarity. (B) Coordination of NADPHX in the active site of YxkO, co-crystallized with ATP and Mg2+ and soaked with NADPH. The FO-FC omit electron density map calculated to 1.5 Å resolution and contoured at 4.0σ demonstrates the modification of the nicotinamide ring of NADPH and hydrolysis of ATP to AMP. Residues from two YxkO subunits forming the active site are colored in pink and yellow. (C) The reaction catalyzed by the orphan enzyme ATP-dependent NAD(P)H-hydrate dehydratase (EC 4.2.1.93) and spontaneous hydratation of NAD(P)H. R is a hydroxyl group in NADH/NADHX and a phosphate group in NADPH/NADPHX.
In contrast to the robust binding of “NAD set” ligands to the YjeF_N site, their ability to bind in the adjoining ATP- and kinase substrate binding sites of PF01256 varied. ATP and AP3A occupied only the ATP-binding site. NADH, NADPH, CoA and acetyl CoA were coordinated only at the kinase substrate site. AMP, ADP, ADPR, ADPG, NAD, and NAAD bound to both sites while NADP, NAADP, AP4A, and AP5A bound to neither site. When the compounds that were able to occupy the kinase substrate site were soaked into Tm0922 crystals simultaneously with ATP/Mg2+, they bound at the same position while ATP/Mg2+ occupied its own binding site (Fig. 5A). The coordination of the ligands at the substrate site involved multiple polar interactions with residues from two Tm0922 subunits. The non-ADP moieties of most compounds were directed away from the ATP location and partially disordered (Fig. 5A). The only exceptions were the NADH and NADPH soaks, where the nicotinamide rings of both ligands approached the position of the γ-phosphate of ATP, while their ADP moieties were shifted by about 4 Å compared to the other bound metabolites. This shift was caused by multiple interactions between the active site residues and both the nicotinamide ring and adjoining ribose in the NAD(P)H soaks (Fig. 5B).
CoA was the only ligand that bound to the PF01256 domain outside its active site. Two molecules of CoA with disordered pantothenate tails were coordinated on opposite sides of the active site. These molecules formed fewer interactions with the Tm0922 residues than CoA bound at the kinase substrate site. The significance of their interaction with Tm0922 is not understood.
Tm0922 and AI-BP – ITC
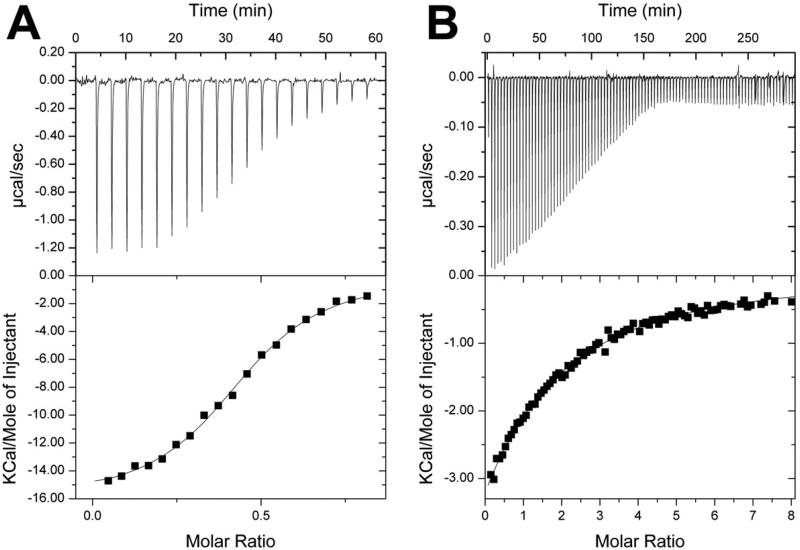
ITC detected the interaction of Tm0922 and AI-BP with six closely related compounds from the “NAD set” (Table 2, Fig. 6). Tm0922 demonstrated significantly stronger binding than AI-BP to these compounds but the relative order of affinities was similar for both proteins: ADP-ribose > NADP ~ NADPH >> NAADP >> NAD ~ NADH. The compounds with highest affinity, ADPR, NADP, and NADPH, bound to both proteins with a 1:2 stoichiometry - only one molecule of the ligand bound per two protein monomers.
Table 2.
Isothermal titration calorimetry of AI-BP, Tm0922 and YxkO.
| Protein/Ligand | Kd, μM | N | ΔH, cal/mol | ΔS, cal/mol/deg |
|---|---|---|---|---|
| AI-BP | ||||
| ADPR | 450.5 ± 30.8 | 0.60 ± 0.18 | -21730 ± 6949 | -57.6 |
| NADP, NADPH | Weak binding1 | |||
| Tm0922 | ||||
| ADPR | 5.1 ± 0.4 | 0.45 ± 0.01 | -16060 ± 212 | -29.60 |
| NADPH | 5.3 ± 1.2 | 0.46 ± 0.01 | -9714 ± 458 | -8.44 |
| NADP | 6.0 ±0.9 | 0.46 ± 0.01 | -9737 ± 355 | -8.76 |
| NAADP | 104.8 ± 11.1 | 0.980 ± 0.05 | -5151 ± 424 | 0.93 |
| NAD, NADH | Weak binding1 | |||
| YxkO | ||||
| NADH | 9.6 ± 0.7 | 1.11 ± 0.02 | -24730 ± 482 | -59.9 |
| NADPH | Weak binding1 | |||
| ATP | 595.2 ± 133.9 | 1.00 ± 0.05 | -7944 ± 1294 | -11.9 |
The binding of NADP(H) to AI-BP, NAD(H) to Tm0922, and NADPH to YxkO was too weak to reliably determine its parameters at achievable protein concentrations.
Figure 6.
Isothermal titration calorimetry profiles and fitting curves for the binding of ADP-ribose to Tm0922 (A) and AI-BP (B) (Table 2).
The affinities of the ligand binding observed by ITC correlated with the coordination patterns observed in the YjeF_N site of Tm0922 but not in its PF01256 site. In the ITC tests, both NADP and NADPH bound to Tm0922 with similar affinities that significantly exceed those of NAD and NADH. In the crystal structures, the YjeF_N site coordinated all four compounds through the same set of interactions with binding of NADP and NADPH being reinforced by the interaction of their 2’-phosphate with K78. In contrast, the residues of the PF01256 site formed numerous polar interactions with the nicotinamide and nicotinamide ribose moieties of both NADPH and NADH but not NAD, and NADP did not bind at all (Fig. 5A). In addition, the 1:2 molar ratio between high affinity ligands and Tm0922 corresponded to the stoichiometry observed with AI-BP which contains only one YjeF_N site. Therefore, it is likely that ITC detected the interaction of the “NAD set” ligands only with the YjeF_N site of Tm0922.
Comparison of structural and ITC binding data for Tm0922 highlighted differences in the detection of protein-ligand binding in crystals versus solution. Due to the sensitivity of the method, ITC was able to detect interactions of the YjeF_N sites of both Tm0922 and AI-BP only with high affinity ligands. The affinity of the other ADP-containing ligands to YjeF_N sites was too low to be detected by ITC. The ability of the PF01256 site of Tm0922 to tightly bind its ligands appears to be compromised as no interaction with this site was detected by ITC for any “NAD set” ligand including expected substrates ATP and ADP. On the other hand, crystallographic analysis led to detection of both low and high affinity binding in the YjeF_N sites of AI-BP and N-Tm0922 as well as in the PF01256 site of C-Tm0922. Notably, ITC provided qualitative affinity and stoicheometric characterization of the high affinity binding not achievable by crystallographic analysis. Thus, the combination of the two methods provided a powerful tool for the characterization of the interactions between proteins and their ligands.
Binding of thymine-related metabolites
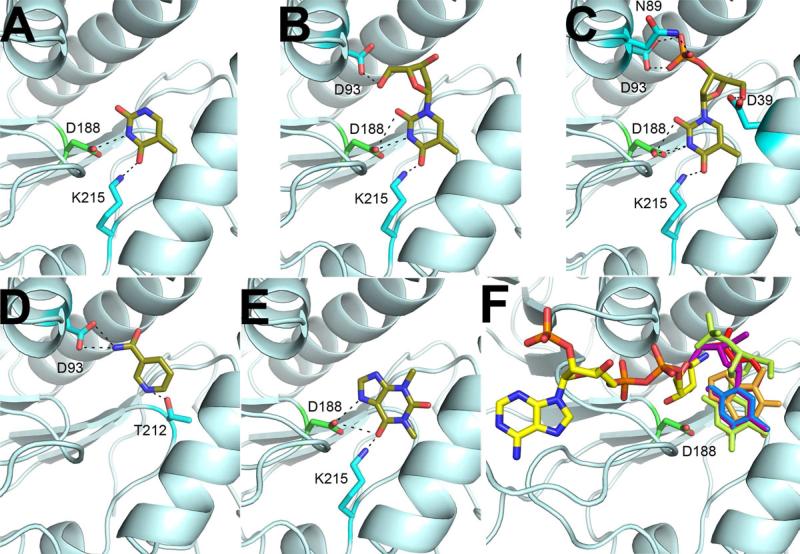
Thymidine 3′-monophosphate (T3′MP) was the only “thymine set” compound besides thymine and thymidine that bound in the AI-BP active site in crystals (Table S2). The position and orientation of T3′MP in the pocket compartment of the YjeF_N site of AI-BP was similar to those of thymine and thymidine (Fig. 7A-C). No binding of “thymine set” ligands in the YjeF_N site of Tm0922 crystals was detected.
Figure 7.
Coordination of putative inhibitors in the YjeF_N site of AI-BP. Thymine (A), thymidine (B), thymidine 3′-monophosphate (C), nicotinamide (D) and theophylline (E) are colored by the atom type. (F) Binding of these ligands (colored by the compound) in the AI-BP active site would interfere with the binding of the NAD-related substrate due to the steric hindrance with a nicotinamide ring.
ITC did not detect binding of any compound from the “thymine set” to either AI-BP or Tm0922. The failure to detect binding of thymine and thymidine to AI-BP observed in the crystals can be related to a low affinity (Kd > 10 mM) or to a low enthalpy component of binding that was not detected by ITC at the tested protein concentrations.
Identification of the PF01256 substrate and function
Although the putative kinase substrate binding site in Tm0922 accommodated various ligands in crystal soaks, interactions with these ligands were not detected in solution by ITC. The reasons for the inhibition of the binding in the PF01256 site of Tm0922 are not clear from the crystal structures but a likely reason is an allosteric regulation of ligand binding among two domains present in Tm0922. We studied the interaction of the “NAD set” metabolites with YxkO, which contains a single PF01256 domain and thus should not exhibit cross-domain allosteric regulation.
ITC detected the tight binding of YxkO to NADH but not to other tested compounds (Table 2). When the crystals of the apo-form of YxkO were soaked with individual metabolites, several compounds bound at the active site (Table S3). However, in contrast to Tm0922 all bound ligands mimicked ATP binding, leaving the kinase substrate site vacant. The loop Asp19-Gly25, which is disordered in the apo-form of YxkO, became ordered upon ligand binding at the ATP site.
When YxkO*ATP*Mg2+ crystals obtained by co-crystallization were soaked with either NADH or NADPH (Table S4), a well-defined ligand was observed in the kinase substrate site while the ATP binding site was occupied by AMP/Mg2+ (Fig. 5B). The ligand coordination was similar to that observed in the NAD(P)H soaks of Tm0922. The Asp19-Gly25 loop was involved in coordination of both NAD(P)H and AMP. A modification of the nicotinamide ring of NAD(P)H due to the saturation of the double bond between its C5 and C6 atoms was clearly visible at 1.5 Å resolution (Fig. 5B). The same modification was present in the Tm0922 soaks, but was not initially identified due to lower resolution.
NAD(P)H is known to undergo a spontaneous non-physiological 5,6-hydratation converting it to NAD(P)H-hydrate (NAD(P)HX) (Johnson and Tuazon, 1977). In the cell, NAD(P)HX is converted back to NAD(P)H in an ADP/ATP-dependent reaction (Fig. 5C). The enzyme catalyzing this dehydratation, EC 4.2.1.93, has been characterized biochemically but was not associated with a gene (Acheson et al., 1988; Meinhart et al., 1956). The ligands bound in the kinase substrate sites of both YxkO and Tm0922 crystals soaked with NAD(P)H were successfully modeled as NAD(P)HX (Fig. 5B), suggesting that the PF01256 proteins are responsible for this orphan “metabolite repair” activity. Kinetic studies revealed that YxkO efficiently catalyzes dehydratation of both NADHX and NADPHX in the presence of ADP or ATP. In the reaction between NADHX and ADP, KmNADHX, KmADP, and kcat values were 0.30±0.04 μM, 6.43±0.49 μM, and 0.35±0.01 sec−1, respectively. Thus, YxkO is an ADP/ATP-dependent NAD(P)H-hydrate dehydratase (EC 4.2.1.93), a function likely shared by other members of the PF01256 family. After the results of this work including the assignment of the PF01256 function to EC 4.2.1.93 were deposited in the PDB, the same function of the PF01256 proteins was independently reported (Marbaix et al., 2011) by purifying the previously described enzymatic activity from yeast (Acheson et al., 1988; Meinhart et al., 1956) and identifying the protein by mass spectrometry.
Closing in on a YjeF_N function
The results of our studies indicate that the putative active sites of YjeF_N proteins are designed to bind ADP-ribose/ADP-ribose phosphate (ADPR(P))-containing ligands. The KEGG database (Kanehisa and Goto, 2000) contains only 16 metabolites that include an ADPR(P) moiety, 15 of which are either substrates or products of NAD(P)-dependent reactions. Thus, the function of YjeF_N proteins is likely to involve an NAD(P)-related metabolite.
The YjeF_N site contains an invariant aspartate (Asp147Tm0922/Asp188AI-BP) that interacts with the nicotinamide ribose of bound NAD(P). Among NAD(P)-dependent enzymes, a similar acidic residue is present in poly(ADP-ribose) polymerases (PARPs), mono-(ADP-ribosyl) transferases (ARTs) and some dehydrogenases (Lin, 2007; Schubert et al., 2002).
The reactions catalyzed by PARPs and ARTs deplete an NAD(P)(H) pool in the cell by transferring the ADPR moiety to various acceptors, while the reactions that involve dehydrogenases do not alter the total NAD(P)(H) concentration. The YjeF_N domain is found in fusions with enzymatic domains linked to the replenishing of the NAD(P)(H) pool, PF01256 and pyridoxal 5'-phosphate (PLP) synthase. The latter fusion is found in plants where the major pathway of NAD(P) biosynthesis includes the PLP-dependent conversion of nicotinate to nicotinamide (Noctor et al., 2006).
Both thymine and thymidine, which bind in the active site of mouse AI-BP but not bacterial Tm0922, are known inhibitors of PARP activity, which is mainly present in eukaryotes (Lin, 2007). When AI-BP and Tm0922 crystals were soaked with other known PARP inhibitors, theophylline and nicotinamide (Skidmore et al., 1979), binding was observed only in the active site of AI-BP (Fig. 7 D-E, Table S2). The chemically diverse PARP inhibitors are unlikely to serve as substrates of a common enzymatic reaction catalyzed by AI-BP, but rather demonstrate a similar inhibition pattern of AI-BP and PARP. Indeed, the binding of any of these potential inhibitors in the pocket compartment would prevent binding of NAD(P)-related compounds due to a steric hindrance with the nicotinamide ring (Fig. 7 F). In addition, the invariant aspartate is involved in the coordination of thymine-related ligands.
Thus, several lines of evidence suggest that the YjeF_N proteins are involved in the reactions involving an ADPR(P) transfer although they do not possess a significant similarity to the known PARPs and ARTs in both sequence and structure. We were unable to detect incorporation of labeled NAD(P)(H) into proteins in the presence of AI-BP or Tm0922. However, most ADP-ribosylation reactions are tightly regulated and the activation of AI-BP and Tm0922 may require an additional factor or involve a non-protein target molecule.
Metabolite cocktail screening as a tool for determination of unknown protein function
Proteins are extremely selective binders, yet they are not absolutely selective. The present work demonstrates that this “imperfection” can be successfully exploited in functional analyses. The described approach to functional assignment is reminiscent of fragment-based drug discovery. It includes an initial identification of binding hits with X-ray crystallography followed by the optimization of binding. However, an important constraint that simplifies the search for a natural binding partner is that the compound of interest belongs to a relatively limited metabolic space. The relatively small 87 compound library used in the study provided a much better coverage of the metabolic space (5,000 – 25,000 compounds per individual species (Nicholson et al., 2007)) than the largest available chemical compound collections used for high-throughput screening (~106 compounds) provide for a drug-like chemical space, which is estimated to contain on the order of 1060 compounds (Carr and Jhoti, 2002). However, increasing the size and diversity of the metabolic library would significantly benefit the study. The efficiency of the metabolic libraries can be further improved by incorporating any functional information available from experimental data or predictions. Once the initial binding hits are identified, an expanded set of ligands, still restricted to the metabolic space, is used to identify a natural binding partner that is expected to bind with a higher affinity. The number of metabolites in the “NAD set” (25 compounds) and the “thymine set” (27 compounds) designed after detection of NAD/NADP and thymine/thymidine binding is appropriate for a quantitative binding study.
X-ray crystallography is an effective technique for characterizing the low affinity binding of proteins to small molecule ligands (Carr and Jhoti, 2002). The major advantage offered by protein crystallography in detecting low affinity protein-ligand interactions is related to using high ligand concentrations as well as dramatically greater protein concentrations than in other methods. High ligand concentrations can be used for soaking without a risk of producing false positive hits due to, for example, interfering with high throughput screening (HTS) assays. The concentration of ligands used in the crystallographic experiment is limited only by the stability of protein crystals. In the present study, ligand concentrations used in the crystal soaking reached 50 mM. In addition, the concentrations of AI-BP (29.5 kDa, 59% solvent), Tm0922 (54.5 kDa, 52% solvent), and YxkO (30.1 kDa, 58% solvent) in the crystals were 20.0 mM, 9.5 mM, and 19.2 mM, respectively. The protein concentrations employed in the other methods used to detect binding of a protein to a small molecule range from picomolar (or less) to micromolar when enzymatic assays are used in HTS and from micromolar to low millimolar in NMR. The binding of a ligand to a protein in crystals appears to be comparable to their binding in solution unless specific complications arise, such as blockade of the binding site due to the crystal packing or restriction of domain movement required for the formation of the binding site. The significant increase in both ligand and protein concentrations compared to the other methods of binding detection leads to the proportional increase in the detection limit and enables characterization of the low affinity binding.
An important consideration recommending a crystallographic approach to functional analysis is the resources available from structural genomics, which have determined the structures of 1912 proteins of unknown function including Tm0922 and YxkO described here. In addition to the benefits provided by the structures themselves, follow-up studies of these proteins can make use of the publically available genetic constructs and crystallization protocols overcoming a long recognized bottleneck, the preparation of the well-diffracting crystals.
Crystallographic detection of binding requires that the ligand be able to diffuse into the active site. This restricts the list of acceptable ligands to small molecules. However, information useful to functional analyses of protein-macromolecule complexes might be obtained by investigating a pool of small binding determinants, e.g. peptides or oligonucleotides, or by identifying a small molecule effector. Another condition for successful crystallographic detection is that ligand binding should not be impeded by the crystal contacts. The structure of the apo-protein should be analyzed to ensure that the putative binding site is not shielded due to the crystal packing. Fortunately, the active sites in crystal structures tend to show some degree of disorder in the absence of bound ligands, indicating that they are not significantly constrained. This work shows that small scale conformational changes in a protein, such as a change in a loop conformation required for the substrate to bind, can be tolerated in crystals. However, ligand binding that requires a large scale change, such as domain movement, is less likely to be detected. Crystallographic screening of metabolite library binding can become a powerful new tool for functional analysis of unknown proteins, useful particularly when little or no functional information is available. A significant advantage offered by the crystallographic approach is that in addition to identifying natural ligands, successful studies also provide exhaustive structural characterization of the interactions between the protein and substrates, effectors, or their analogs.
Experimental Procedures
Protein purification and crystallization
Selenomethionine-substituted mature AI-BP was expressed, purified and crystallized as described previously (Jha et al., 2008). The clones encoding YxkO and Tm0922 were obtained from the MCSG and JCSG; the proteins were expressed and purified as described previously (Donnelly et al., 2006; Lesley et al., 2002) and crystallized according to the protocols described in the PDB depositions 1KYH and 2AX3. The crystals of YxkO*ATP*Mg2+ were grown at 20°C using the hanging drop method, where the drop contained a 1:1 mixture of a solution containing 25 mg/ml protein and 10 mM ATP with the crystallization buffer composed of 13.5 % (v/v) PEG 400, 0.09 M MgCl2, 5% glycerol and 0.045 M HEPES pH 7.5.
Preparation of crystal soaks and structure determination
Before soaking, the crystals of YxkO were stabilized by equilibration against the mother liquor containing 35% PEG 400 while the Tm0922 and AI-BP crystals were derivatized without additional treatment. The metabolite cocktails (Table 1) were prepared as aqueous solutions or suspensions at 100 mM concentration of each component. To minimize crystal damage, 0.2 μl of each cocktail was mixed with 0.8 μl of the mother liquor and then combined with a 1 μl crystallization drop and incubated for 4-10 days. X-ray diffraction data were collected from the crystals flash cooled with liquid N2 and maintained at 100 K at the 19-ID, 19-BM, 21-ID-F, and 21-ID-D beamlines at Argonne National Laboratory. HKL-3000 (Minor et al., 2006) was used for the crystallographic data collection and processing, structure solution, and refinement. The structures of AI-BP, Tm0922, and YxkO complexes were solved by molecular replacement using the corresponding apo-structures (PDB: 2O8N, 2AX3, and 1KYH) as models. Depending on the crystal symmetry group and number of subunits per asymmetric unit (I422/one subunit for Tm0922 and YxkO; R32/one subunit or C2/six subunits for AI-BP) the time required to complete initial refinement sufficient to detect the presence of a bound ligand ranged from less than 20 to 45 minutes after mounting the crystal on the goniostat.
Based upon initial binding hits detected in cocktail soaks, two sets of metabolites, the “NAD set” and the “thymine set” and were selected for further study (see Results and Fig. S1-S2). The soaks with individual ligands were prepared similarly to the cocktail soaks using 50-500 mM stock aqueous solutions or suspensions.
The twenty structures of Tm0922 complexes were determined at the resolution 1.95 – 2.6 Å with R/Rfree in the range of 15.7-17.4/19.2-23.7% (Table S1). The six structures of AI-BP complexes were determined at a resolution of 2.05 – 2.8 Å with R/Rfree in the range of 16.5-20.8/20.1-24.3% (Table S2). The eleven structures of YxkO complexes determined at the resolution of 1.5 – 1.9 Å had R/Rfree in the range of 13.4-16.6/14.8-19.0% (Tables S3-S4). All structures had good stereochemistry. Atomic coordinates and structure factors for the reported crystal structures have been deposited with the Protein Data Bank under accession codes reported in Tables S1-S4.
Isothermal calorimetry
Isothermal titration binding assays were carried out at 25°C (the binding to Tm0922 was also tested at 55°C) using an iTC200 instrument (MicroCal). The measurements were carried out in 100 mM HEPES buffer pH 7.5 with 100 mM NaCl, using 70-130 μM protein solutions. The final concentrations of the “NAD set” and “thymine set” ligands exceeded the protein concentration 1.2 to 5 times. Data analysis was conducted with the Origin software (OriginLab).
NAD(P)H-hydrate dehydratase activity of YxkO
NADHX was prepared using the conversion of NADH by glyceraldehyde-3-phosphate dehydrogenase (Sigma-Aldrich) as described (Miksic and Brown, 1978). NADPHX was prepared by incubating 100 mM NADPH in 0.2 M sodium phosphate buffer pH 5.0 and adjusting pH to 8.5 after A340 decreased by 75%. The enzymatic activity of YxkO was assayed as described (Acheson et al., 1988) by monitoring conversion of NAD(P)HX to NAD(P)H at 340 nm (Δε340 = 6200 M-1cm-1) at 25°C. Kinetic parameters for the Michaelis-Menten model were determined by nonlinear curve fitting using Origin software.
Testing ADPR trasferase activities of AI-BP and Tm0922
[32P]NAD was purchased from PerkinElmer. [32P]NADP was produced by ATP-dependent phosphorylation of [32P]NAD catalyzed by chicken liver NAD kinase (Sigma-Aldrich). [32P]NADPH was synthesized by reduction of [32P]NADP by 6-phospho-D-gluconate in the presence of yeast 6-phosphogluconate dehydrogenase (Sigma-Aldrich). [32P]NADH was obtained by reduction of [32P]NAD by formate catalyzed by formate dehydrogenase from Pseudomonas sp. 101 (generous gift from Dr. V.I.Tishkov).
Each of the [32P]-labeled metabolites was added to mouse sperm lysate along with AI-BP and incubated for 30 minutes at 30°C. A similar experiment with Tm0922 and T. maritima lysate was performed at 55°C. The control experiments were done with human PARP (Sigma-Aldrich) and both lysates at 30°C. The samples were subjected to SDS-PAGE, dried, and positions of the proteins labeled by [32P]ADPR were determined using phosphoimager.
Supplementary Material
Highlights.
▶Crystallographic screening of ligand binding can be used in protein function analysis
▶Proteins from family PF01256 serve as ADP/ATP-dependent NAD(P)H-hydrate dehydratases
▶Proteins of family YjeF_N interact with substrate structurally related to ADP-ribose
Acknowledgements
We thank staff at the Advanced Proton Source beamlines 19-ID, 19-BM, and 21-ID for help, Dr. V.I. Tishkov for providing formate dehydrogenase from Pseudomonas, and Drs. M.D. Zimmerman and D.R Cooper for valuable comments on the manuscript. This work was supported by the NIH grants U54 GM074942 and U54 GM094585 (to A.J. and W.M.), R01 GM053163 (to W.M.), U54 HD029099 and U01 HD060491 (to J.C.H.), and U54 GM094586 and U54 GM074898 (to S.A.L). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson SA, Kirkman HN, Wolfenden R. Equilibrium of 5,6-hydration of NADH and mechanism of ATP-dependent dehydration. Biochemistry. 1988;27:7371–7375. doi: 10.1021/bi00419a030. [DOI] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L. Novel conserved domains in proteins with predicted roles in eukaryotic cell-cycle regulation, decapping and RNA stability. BMC Genomics. 2004;5:45. doi: 10.1186/1471-2164-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R, Jhoti H. Structure-based screening of low-affinity compounds. Drug Discov Today. 2002;7:522–527. doi: 10.1016/s1359-6446(02)02245-6. [DOI] [PubMed] [Google Scholar]
- Chance MR, Bresnick AR, Burley SK, Jiang JS, Lima CD, Sali A, Almo SC, Bonanno JB, Buglino JA, Boulton S, et al. Structural genomics: A pipeline for providing structures for the biologist. Protein Science. 2002;11:723–738. doi: 10.1110/ps.4570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Bennett EM, Begley TP, Ealick SE. Crystal structure of 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate kinase from Salmonella typhimurium at 2.3 A resolution. Structure. 2002;10:225–235. doi: 10.1016/s0969-2126(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, et al. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Research. 2012;40:D700–D705. doi: 10.1093/nar/gkr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Teixeira D, Parker R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J Cell Biol. 2007;179:437–449. doi: 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly MI, Zhou M, Millard CS, Clancy S, Stols L, Eschenfeldt WH, Collart FR, Joachimiak A. An expression vector tailored for large-scale, high-throughput purification of recombinant proteins. Protein Expr Purif. 2006;47:446–454. doi: 10.1016/j.pep.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, et al. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY, Koonin EV. ‘Conserved hypothetical’ proteins: prioritization of targets for experimental study. Nucleic Acids Res. 2004;32:5452–5463. doi: 10.1093/nar/gkh885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes SY, Scholle MD, Campbell JW, Balazsi G, Ravasz E, Daugherty MD, Somera AL, Kyrpides NC, Anderson I, Gelfand MS, et al. Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. Journal of Bacteriology. 2003;185:5673–5684. doi: 10.1128/JB.185.19.5673-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya Y, Jones BN, Muhlrad D, Gross JD, Parker R. Identification and Analysis of the Interaction between Edc3 and Dcp2 in Saccharomyces cerevisiae. Mol Cell Biol. 2010;30:1446–1456. doi: 10.1128/MCB.01305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha KN, Shumilin IA, Digilio LC, Chertihin O, Zheng H, Schmitz G, Visconti PE, Flickinger CJ, Minor W, Herr JC. Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation. Endocrinology. 2008;149:2108–2120. doi: 10.1210/en.2007-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Tuazon PT. Acid-catalyzed hydration of reduced nicotinamide adenine dinucleotide and its analogues. Biochemistry. 1977;16:1175–1183. doi: 10.1021/bi00625a023. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato JI, Hashimoto M. Construction of consecutive deletions of the Escherichia coli chromosome. Mol Syst Biol. 2007;3 doi: 10.1038/msb4100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley SA, Kuhn P, Godzik A, Deacon AM, Mathews I, Kreusch A, Spraggon G, Klock HE, McMullan D, Shin T, et al. Structural genomics of the Thermotoga maritima proteome implemented in a high-throughput structure determination pipeline. Proc Natl Acad Sci U S A. 2002;99:11664–11669. doi: 10.1073/pnas.142413399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. Nicotinamide adenine dinucleotide: beyond a redox coenzyme. Org Biomol Chem. 2007;5:2541–2554. doi: 10.1039/b706887e. [DOI] [PubMed] [Google Scholar]
- Ling SHM, Decker CJ, Walsh MA, She MP, Parker R, Song HW. Crystal structure of human Edc3 and its functional implications. Mol Cell Biol. 2008;28:5965–5976. doi: 10.1128/MCB.00761-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbaix AY, Noel G, Detroux AM, Vertommen D, Van Schaftingen E, Linster CL. Extremely Conserved ATP- or ADP-dependent Enzymatic System for Nicotinamide Nucleotide Repair. Journal of Biological Chemistry. 2011;286:41246–41252. doi: 10.1074/jbc.C111.310847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhart JO, Chaykin S, Krebs EG. Enzymatic conversion of a reduced diphosphopyridine nucleotide derivative to reduced diphosphopyridine nucleotide. J Biol Chem. 1956;220:821–829. [PubMed] [Google Scholar]
- Miksic JR, Brown PR. Reactions of Reduced Nicotinamide Adenine-Dinucleotide in Acid - Studies by Reversed-Phase High-Pressure Liquid-Chromatography. Biochemistry. 1978;17:2234–2238. doi: 10.1021/bi00604a034. [DOI] [PubMed] [Google Scholar]
- Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Lindon JC. The handbook of metabonomics and metabolomics. 1st edn Elsevier; Amsterdam ; Oxford: 2007. [Google Scholar]
- Noctor G, Queval G, Gakiere B. NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J Exp Bot57. 2006:1603–1620. doi: 10.1093/jxb/erj202. [DOI] [PubMed] [Google Scholar]
- Ritter M, Buechler C, Boettcher A, Barlage S, Schmitz-Madry A, Orso E, Bared SM, Schmiedeknecht G, Baehr CH, Fricker G, et al. Cloning and characterization of a novel apolipoprotein A-I binding protein, AI-BP, secreted by cells of the kidney proximal tubules in response to HDL or ApoA-I. Genomics. 2002;79:693–702. doi: 10.1006/geno.2002.6761. [DOI] [PubMed] [Google Scholar]
- Rudolph C, Sigruener A, Hartmann A, Orso E, Bals-Pratsch M, Gronwald W, Seifert B, Kalbitzer HR, Verdorfer I, Luetjens CM, et al. ApoA-I-binding protein (AI-BP) and its homologues hYjeF_N2 and hYjeF_N3 comprise the YjeF_N domain protein family in humans with a role in spermiogenesis and oogenesis. Horm Metab Res. 2007;39:322–335. doi: 10.1055/s-2007-977699. [DOI] [PubMed] [Google Scholar]
- Schubert HL, Raux E, Brindley AA, Leech HK, Wilson KS, Hill CP, Warren MJ. The structure of Saccharomyces cerevisiae Met8p, a bifunctional dehydrogenase and ferrochelatase. EMBO J. 2002;21:2068–2075. doi: 10.1093/emboj/21.9.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore CJ, Davies MI, Goodwin PM, Halldorsson H, Lewis PJ, Shall S, Zia'ee AA. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979;101:135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Suhre K. Inference of gene function based on gene fusion events: the rosetta-stone method. Methods Mol Biol. 2007;396:31–41. doi: 10.1007/978-1-59745-515-2_3. [DOI] [PubMed] [Google Scholar]
- Tritschler F, Braun JE, Eulalio A, Truffault V, Izaurralde E, Weichenrieder O. Structural Basis for the Mutually Exclusive Anchoring of P Body Components EDC3 and Tral to the DEAD Box Protein DDX6/Me31B. Mol Cell. 2009;33:661–668. doi: 10.1016/j.molcel.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Wolf YI, Brenner SE, Bash PA, Koonin EV. Distribution of protein folds in the three superkingdoms of life. Genome Research. 1999;9:17–26. [PubMed] [Google Scholar]
- Zhang RG, Grembecka J, Vinokour E, Collart F, Dementieva I, Minor W, Joachimiak A. Structure of Bacillus subtilis YXKO--a member of the UPF0031 family and a putative kinase. J Struct Biol. 2002;139:161–170. doi: 10.1016/s1047-8477(02)00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.