Abstract
Retinal diseases such as age-related macular degeneration (ARMD) and retinitis pigmentosa (RP) affect millions of people. Replacing lost cells with new cells that connect with the still functional part of the host retina might repair a degenerating retina and restore eyesight to an unknown extent. A unique model, subretinal transplantation of freshly dissected sheets of fetal-derived retinal progenitor cells, combined with its retinal pigment epithelium (RPE), has demonstrated successful results in both animals and humans. Most other approaches are restricted to rescue endogenous retinal cells of the recipient in earlier disease stages by a ‘nursing’ role of the implanted cells and are not aimed at neural retinal cell replacement. Sheet transplants restore lost visual responses in several retinal degeneration models in the superior colliculus (SC) corresponding to the location of the transplant in the retina. They do not simply preserve visual performance – they increase visual responsiveness to light. Restoration of visual responses in the SC can be directly traced to neural cells in the transplant, demonstrating that synaptic connections between transplant and host contribute to the visual improvement. Transplant processes invade the inner plexiform layer of the host retina and form synapses with presumable host cells. In a Phase II trial of RP and ARMD patients, transplants of retina together with its RPE improved visual acuity.
In summary, retinal progenitor sheet transplantation provides an excellent model to answer questions about how to repair and restore function of a degenerating retina. Supply of fetal donor tissue will always be limited but the model can set a standard and provide an informative base for optimal cell replacement therapies such as embryonic stem cell (ESC)-derived therapy.
Keywords: retinal degeneration, retinal transplantation, retinal progenitor sheets, electrophysiology, superior colliculus, trans-synaptic tracing, electron microscopy
1. Introduction: Cell replacement therapies for retinal degeneration
The transplanting of immature, freshly harvested retina together with or without its RPE as a sheet is currently the method that can lead to fully developed functional photoreceptors in severely degenerated recipients that have lost most photoreceptors at the time of transplantation. Since our previously published reviews in this journal (Aramant and Seiler, 2002a; Aramant and Seiler, 2004), much progress has been made toward demonstrating the mechanism of visual restoration by retinal sheet transplants.
The purpose of this article is to describe the positive results of fetal sheet transplantation compared to other cell transplantation approaches, with a special emphasis on the evidence of functional connectivity and long-term retinal restoration. We define “restoration” either as a morphological repair of a retinal area with lamination resembling normal retina, or as a stable improvement of visual function compared to the stage of retinal degeneration of the host at time of surgery. It does not mean to have reached a level of vision of a normal retina. “Rescue” means preservation of remaining photoreceptors so that there is better visual function at a later time compared to the corresponding untreated stage of retinal degeneration.
1.1 Short overview of retinal degenerative diseases
Retinal degenerative diseases first induce destruction of photoreceptors or RPE, and then affect the nearest inner retinal cell layers. These cells can look histologically normal but have started the disease process (review: Marc, 2010). Diseases such as age-related macular degeneration (ARMD) or retinitis pigmentosa (RP) affect millions of people and lead to irreversible vision loss. In ARMD, loss of vision occurs in the central retina first; in RP, peripheral vision is lost first. In spite of the synaptic rewiring (“remodeling”) that occurs after loss of photoreceptors and RPE (review: Jones and Marc, 2005; Marc et al., 2007), the remaining inner retinal layers maintain their thickness for a long time (Humayun et al., 1999). Retinal ganglion cells can also still produce action potentials after electrical stimulation in the absence of photoreceptor input (Margolis et al., 2008; Jensen and Rizzo, 2009; Kolomiets et al., 2010).
1.2. Retinal degeneration models
We have provided an extensive, more detailed review of retinal degeneration models in our previous reviews (Aramant and Seiler, 2002a; Aramant and Seiler, 2004). Important is to keep in mind that each retinal degeneration model has its own unique features and causes. The results achieved in one model often cannot be reproduced in another. In particular, trophic effects on photoreceptors can vary across models (see section 5.5).
1.2.1 Induced retinal degeneration models
Light damage models had been widely used before inherited retinal degeneration models became widely available. Both Silverman and Del Cerro used light damaged albino rats as recipient for retinal transplants. Rats were exposed to continuous white light for 4-5 weeks at 3500 lux (del Cerro et al., 1991) or 2-4 weeks at 1900 lux (Silverman and Hughes, 1989). In contrast, exposure to 420 nm blue light of moderate intensity (680-1290 lux) requires only 2-4 days to specifically destroy almost all photoreceptors while sparing the RPE (Seiler et al., 2000). This procedure was used in early retinal sheet transplantation studies (Seiler and Aramant, 1998). In addition, blue light damage has been used to accelerate retinal degeneration in the S334ter line 3 transgenic rat (Thomas et al., 2007). However, light damage cannot be achieved in albino rabbits (unpublished observations) possibly due to the avascular retina. Light damaged minipigs have been used for transplantation of human fetal retina/RPE sheets (Li et al., 2009). Further discussion of different light damage models can be found in other reviews (Wenzel et al., 2005; Organisciak and Vaughan, 2010).
Drugs have been used to induce retinal degeneration, such as sodium iodate (Henkind and Gartner, 1983) for destruction of RPE and MNU (N-Methyl-N-nitrosourea) for destruction of photoreceptors (review: Tsubura et al., 2011). However, if used at a dosage that is not generally toxic, this kind of drug treatment only results in patchy and incomplete damage which makes it difficult to study the functional effect of transplants.
1.2.2. Inherited and transgenic rodent retinal degeneration models
A recent review of naturally occurring models of retinal degeneration can be found in Baehr and Frederick (2009). The Royal College of Surgeons (RCS) rat which has a defect in RPE phagocytosis (D'Cruz et al., 2000) and the retinal degenerate (rd) 1 mouse with a defect in the β-phosphodiesterase gene (Bowes et al., 1990) have been the most commonly used models for retinal cell replacement and rescue strategies. Photoreceptors degenerate relatively slowly in the RCS rat secondarily to RPE dysfunction, whereas rd mice show loss of photoreceptors early on and never develop outer segments. Rds mice have a mutation in the rds/peripherin gene and show slow photoreceptor degeneration over several months.
With the advancement of transgenic technologies, many human mutations identified in retinal diseases have been cloned into animals, commonly mice (review: Chang et al., 2005). Fewer transgenic rat models have been created on an albino Sprague-Dawley rat background, using the P23H and S334ter mutation of rhodopsin (Steinberg et al., 1996; Pennesi et al., 2008; Martinez-Navarrete et al., 2011). For most of our latest transplantation studies, we have used transgenic pigmented S334ter line 3 rats, a model of dominant RP with fast retinal degeneration. Because there is a homozygous strain available, mating with pigmented rats results in pigmented heterozygous rats that are more useful for functional testing than albinos. The rate of retinal degeneration is not affected by the pigmentation. Eye surgery is also easier in rats than in mice. For testing of human tissue without immunosuppression, we have recently developed a pigmented immunodeficient retinal degenerate rat strain, a cross between S334ter line 3 and NIH nude rats [SD-Foxn1 Tg(S334ter)3Lav], which is now available through the Rat Research Resource Center at the University of Missouri (www.rrrc.us).
1.2.3 Large animal models of retinal degeneration
Many naturally occurring mutations that lead to retinal degeneration have been found in dogs (review: Tsai et al., 2007), and cats (review: Narfstrom et al., 2011). In addition, rhodopsin Pro347Leu-transgenic retinal degeneration models have also been created in pigs (Li et al., 1998) and rabbits (Kondo et al., 2009). The rate of retinal degeneration is, however, very slow in most larger transgenic models. Recently, a transgenic minipig has been developed that more closely mimics RP with a faster rate of degeneration (Ross et al., 2012).
1.3. Treatment strategies for retinal degeneration
Most current experimental approaches target early disease stages, with the aim of preventing degeneration of cones. Micronutrient supplements (Berson et al., 2004) and gene therapy to introduce trophic factors or to correct mutated genes (Liu et al., 2011) may help in the early stages. Many factors (e.g., basic fibroblast growth factor [bFGF], ciliary derived neurotrophic factor [CNTF], pigment epithelium derived factor [PEDF], glial cell-line derived neurotrophic factor [GDNF], brain-derived neurotrophic factor [BDNF]) delay degeneration of retinal cells, and protect photoreceptors in different models of retinal degeneration (review: (LaVail, 2005). Phase II clinical trials with encapsulated RPE cells producing CNTF have shown some photoreceptor protection in ARMD and RP patients with early stages of retinal degeneration (Talcott et al., 2011; Zhang et al., 2011; review: Wen et al., 2012). Although the effect of most factors on photoreceptor survival is indirect via microglia and Müller cells (Taylor et al., 2003), red-green cones express the BDNF receptor trkB and can directly respond to BDNF (Di Polo et al., 2000). CNTF treatment up-regulates both BDNF and bFGF in Müller cells (Harada et al., 2002). In rd mice, transplants of rods slow cone degeneration (Mohand-Said et al., 2000). This so-called rod-derived cone viability factor (RdCVF) is a diffusible factor, synthesized by rods, and distinct from known trophic factors (Leveillard et al., 2004).
In contrast, retinal sheet transplantation targets extended, especially later-stage retinal degeneration when photoreceptors and/or RPE have been irreversibly damaged. Two other treatment strategies that are targeting later disease stages are not covered in this review: development of a retinal prosthesis (reviews: Matthaei et al., 2011; Ong and Cruz, 2011) and gene transfer to make either retinal ganglion cells or bipolar cells responsive to light by introducing light-sensitive bacterial or algae proteins (Tomita et al., 2009; Busskamp and Roska, 2011).
1.4. Criteria for successful transplants
To be successful, transplants should (1) replace lost photoreceptors with new, functional and morphologically differentiated cells, (2) make appropriate synaptic connections with the host retina, and (3) restore visual function to an objectively measurable degree in the brain that can be demonstrated by different functional tests (electrophysiology, behavior, etc.). (4) In addition, transplants should not form tumors or in any other way be harmful to recipients.
2. History of retinal transplantation
This review concentrates on cellular replacement therapy, with emphasis on sheets of retina together with its RPE (Figure 1) that established proof of principle in 2001 (Woch et al., 2001). Retinal sheet transplantation is based on the hypothesis that degenerated cells can be replaced with healthy cells that can connect with the remaining inner retina. Because many patients with retinal degeneration have lost both photoreceptors and RPE, both tissues should be replaced together. Transplantation of photoreceptors can only have a limited effect when the patient needs new RPE. Or in reverse, it makes little sense of transplanting RPE when the patient has almost no photoreceptors left to be rescued.
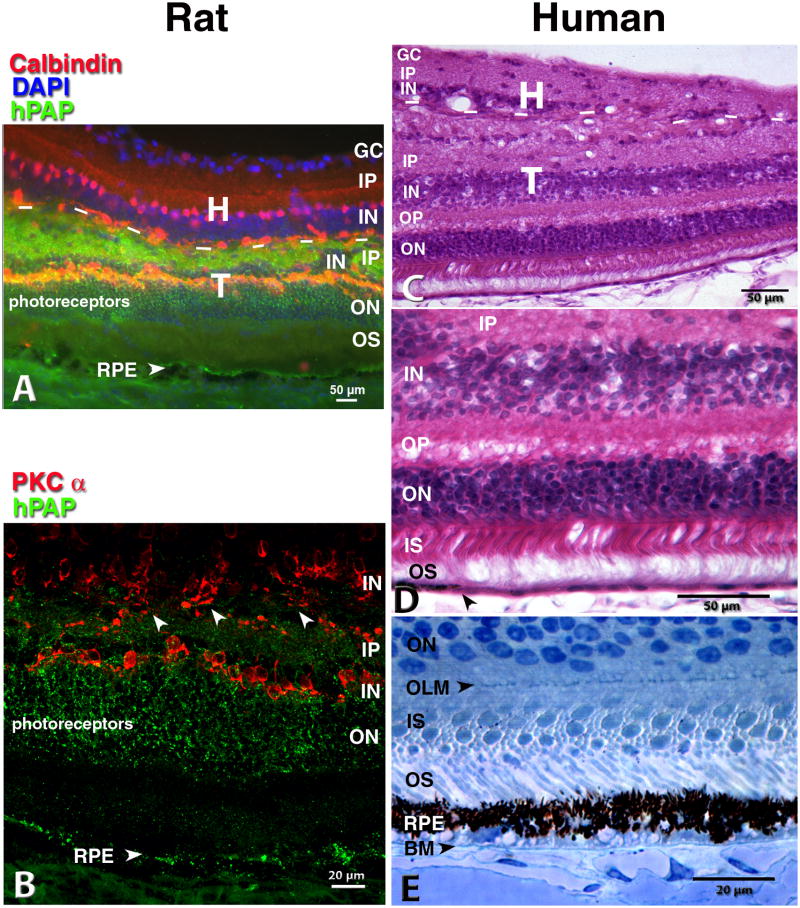
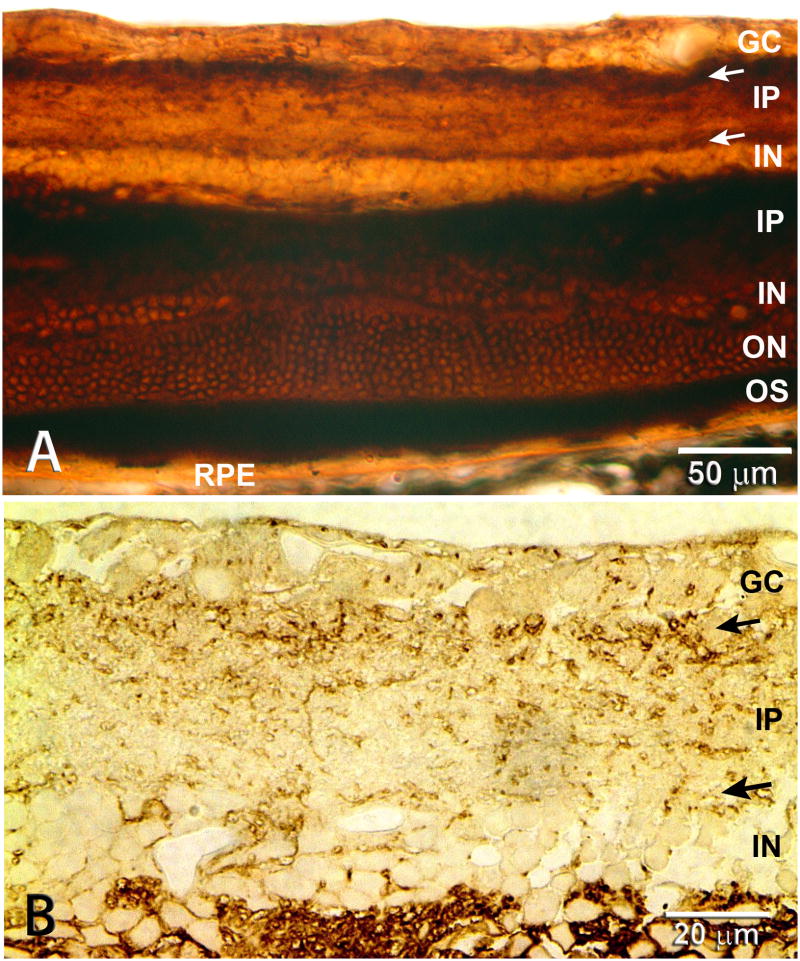
Figure 1. Rat and human retina, transplanted together with its RPE to the subretinal space.
From a sheet of neuroblastic cells, the transplant develops most retinal layers and cell types together with a monolayer of RPE seemingly in interaction with host choroid. A) B) Rat transplant to RCS rat 5.6 mo. after surgery; C) D) E) human transplant to nude rat 11.7 mo. after surgery.
(A) Double staining: Green hPAP label of all donor cells' cytoplasm, including processes, in combination with Calbindin (red) that labels horizontal and some amacrine cells. Nuclei are stained with DAPI (blue). Dashed lines: approximate border between transplant and host. Orange band of transplant horizontal cells double-stained for hPAP and Calbindin. The host horizontal cells border the transplant-host interface. The choroid shows some unspecific green autofluorescence which can be clearly distinguished from specific staining in the confocal microscope (B). (B) Single confocal scan of adjacent section: donor hPAP (green) and rod bipolar cells (PKC alpha, red). Arrow heads indicate areas with potentially crossing processes. Note the hPAP label of the co-transplanted RPE cells in A) and B). (C, D,E) Human transplant to normal albino athymic nude rats. C),D), Donor 12 weeks post-conception, 11.7 months after surgery, H-E staining. The transplant has developed all retinal layers with the exception of ganglion cells. (D) Enlargement of C). Arrow head in D) indicates transition from pigmented to non-pigmented RPE. (E) Toluidine blue-stained 1 μm semithin section. Human donor 14 weeks post-conception, 8.9 months post-surgery. Inner segments of individual transplant cones and rods clearly outlined. Normal appearing donor RPE with apical melanosomes adjacent to transplant photoreceptor outer segments. Close to the human Bruch's membrane, many rat host choroidal blood vessels can be seen. No trace of host albino rat RPE. Image in A) taken with standard Nikon FXA fluorescence microscope and deconvoluted (Autoquant, Autodeblur software 9.2 and 9.3). Scale bars: 50 μm (A,C,D), 20 μm (B,E). (A) Reprinted with permission from Seiler et al., 2008: Transplants of retinal layers– a hope to preserve and restore vision? Optonics and Photonics News, 19(4): 37-42. Copyright The Optical Society.
2.1 Transplantation of RPE and other supporting cells
RPE cells support photoreceptor function by maintaining the blood-retinal barrier, maintaining the Vitamin A cycle, providing nutrition and trophic support to the photoreceptors and phagocytosis of outer segments (Binder et al., 2007; Lee and Maclaren, 2011). The possibility of RPE transplantation was first investigated by Gouras et al. (1984). Since the demonstration that RPE cells can delay photoreceptor degeneration in RCS rats (Li and Turner, 1988; Lopez et al., 1989), this rescue effect and its functional implications have been studied extensively (Yamamoto et al., 1993; Sauvé et al., 1998; Girman et al., 2003; Gias et al., 2007), leading to clinical trials (Algvere et al., 1999) (see section 7). Because of supply issues, several laboratories have derived RPE cells from human embryonic stem cells (hESC) (Klimanskaya et al., 2004; Vugler et al., 2008a) and induced pluripotent stem cells (iPSC) (Buchholz et al., 2009; Kokkinaki et al., 2011) which have shown photoreceptor rescue in RCS rats (Lund et al., 2006; Carr et al., 2009) and in RPE65 −/− mice (Wang et al., 2010b). This research is now finally leading to clinical trials (see section 7). However, RPE transplants can only delay retinal degeneration and only have an effect as long as there are photoreceptors to rescue.
It has also been shown in the RCS rat that a trophic effect can be achieved by transplanting non-RPE cells, such as iris pigment epithelium (IPE) (Abe et al., 2000a; Schraermeyer et al., 2000), Schwann cells (Lawrence et al., 2000), bone marrow stem cells (Inoue et al., 2007; Lu et al., 2010), fetal brain-derived neural progenitors (Wang et al., 2008a) and ESC-derived neural progenitors (Schraermeyer et al., 2001).
Although dissociated RPE can delay photoreceptor degeneration in the RCS rat, they do not form a monolayer and thus do not contribute to the blood-retinal barrier. In addition, the aged Bruch's membrane in the patient's eye does not provide a good attachment substrate for RPE cells which inhibits RPE function (Gullapalli et al., 2004; Sun et al., 2007). There is also concern that dissociated cells may escape into the vitreous after transplantation and cause retinal detachment. Therefore, many laboratories have worked on culturing and transplanting RPE sheets isolated as patches (Sheng et al., 1995), embedded in gelatin (Del Priore et al., 2004) or grown as sheets on a biodegradable membrane carrier (Thumann et al., 1997; Lu et al., 1998) (review: Treharne et al., 2011) or on a parylene membrane (Zhu et al., 2011; Lu et al., 2012).
2.2. Transplantation of neural retina
Retinal transplantation in mammals started with a study transplanting rat fetal retina to the anterior chamber of the pregnant mother's eye (Royo and Quay, 1959). Several decades later, retinal transplantation to the anterior chamber was taken up again (del Cerro et al., 1985), followed by transplantation of retinal cell aggregates or microaggregates to the retina (Turner and Blair, 1986; del Cerro et al., 1991; Gouras et al., 1992).
2.2.1. Transplantation of dissociated cells or cell aggregates
Early on, most studies used freshly harvested early neonatal or fetal retinal cells. Del Cerro et al. transplanted dissociated postnatal cells to the subretinal space of light-damaged rats (del Cerro et al., 1991). After retrograde labeling retinal ganglion cells of P4 rat retina by rhodamine injection into the superior colliculus (SC), inner and outer retinal layers were horizontally separated using trypsin and a filter membrane, and transplanted to the vitreous of light-damaged rats (del Cerro et al., 1990). Isolated outer retinal layers did not survive well in the vitreous, whereas isolated inner retinal layers developed primitive photoreceptors. Later studies included transplantation of dissociated human retinal cells to the subretinal space (DiLoreto et al., 1996), leading to a Phase I clinical trial in India (Das et al., 1999) (see section 7).
Gouras et al. transplanted dissociated adult rat photoreceptors to 4-6 months old RCS rats (Gouras et al., 1991a). Transplanted cells slowly degenerated over time and only showed rudimentary outer segments at 2 weeks post surgery although some surviving photoreceptor cell bodies could be seen after several months. Then, transplantation of dissociated cells was compared with transplants of microaggregates (small retinal pieces) of neonatal mouse retina to rd mice (Gouras et al., 1992). In contrast to transplants of dissociated cells, microaggregate transplants developed small patches of outer segments in 50% of the cases where the small pieces were randomly placed in the correct orientation into the subretinal space, with photoreceptor progenitors facing the RPE. Such transplanted photoreceptors could survive and maintain their outer segments long-term (Gouras and Tanabe, 2003). However, after transplantation of retinal microaggregates to retinal degenerate cats, photoreceptors mostly formed rosettes (spheres of photoreceptors with outer segments in the center) (Ivert et al., 1998). Rosette formation thus showed some level of laminar organization that could not be achieved with dissociated cells.
Similarly, another group compared transplants of dissociated cells and aggregates and found photoreceptor rosette formation only in aggregate transplants (Juliusson et al., 1993).
The model of transplanting cell aggregates (in contrast to dissociated cells) into a retinal injury site (Turner and Blair, 1986) showed that immature cells can develop retinal layers in rosettes, that embryonic donor ages develop better lamination than postnatal donor ages (Aramant et al., 1988), express glial and neuronal retinal cell markers (Seiler and Turner, 1988; Aramant et al., 1990a), grow processes into the host inner plexiform layer and form synapses (Aramant and Seiler, 1995) and that xenografts of human fetal retinal cells develop most retinal cell types in immunosuppressed or immunodeficient rat recipients (Aramant et al., 1990b; Aramant and Seiler, 1994). After 8 months of cryopreservation, fetal retinal aggregates can be transplanted and survive (Aramant and Seiler, 1991). Early on in Turner's laboratory it was found out that less disrupted cell aggregates formed transplants containing many more photoreceptors with outer segments in form of rosettes (Aramant et al., 1988). However, the organization of such transplants was still very unsatisfactory.
In summary, cell aggregates clearly showed better results than dissociated cells.
2.2.2. Transplants of retinal or photoreceptor sheets
Silverman developed a method to isolate photoreceptor sheets of 8d postnatal and adult retina by gelatin embedding and vibratome sectioning retinal wholemounts down to the photoreceptor layer (Silverman and Hughes, 1989). These sheets were then transplanted into light-damaged rats using a special instrument. The transplantation method consisted of rolling up the gelatin embedded donor tissue to fit into a round nozzle, and then unfolding it after insertion into the subretinal space. This required the formation of a subretinal bleb prior to delivering the tissue, and subsequent retinal reattachment. The amount of fluid required to inject the tissue caused some trauma to the host and the donor tissue. However, the transplanted isolated sheet photoreceptors did not maintain outer segments; outer segments were only seen when portions of inner retina were left together with the photoreceptor sheet (Silverman and Hughes, 1989; Silverman et al., 1992a). Photoreceptor isolation was improved by using an excimer laser instead of a vibratome (Huang et al., 1998; Tezel and Kaplan, 1998). This procedure was also used for early clinical safety trials with transplanting adult photoreceptor sheets to RP patients (Kaplan et al., 1997) (see section 7).
The Silverman method (with modifications) was taken up by several researchers who either transplanted photoreceptor sheets (Mohand-Said et al., 1997; Ghosh et al., 1999c), full-thickness fetal (Ghosh et al., 1998) or adult retina (Schuschereba and Silverman, 1992; Ghosh et al., 1999c; Wasselius and Ghosh, 2001). Ghosh at al. compared the vibratome sectioning method with their “full-thickness” retinal transplant method and concluded the trauma induced by vibratome sectioning led to disturbed lamination and lower transplant survival (Ghosh et al., 1999c).
Independently, our group developed an instrument and procedure to transplant intact fetal retinal sheets - freshly isolated alone or together with its RPE - to the subretinal space of a degenerating retina (Seiler and Aramant, 1998; Aramant et al., 1999; Aramant and Seiler, 2002b) (see section 4). The implantation instrument (reviewed in Aramant and Seiler, 2002a) does not inject the sheet, but allows the fragile donor tissue to be gently placed in its correct orientation in a minimal amount of fluid. No retinal reattachment is necessary after delivering the donor tissue in this way. These sheet transplants can integrate with a degenerating retina and restore visual responses as shown in several rat models of retinal degeneration (Woch et al., 2001; Sagdullaev et al., 2003; Thomas et al., 2004b; Seiler et al., 2005; Seiler et al., 2008b; Seiler et al., 2010a) (see sections 4 and 6, Figures 1-8).
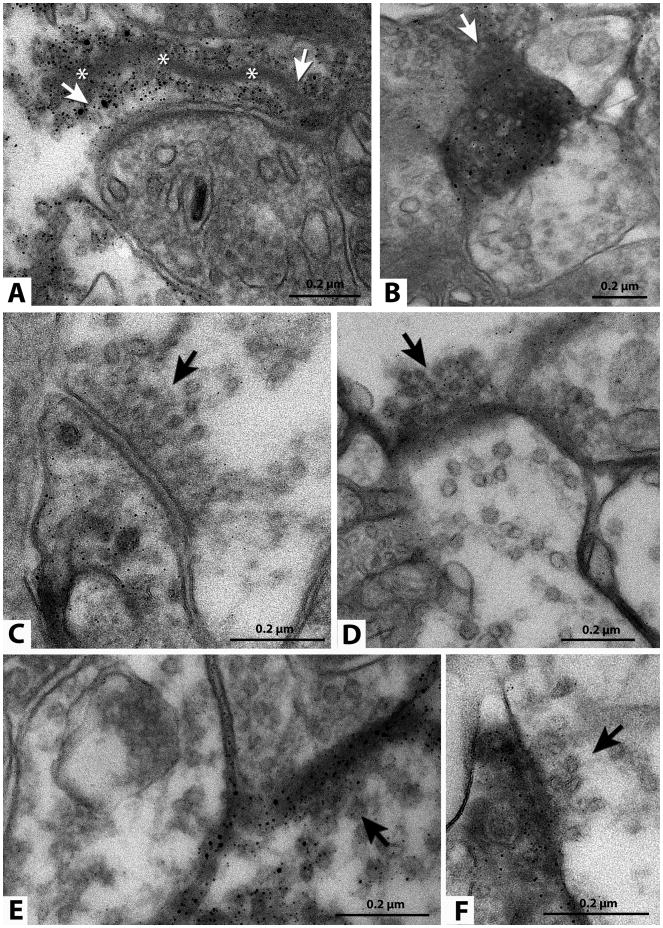
Figure 8. Ultrastructural demonstration of synaptic connectivity. Direct evidence.
Transplant processes and synapses in the inner plexiform layer of the host retina (images from 4 different rats). Immunohistochemistry for human placental alkaline phosphatase, recognizable as silver grains. The sections could not be counterstained therefore many apparent synapses were too diffuse to document clearly. Arrows indicate a presynaptic element of an apparent synapse between transplant and host cell. (A) Labeled ribbon synapse. A long synaptic ribbon is indicated by asterisks. Labeled processes are presynaptic in A, D and E, and postsynaptic in B, C and F. Scale bars: 0.2 μm.
Reprinted with permission from Seiler et al. 2010: Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets. Eur J. Neurosci, 31(3):508-520. Copyright J. Wiley & Sons.
In summary, more-or-less inferior results occur when the retina is sliced on different levels; this is likely due to the damage to the scaffold of Muller glial cells that are responsible for organization and nourishment of the neuronal cells.
3. Retinal donor tissue
Preparation of the retinal donor tissue is crucial for the survival and development of the transplant. The dissection has to be done very carefully, with minimal touching of the retina. In our experiments, donor tissue is kept cold in Hibernate E medium which can protect the tissue for several days. Ghosh's group is using oxygenated Ames' medium, originally used for electrophysiology of retinal wholemounts (e.g., Ghosh et al., 1998; Wasselius and Ghosh, 2001).
3.1. Fetal cells
Extensive preclinical studies to find the optimal donor tissue for transplantation in the central nervous system (CNS) took place around the 1980s in Sweden for Parkinson's patients (Lindvall et al., 1988). Unequivocally, these studies indicated that fetal donor tissue was the tissue of choice (Brundin et al., 1988; Lindvall and Bjorklund, 2004). A 2004 review stated “… intrastriatal transplants of fetal mesencephalic tissue in Parkinson's disease (PD) patients have provided proof-of-principle for the cell replacement strategy in this disorder” and “Most probably, fetal mesencephalic grafts will continue to be the golden standard …” (Lindvall and Bjorklund, 2004). In a 2011 review, the same authors discuss a European Research project called TransEuro, started in 2010, where fetal cell grafting was taken up again to optimize and standardize the therapy for PD “while waiting for the new opportunities offered by stem cell technology” because “… stem cell-derived DA neurons, suitable for use in clinical trials, are not going to be available any time soon” (Lindvall and Bjorklund, 2011).
We can presume that the overall principles apply throughout the CNS including the eye, i.e., fetal cells are optimal for transplantation. Many retinal transplantation studies from 1980s and 1990s indicated that fetal cells were optimal in a progressive scale from dissociated cells to intact sheets (see section 3.2 below).
In spite of the ethical and supply issues, fetal transplants can be done ethically and safely following the protocol of Helsinki from otherwise discarded tissue provided that there is complete separation between donor and tissue use for the recipient, clean medical history of the donor, informed consents (both donor and recipient), and no incentive for abortions. Results with fetal tissue provide the basis for the proof of principle of what retinal transplants can do in the best case. In this interim, while aiding to develop other approaches like stem cells for clinical use, many patients might be helped.
3.2. Advantages of fetal retinal sheets
As a general overview, the retinal sheets are part of an organ: the eye. Science has been successful with organ transplants like heart, lung, kidney, liver etc. Transplants of intact fetal sheets with its pigment epithelium have clear advantages. It is a method that takes into account different sick parts of a degenerating retina. Degeneration of RPE or photoreceptors is either directly or indirectly related to each other, and then the damage progressively spreads to the nearest layers. Results have shown that restored communication with the host retina can take place not necessarily by the photoreceptors but through other transplant retinal cells (Seiler et al., 2008b; Seiler et al., 2012) (see section 6.2). As a piece of a developing organ, fetal retinal tissue has already early on developed a primordial circuitry and the cells are committed to be retinal cells.
Allografts of fetal tissue face a lower chance of rejection because of the “immune privilege” of the subretinal space (Streilein et al., 2002; Niederkorn, 2006). Although neural retina is non-immunogenic, the RPE and the microglial cells in the donor retina are immunogenic (Ma and Streilein, 1998; Streilein et al., 2002). Microglial cells are associated with blood vessels and migrate postnatally into the rat retina (Ashwell et al., 1989) and, into the human retina, from 16 weeks gestation (Provis et al., 1997). Fetal retinal tissue is less immunogenic than adult tissue because it contains less microglia and fewer, immature blood vessels (Ashwell et al., 1989; Diaz-Araya et al., 1995). Fetal cells have few surface markers that make them recognizable as foreign cells. However, macrophages can act as antigen presenting cells in the subretinal space (McMenamin and Loeffler, 1990) when cografting RPE and retina. Interestingly, xenografts of full-thickness fetal rat retina can survive as laminated sheets in rabbits – however, without development of outer segments and only rudimentary inner segments – whereas fragmented fetal rat grafts are rejected (Ghosh et al., 2008). Similarly, allografted sheets of neonatal mouse RPE survive when transplanted to the kidney capsule whereas dissociated cells are rejected (Wenkel and Streilein, 2000).
The fetal cells have a high capacity to sprout processes, and to produce trophic substances that will aid host and transplant cells in different ways. Fetal cells can multiply, so that the transplants can grow to cover a larger area, usually doubling in size. A fully developed sheet transplant contains much more cells than at the time of surgery. Fetal cells can overcome the trauma of transplantation much easier than adult cells because fetal retinal cells do not depend as much on oxygen as adult cells (Wasselius and Ghosh, 2001).
However, photoreceptors and their precursors develop mostly postnatally; thus it is impossible to isolate photoreceptor progenitors from fetal retinal sheets without disrupting the sheets. Gosh and collaborators have developed procedures for culturing fetal retinal sheets (Ghosh et al., 2009) and whole eye cups (Engelsberg and Ghosh, 2011). Tissue culture induces selective loss of inner retinal cells. They argue that this is a better way to enrich photoreceptor progenitors than cutting photoreceptor sheets from retinal wholemounts or enriching photoreceptor progenitors from dissociated retinal cells. No transplantation studies have been published yet.
Yang et al. transplanted either photoreceptor sheets (sliced from retinal wholemounts) or whole retinal sheets of postnatal P8 retina to 3-month-old P23H rats, a slow retinal degeneration model (Yang et al., 2010b) using a modification of Silverman's method. Both types of transplants improved the amplitude of the ERG b-wave to same extent and had a positive paracrine effect to rescue host cones.
A comparison of different rat donor ages in aggregate transplants showed that embryonic donor tissue developed better lamination and integrated better than postnatal donor tissue (Aramant et al., 1988). In a study comparing different donor ages of retinal aggregate transplants after 8 months of cryopreservation, rat E16 donor retina developed better than older or younger donor ages. However, freshly harvested retinas developed better laminated transplants than cryopreserved retinas (Aramant and Seiler, 1991). Similarly, aggregate transplants of human fetal retina grew larger and were better organized when derived from donor tissue of 9-11 weeks post-conception compared to older human donor tissue (Aramant and Seiler, 1994).
Our first published study of intact fetal rat retinal sheet transplants to light-damaged rats used E15 to E21 donors (Seiler and Aramant, 1998). Most of our later studies used E19-20 donor tissue (Woch et al., 2001; Sagdullaev et al., 2003; Thomas et al., 2004b; Seiler et al., 2010a; Yang et al., 2010a).
Other laboratories have transplanted adult retinal sheets in normal rabbits (Schuschereba and Silverman, 1992; Wasselius and Ghosh, 2001). Rabbit retina is special because it contains no blood vessels outside the visual streak, and can be maintained viable in vitro (e.g. for recording) for relatively long time. Schuschereba's study reported that the transplant photoreceptor layer was reduced to 50% thickness after only 2 weeks survival (Schuschereba and Silverman, 1992). Wasselius' study showed long-term survival of adult retinal sheet transplants in normal rabbits, using optimized medium and storage conditions before transplantation (Wasselius and Ghosh, 2001). In contrast, fragmented transplants did not survive. However, all layers of the normal host retina severely degenerated in the transplant area, and the transplants were mostly separated from the host retina although small areas of connections were seen. This study was not repeated in retinal degeneration models.
On the other hand, transplantation of intact fetal retinal sheets can consistently achieve a large continuous layer of transplant photoreceptors with outer segments in contact with host or donor RPE, in recipients that have lost their photoreceptor layer (Seiler and Aramant, 1998; Aramant et al., 1999; Seiler and Aramant, 2001; Aramant and Seiler, 2002b).
In summary, early in the 1990s, we hypothesized that the optimal donor tissue for transplantation in the eye should be freshly harvested fetal retinal sheets because transplants of intact fetal retinal sheets preserve the environment of the developing donor retina including the Muller cells that are responsible for the organization and nourishment of the retinal neurons.
3.3. Retinal stem cells, photoreceptor precursors - Single cells and neurospheres
Transplantation of dissociated cells has the advantage that only a small, self-sealing incision is necessary to deliver the cells. However, long-term survival of photoreceptors is poor in transplants of dissociated retinal cells (Gouras et al., 1991a; Gouras et al., 1991b; Mansergh et al., 2010); photoreceptors in microaggregates fare better if photoreceptors come in contact with RPE (Gouras et al., 1994; Gouras and Tanabe, 2003). Only one study in 1999 showed the development of a new photoreceptor layer with outer segments from mechanically dissociated postnatal retinal cell transplants to rd mice (Kwan et al., 1999). No other study using dissociated cells has replicated these results although transplanted photoreceptor precursors can develop outer segments and synaptic connections when they integrate into an existing outer nuclear layer (MacLaren et al., 2006; Bartsch et al., 2008; Pearson et al., 2012).
Since the source of fetal tissue is limited and controversial, most research is now focused on expanding cells (either derived from fetal origin, adult tissue or pluripotent stem cells) in tissue culture before transplantation. Recent research advances have made it possible to create three-dimensional optic eye cup-like tissue from pluripotent stem cells (Eiraku et al., 2011; Meyer et al., 2011; Phillips et al., 2012; Nakano et al. 2012) (see section 7.6).
3.3.1. Retinal stem cells
Retinal stem cells have been isolated from fetal retina (Chacko et al., 2000; Qiu et al., 2004; Klassen et al., 2008), postnatal retina (Klassen et al., 2004a), Muller glial cells (Das et al., 2006) or from the ciliary margin of the eye (review: Djojosubroto and Arsenijevic, 2008). However, it has been questioned whether adult ciliary epithelial cells represent retinal stem cells (Cicero et al., 2009). In addition, ciliary epithelial cells derived from NRL-GFP mice fail to differentiate into rod photoreceptors (Gualdoni et al., 2010).
Undifferentiated retinal stem cells remain proliferative in culture over several passages (usually by the addition of EGF and/or FGF). Differentiation to various retinal cell types is induced by removing growth factors and adding retinoic acid and/or serum. The photoreceptor differentiation potential of adult ciliary stem cells can be much improved by combined transduction of OTX2 and CRX together with the modulation of Chx10 (Inoue et al., 2010).
After transplantation to the vitreous or subretinal space, a subpopulation of retinal stem cells can integrate into the retina, differentiate and show transient trophic effects in retinal degeneration models (Klassen et al., 2004a). Klassen et al. transplanted neurospheres of retinal progenitor cells to the subretinal space of rho −/− mice (Klassen et al., 2004a), whereas other laboratories transplanted dissociated cells (Yang et al., 2002; Chacko et al., 2003; Qiu et al., 2005). Survival, differentiation and integration of retinal progenitor cell transplants can be somewhat improved by matrix embedding (Redenti et al., 2009; Tucker et al., 2010), indicating that maintaining cell-cell interactions is important.
3.3.2. Freshly isolated or cultured photoreceptor precursors
Rod precursor cells are postmitotic and express the transcription factors NRL and CRX. MacLaren et al. (MacLaren et al., 2006) isolated mouse photoreceptor precursor cells from freshly harvested postnatal retinas (P1-7) and transplanted them as dissociated cells. Using the NRL-GFP mouse as donor, photoreceptor precursors could be enriched by FACS sorting. A small percentage (0.1 – 0.2%) of the transplanted cells integrated into the photoreceptor layer of normal and rho−/− retina (see below). More recently, photoreceptor progenitor cells were isolated by FACS sorting from CRX-GFP mice and transplanted to two mouse models of Leber's Congenital Amaurosis (Lakowski et al., 2010). Dissociated cells (derived from the rho-GFP mouse) survived and differentiated better after transplantation when freshly isolated than after several passages of cell culture (Mansergh et al., 2010). After transplantation of freshly harvested photoreceptor precursors derived from NRL-GFP mice to normal adult mice, a significant decrease of integrated photoreceptors was seen starting at 4 months due to invasion of macrophages, and very few cells could be found surviving in the retina up to 12 months after transplantation (West et al., 2010). At 4 months after transplantation, immune suppression with cyclosporine A increased the number of the few integrated cells about 4-fold. One main inference of the study was that the problem with survival could be solved with immunosuppression. However, immunosuppression has many side effects, both on the recipient and on the transplants. An important comment: fetal retinal sheet transplants survive long-term without immunosuppression (e.g. Ghosh et al., 1999b; Thomas et al., 2004b; Seiler et al., 2010a; Yang et al., 2010a). It would be very difficult to justify systemic immunosuppression in a clinical setting for treating an eye disease because it is not life-threatening like heart or kidney diseases.
Sorting of postnatal retinal cells for rod precursor cell surface markers CD73 (and absence of CD24) increases the efficiency of photoreceptor integration into the outer nuclear layer and is 2-3 times more effective than only sorting for the expression of the GFP-NRL transgene (Eberle et al., 2011; Lakowski et al., 2011). At 3 weeks after transplantation, the percentages of integrated cells varied greatly between these 2 publications. Lakowski (Lakowski et al., 2011), injecting 200,000 cells, reported integration of in average 6%, up to 16% of the transplanted cells when sorting for CD74 and excluding CD24, compared to an average integration of 2.3%, up to maximum 4% for NRL-GFP sorted cells. Eberle (Eberle et al., 2011), injecting 400,000 cells, reported integration of 0.55% of CD73-sorted cells, and only integration of 0.17% unsorted cells.
Recently, mouse NRL-GFP+ sorted rod precursors have been transplanted into a mouse model of rod degeneration lacking rod transducin (Gnat1−/− mice) using an improved transplantation procedure, either performing scleral puncture or retinal detachment before cell injection to two transplantation sites instead of one (Pearson et al., 2012). This resulted in the integration of in average 8-9%, up to 16% of transplanted cells into the outer nuclear layer. Experiments were analyzed 4-6 weeks after transplantation, with many different methods, from cellular to behavioral, demonstrating that transplanted cells developed photoreceptor morphology, synaptic connectivity and light responses and could elicit light-evoked responses in the brain (although no full-field ERGs). Transplanted mice showed visual improvements in dark-adapted optokinetic testing and water maze (in dim light). The authors claimed that “So far there have been no convincing reports of photoreceptor-cell transplantation actually improving the recipient's vision …”, and thus their study was the first showing visual restoration by photoreceptor transplantation, ignoring many previous studies (see section 5).
3.3.3. Retinal progenitors and photoreceptor precursors derived from pluripotent stem cells
Mouse postnatal day 5 (the best age to isolate photoreceptor precursors) would correspond to late second or early third trimester human fetal tissue. This would be impossible to justify and obtain. Therefore, another cell source needs to be found. Retinal and photoreceptor progenitor cells have been derived from human embryonic stem cells (hESCs) (Lamba et al., 2006; Meyer et al., 2009; Yue et al., 2010; Meyer et al., 2011; Hambright et al., 2012; Clarke et al. 2012) and from induced pluripotent stem cells (iPSCs) (Lamba et al., 2010; Parameswaran et al., 2010; Meyer et al., 2011; Tucker et al., 2011). Lamba et al. (Lamba et al., 2009) transplanted hESC-derived retinal progenitors, differentiated according to a previously published protocol to newborn and adult wild-type mice and to adult transgenic CRX −/− mice, a retinal degeneration model. Transplanted cells migrated into all cell layers in newborn mice and preferentially into the outer nuclear layer in adult mice. Cells that developed into photoreceptors did not develop outer segments in the CRX −/− mice. A study in 2010 showed a differentiation protocol for iPS towards photoreceptors and a picture of three transplanted cells that had successfully integrated into the outer nuclear layer of a normal mouse, expressing recoverin and OTX2 (Lamba et al., 2010). Integrated transplanted cells also expressed rhodopsin in their cytoplasm, but no outer segments were shown. Using mouse iPS cell-derived retinal progenitors, Tucker et al. (2011) showed that transplanted cells (labeled with dsRed) integrated into the outer nuclear layer of rho−/− mice and expressed the outer segment marker ROM1 (shown 3 weeks after transplantation). Undifferentiated cells expressing the marker SSEA1 needed to be removed twice prior to transplantation to avoid teratoma formation. A recent study (Hambright et al., 2012) compared the survival and differentiation of hESC-derived retinal progenitor cells that were injected either into the subretinal space or vitreous of normal mice, and analyzed at 3 weeks and 3 months after transplantation. Surprisingly, the xenografts survived without immunosuppression provided there was no break of the blood-retinal barrier. However, this surgery approach (injection through the cornea) would not apply to human vitreoretinal surgery, and the study was not performed in a retinal degeneration model. Cells transplanted to the subretinal space developed photoreceptor markers whereas cells in the vitreous failed to differentiate. Cells transplanted to the vitreous migrated and integrated into the inner retinal layers, but cells from subretinal grafts only migrated into the outer nuclear layer when there was damage to the outer retina due to the injection.
3.3.4. Summary
As the studies above indicate, the foremost issue with transplantation of dissociated cells is cell survival and integration. Although some cells will integrate into the host retina, a high percentage of dissociated transplanted photoreceptor precursors remain in the subretinal space without contact to the host retina (MacLaren et al., 2006; Bartsch et al., 2008; Hambright et al., 2012). Retinal integration and morphological development of dissociated photoreceptor precursors depends on the developmental stage, process of mitosis, the donor age, selection of photoreceptor precursors, disruption of the glial barrier of the host retina and on the status of the host.
Development of normal photoreceptor morphology of the integrated transplanted photoreceptor precursors depends on the presence of the host outer nuclear layer; transplanted cells will not develop normal morphology of outer segments when transplanted to a recipient that has lost most photoreceptors such as rd or severely light-damaged mice (MacLaren et al., 2006; Lamba et al., 2009). When transplanting freshly harvested dissociated retinal cells, it appears that photoreceptor progenitor cells derived from early postnatal ages (P1 – P7) integrated into the host retina more efficiently than donor cells derived from either embryonic or older ages (MacLaren et al., 2006). P1 – P7 photoreceptor progenitor cells have stopped dividing, but are still immature and do not express markers of mature photoreceptors such as opsin and recoverin. They are in their strongest morphological and most suitable stage for single cell transplantation.
On the other hand, integration of donor cones after transplantation into two genetic models of Leber's congenital amaurosis (Crb1rd8/rd8 and Gucy2e−/− mouse) was only achieved when transplanting embryonic donor tissue (FACS-sorted from transgenic GFP-CRX mice that express GFP in all photoreceptor progenitors) (Lakowski et al., 2010). Cone integration efficiency was highest in the cone-deficient Gucy2e−/− retina. However, dissociated adult photoreceptors can also integrate into the outer nuclear layer of normal mouse retina when analyzed 2 weeks after transplantation (Gust and Reh, 2011), but they died rapidly after plating on coverslips in vitro. Long-term survival of such transplants is unknown but is not expected.
In summary, dissociated retinal cells (retinal stem cells, photoreceptor precursors, or adult retinal cells) survive less well after transplantation and are more easily rejected than sheets or microaggregates. Only a small percentage of transplanted cells integrate into the host retina and develop morphology of photoreceptors provided there is an existing host outer nuclear layer. All this indicates that a scaffold of Muller cells and also likely a healthy RPE is important for full photoreceptor development in vivo. The optimal donor age timing for cell transplantation is likely different for diverse retinal cell types depending on their last mitosis and applies only to transplantation of single cell suspensions, not to sheets.
3.4. Donor cell label
It is necessary to identify the donor tissue in the host to demonstrate cell differentiation, survival and integration after transplantation. Several groups have used nuclear labels that are taken up by dividing cells during DNA synthesis such as 3H-thymidine (del Cerro et al., 1990; Gouras et al., 1991a; Du et al., 1992) and bromodeoxyuridine (BrdU) (Seiler and Aramant, 1995, 1998; Aramant et al., 1999); and the nuclear label DAPI (4′,6-diamidino-2-phenylindole hydrochloride) (Wasselius and Ghosh, 2001). DAPI has however the disadvantage that it can be taken up by host macrophages, especially when dissociated cells are transplanted (Castanheira et al., 2009). Nuclear labels can only show cell migration, not extension of processes into the host. Donor tissue has also been prelabeled using dyes such as DiI (Silverman and Hughes, 1989; Tian et al., 2010), CFDA (Chacko et al., 2000), quantum dots (Wang et al., 2010a), or by gene transfection (Chacko et al., 2003; Bartsch et al., 2008).
However, genetically labeled donor tissue provides a label that also shows cytoplasmic processes and cannot be transferred by diffusion to host cells. Gouras et al. used donor tissue derived from transgenic mice expressing E.coli β-galactosidase (β-gal) in rods transplanted to a recipient that expressed β-gal in rod bipolar cells (Gouras and Tanabe, 2003). Transgenic animals have been created which express fluorescent proteins such as EGFP (enhanced green fluorescent protein) - either in all cells (Okabe et al., 1997; Park et al., 2001; Hadjantonakis et al., 2002) or specifically in photoreceptor precursors (Chan et al., 2004; Akimoto et al., 2006). Such animals have been used in a variety of studies, ranging from transplantation of cultured retinal progenitors (Klassen et al., 2004b; Klassen et al., 2008; Mansergh et al., 2010), freshly isolated photoreceptor precursors (MacLaren et al., 2006; Bartsch et al., 2008) to retinal sheets (Arai et al., 2004). A rat strain in which all cells express human placental alkaline phosphatase (hPAP) developed by Dr. Sandgren, University of Wisconsin (Kisseberth et al., 1999), has been used by our laboratory since 2001 in all our experiments. This strain was cross-bred with pigmented ACI rats (RT1av1, Harlan Laboratories) to obtain pigmented donors. 50% of the offspring will express hPAP which can be detected by a simple histochemical reaction or by immunohistochemistry. Figure 1 A,B; 2A, 5 – 8 show examples of hPAP stained transplants. The label is expressed in the cytoplasm, not in nuclei, and therefore shows up much fainter in nuclear layers. A list of the antibodies used for the figures is shown in Table 1.
Table 1. List of antibodies used in figures.
| Antibody Specificity | Species | Description | Dilution | Supplier | Shown in Figure |
|---|---|---|---|---|---|
| Blue opsin | rabbit | Blue-sensitive cones | 1:2000 | Chemicon, Temecula CA | 2C |
| Calbindin | rabbit | Marker for horizontal and some amacrine cells | 1:1000 | Chemicon | 1A |
| hPAP | mouse | Human placental alkaline phosphatase (donor label) Punctate stain | 1:500 (fl. 2nd ab) | Chemicon | 1A, B |
| 1:1K -1:2K (Elite ABC method) | Chemicon | 7, 8 | |||
| hPAP | mouse | Human placental alkaline phosphatase (donor label) Punctate stain | 1:600 | Sigma, St. Louis, MO | 5A,B |
| hPAP | rabbit | Human placental alkaline phosphatase (donor label) smooth stain | 1:50 | Epitomics, Burlingame CA | 2A, 6A-C |
| PKC alpha | rabbit | Rod bipolar cells | 1:125 | Oxford Biomedical, Oxford MI | 1B |
| PKC alpha | rabbit | Rod bipolar cells | 1:100 | Biodesign, Saco ME | 2C |
| PRV | rabbit | Pseudorabies virus | 1:5000 | gift of Dr. Enquist, Princeton University (Card et al., 1990) | 5A,B |
| PSD-95 | mouse | Post-synaptic density protein 95 | 1:500 | Stressgen, Victoria, BC, Canada | 6C |
| Recoverin | mouse | Rod and cone photoreceptors, cone bipolar cells | 1:500 | gift of Dr. McGinnis, University of Oklahoma (McGinnis et al., 1997) | 6 B1,B2 |
| Red-green opsin | rabbit | Red-green sensitive cones | 1:2000 | Chemicon | 2B |
| Rhodopsin (rho1D4) | mouse | rods | 1:50 | gift of Dr. Molday, University of British Columbia (Molday and MacKenzie, 1983) | 2B |
| Synapsin-1 | mouse | Synaptic layers | 1:500 | Chemicon | 6 A1,A2 |
| Syntaxin-1 (HPC-1) | mouse | Synaptic layers, amacrine cells | 1:500 | gift of Dr. Colin Barnstable, now Penn State Univ. (Barnstable et al., 1985) | 2A |
4. Transplantation of freshly harvested retinal progenitor sheets to repair degenerated retinas
4.1. Introduction
We hypothesized that the optimal organized transplant in the eye should be from a fetal intact piece, a retinal sheet with its primordial circuitry formed and cells that were committed to differentiate into various retinal cells.
In all studies, the ARVO statement for use of animals in research was followed, and all protocols were approved and monitored by the local IACUC committees (of the University of Louisville, the Doheny Eye Research Institute, and UC Irvine).
Our first published study showed transplantation of fetal retina to a rat light damage model (Seiler and Aramant, 1998). Using a custom-made instrument and procedure, the donor tissue was gently placed – not injected – into the subretinal space. The procedure has been described and reviewed in detail in Aramant and Seiler (2002a). The method was first presented at the Neuroscience Meeting in 1995.
A Swedish group also transplanted fetal “full thickness” retina, but, - in contrast to our group, to normal rabbit retina (Ghosh et al., 1998; Ghosh et al., 1999a; Ghosh et al., 1999b) and normal pig retina (Ghosh and Arner, 2002). They switched to the pig model of retinal degeneration later (Ghosh et al., 2004). Their transplants could develop good lamination, but they only showed integration with the inner layers of the avascular rabbit retina which appeared to degenerate over the transplant. However, in the vascularized pig and cat retina, the transplant remained mostly separated from the host retina (Ghosh and Arner, 2002; Bragadottir and Narfstrom, 2003; Ghosh et al., 2004). This was likely due to the more traumatic surgery method that required a retinal bleb and an extra retinotomy for relieving pressure to prevent the tissue to slip out after injection.
In contrast, using our implantation method, fetal sheet transplants could fuse well with the host retina in several rat degeneration models (Seiler and Aramant, 1998; Seiler et al., 2005; Seiler et al., 2008b) and in Abyssinian cats (Seiler et al., 2009). Differences between the results of the Swedish work and ours could be the result of the different instrument and procedure used for transplantation.
4.2. Efforts to support donor tissue by matrix embedding
Fetal donor tissue is very fragile, so we thought it may be necessary to embed it in a gel for protection. Early studies used growth factor reduced matrigel (Seiler and Aramant, 1998; Seiler et al., 1999), and later medium viscosity (MVG) alginate (Pronova, Oslo, Norway) (Seiler et al., 1999; Seiler and Aramant, 2001). We then decided to omit gel embedding entirely because of complications, such as partial separation of the neural retinal transplant from the host retina with the embedding matrix alginate, as can be seen in Aramant et al. (1999). However, it may be possible to improve the delivery of retina/RPE co-transplants with biomaterial scaffolds of which there are many possibilities available (review Treharne et al., 2011).
4.3. Cografts of fetal retina with RPE (rat and human) - an unique approach (Figure 1)
Many patients with advanced retinal degeneration need transplantation of both RPE, photoreceptors, and other retinal cells. Our retinal transplantation model has successfully demonstrated replacement of RPE and photoreceptors together in animals (Aramant et al., 1999; Woch et al., 2001; Aramant and Seiler, 2002b) and humans (Radtke et al., 2008). This was accomplished by transplanting freshly dissected sheets of fetal-derived (rat or human) neural retina progenitor cells with its RPE into the subretinal space. Such transplants have the potential to benefit retinal diseases with dysfunctional RPE and photoreceptors such as in ARMD and RP.
Dissection of fetal retina together with its RPE is a challenge since there is no adhesion between retina and RPE without developed photoreceptor outer segments. To dissect the RPE cleanly, eye cups were incubated in Dispase (Collaborative Biomedical Products, Bedford, MA) for 30 minutes at 37°C. Using ultrafine forceps, sclera and choroid were gently peeled away, and alginate-embedded pieces of retina with its attached RPE were then transplanted to the subretinal space (Aramant et al., 1999; Woch et al., 2001; Aramant and Seiler, 2002b). The first successful co-transplantation of fetal retina with its RPE was done in RCS rats (Aramant et al., 1999) (Figure 1 A,B). These transplants could restore visual responses in the SC (Woch et al., 2001). In another important preclinical study, we showed that human fetal retina with its RPE could be transplanted to immunodeficient albino athymic nude rats, survive for 8 - 11 months and develop normal lamination with its monolayer of RPE (Aramant and Seiler, 2002b) (Figure 1 C, D, E). This study was the basis for FDA-controlled clinical trials (see section 7).
Gel embedding was later omitted for the clinical trials because the instrument could gently deliver the co-transplants without any protection (Radtke et al., 2002; Radtke et al., 2004; Radtke et al., 2008). In addition, we had noticed that alginate sometimes caused graft-host separation (see section 4.2), and FDA approval would have been needed for using bioscaffolds in patients.
Another group has transplanted gelatin-embedded sheets of fetal human retina and RPE to light-damaged minipigs (without immunosuppression), and reported an improvement in multifocal ERG (Li et al., 2009). These transplants did not differentiate photoreceptors, and contained mostly glial cells. The transplants degenerated over time, likely due to the lack of immunosuppression, with complete degeneration of host and graft retina in the transplant area at 9-12 months.
4.4. Why do not all transplants develop perfectly?
In the rodent eye, a posterior approach had to be used to place the donor tissue into the subretinal space in the center of the eye near the optic disk. A cut is made behind pars plana through sclera, choroid and retina. Then the surgeon “feels” his/her way with the instrument into the subretinal space without seeing how the transplant is placed. Therefore, only 20 - 30 % of the surgeries result in transplants with large laminated areas (e.g. Seiler and Aramant, 1998; Seiler et al., 2008b). Transplants only develop lamination if the transplant photoreceptors can develop in normal interaction with the host or donor RPE. The fetal tissue is very fragile; any misplacement and disturbance can lead to rosette formation.
Transplantation of retina together with its RPE adds additional difficulties since the RPE does not attach well to the retina when outer segments have not yet developed. Treatment of the eye cup with Dispase before dissection is important so choroidal vessels can easily be teased away.
In larger eyes, the surgery is a completely different scenario. The instrument can be used with a standard vitreoretinal surgery approach so that the surgeon can see and be in full control of the delivery with a higher success rate than in rodent eyes (Radtke et al., 2002; Radtke et al., 2008; Seiler et al., 2009) (see success criteria in section 1.4).
However, the study in cats (Seiler et al., 2009) used nozzles for human surgery that were too large and not adapted to the smaller cat eye. In addition, as discussed in that paper, posterior surgery in cats is technically more difficult because more bleeding occurs. Therefore, only 2 of the 4 cats contained transplants, and the donor tissue had folded in on itself. Despite of these issues, the transplants were well integrated with the host retina (Seiler et al., 2009), in contrast to another cat study using a different instrument (Bragadottir and Narfstrom, 2003).
We have not done extensive studies in larger animal models for two reasons: (1) the high costs involved and lack of funding; and (2) the lack of available models at the time we did the experiments. In addition, results can be obtained much faster and in higher numbers with rats although the surgery is more difficult. The transplantation into Abyssinian cats (Seiler et al., 2009) was done at a stage of retinal degeneration when most of the outer nuclear layer was still present, with a short follow-up of 2 months. Therefore, no transplant effect could be seen by electroretinograms (ERGs).
4.5. Imaging of transplants in live rats by 3-D Ocular coherence tomography
To evaluate the placement and structural quality of the transplants in live animals, retinal transplants were analyzed using optical coherence tomography (OCT) (Thomas et al., 2006a; Seiler et al., 2010b). Initial studies were done with single scans of a Stratus OCT-3 (Thomas et al., 2006a) which gave mainly information whether the transplant had been correctly placed into the subretinal space. However, it was difficult to clearly identify laminated transplants. In a later study (Seiler et al., 2010b), using a better setup with a Fourier domain optical coherence tomography (FDOCT) system (scanning of 139 or 199 consecutive slices), the laminar structure of the transplants and surgical defects, such as RPE/choroid damage could be detected with an accuracy rate between 83 and 99%. Three-dimensional projections showed the transplant position in the retina in relation to the optic disc and the growth of the transplant.
4.6 Differentiation of retinal cell types in retinal sheet transplants and comparison with the degenerated host retina (Figures 1 + 2)
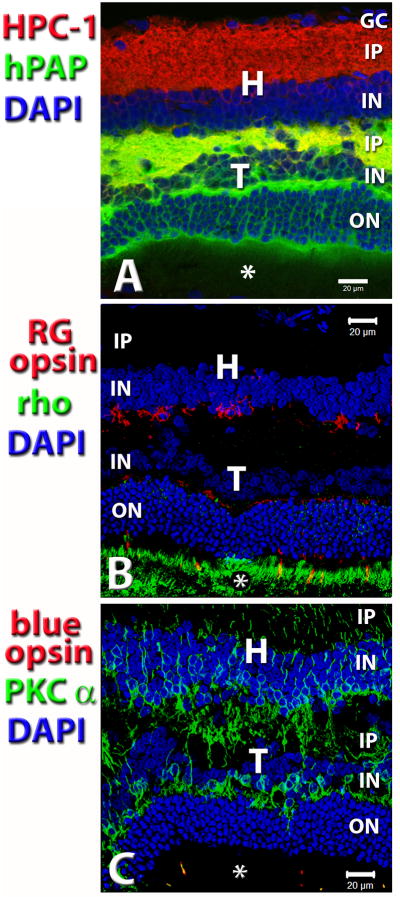
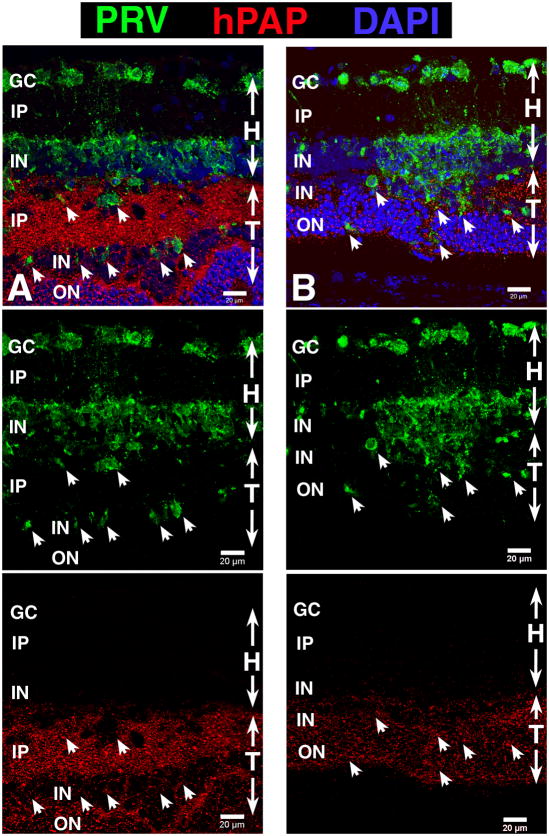
Figure 2. Analysis of marker expression and integration between transplant and host – indirect evidence that transplant is responsible for restoration of visual function.
Laminated transplant in S334ter-3 rat with responses in the superior colliculus (SC). Confocal projection stacks, stained for antibodies listed on the left side. Nuclei are stained with DAPI (blue). Photoreceptor outer segments are indicated by asterisks. Retinal transplant, age 8.5 mo., 7.4 mo. post surgery. This rat had very good visual sensitivity; the threshold was at −2.2 log cd/m2. (A) Donor cell label hPAP (green) in combination with anti-Syntaxin-1 (HPC-1; red). HPC-1 stains synaptic layers and, more faintly, the cytoplasm of amacrine cells in the inner nuclear layers. (B) red-green (RG) opsin (red) in combination with rhodopsin (green). Strong staining of transplant outer segments for rhodopsin. Note that there is no rhodopsin staining in the host retina. There are scattered cell bodies with processes of residual RG opsin immunoreactive host cones (red) at the transplant-host interface. Cone outer segments can only be seen in the transplant. Consequently, only transplant photoreceptors can send strong signals to the brain. Cone opsin also stains cone terminals in the outer plexiform layer of the transplant. (C) Double label of PKC alpha (green) which stains rod bipolar cells and blue opsin (red) that stains blue-sensitive cones. PKC staining of bipolar cells is stronger in the transplant than in the host retina. Note the good integration. Outer segments of blue cones (two samples) can only be seen in the transplant. Scale bars: 20 μm. - Reprinted with permission from Yang et al., 2010: Trophic Factors GDNF and BDNF Improve Function of Retinal Sheet Transplants. Exp Eye Res 91: 727-738 (part of Figure 7). Copyright Elsevier.
With the exception of ganglion cells, retinal sheet transplants can develop all retinal cell types, including fully differentiated photoreceptors in recipients of several retinal degeneration models (Seiler and Aramant, 1998; Aramant et al., 1999; Aramant and Seiler, 2002b) (see Figures 1A,B, 2B, 6B1). Both rods and cones develop outer segments when in contact with either host or donor RPE. In general, the inner retinal layers of the transplants are less developed, specifically the inner nuclear and plexiform layer is thinner than of the host retina. Horizontal cells often show stronger immunoreactivity for Calbindin in the transplant than in the host (Figure 1A). In addition, transplants contain many rod bipolar cells which show stronger PKC alpha immunoreactivity and a more normal mGluR6 immunoreactivity at their dendritic tips in the outer plexiform layer than bipolar cells in the host retina (Seiler et al., 2008a; Yang et al., 2010a) (examples in Figures 1B, 2C).
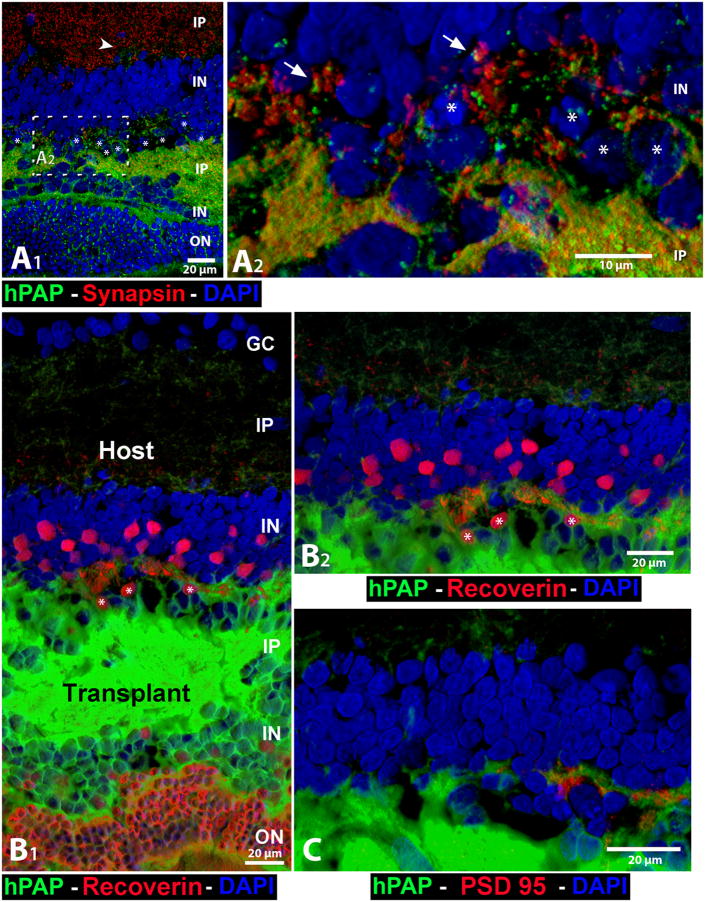
Figure 6. Interface transplant-host: cell and synaptic markers.
The cytoplasm of all donor cells, including their processes, is labeled with hPAP (green) in their cytoplasm (not the nuclei). Note the green donor cell processes in the host inner plexiform layer (IP), indicating outgrowth of processes from the transplant. All images are oriented with the host ganglion cell layer (GC) up. White asterisks (*) indicate nuclei of remnant host cones (containing clumped chromatin). All images are three-dimensional renderings of confocal stacks (not maximum intensity projections). (A1 and A2) Combination of donor label hPAP (green) and synapsin 1 (red, marker for synaptic vesicles and synaptic terminals), and DAPI nuclear label (blue). (A1) Overview. Transplant processes extend past remnants of host cones to the outer plexiform layer of the host. The arrowhead points to a group of transplant processes in the host IP that is slightly visible at this magnification, but can be clearly seen in B1, B2 and in Figure 7. The white dashed box indicates enlargement of the transplant–host interface in A2. (A2) Plentiful areas with potential transplant–host synaptic interactions (transplant processes close to red-stained synaptic structures inside the host area; only 2 examples indicated by arrows. (B1), B2), and C) Unpublished pictures of the same transplant. (B1) Recoverin (red), a marker for photoreceptors and cone bipolar cells and their processes, in combination with hPAP (green). The transplant photoreceptor layer stains strongly for recoverin. There are less, smaller-appearing recoverin-immunoreactive cone bipolar cells in the transplant IN than in the host IN. (B2) Enlargement of transplant-host interface. (C) PSD95 (marker for postsynaptic densities, red) in combination with hPAP (green) at the transplant-host interface. Note transplant process on the right, closely adjacent to PSD95-immunoreactive structures, indicating synaptic connections. E19 retinal transplant (no BDNF-treatment), age 3.0 months, 2.1 months after surgery. Scale bars: 20 μm (A1, B1, B2, C), 10 μm (A2).
(A1, A2) Reprinted with permission from Seiler et al. 2010: Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets. Eur J. Neurosci, 31(3):508-520. Copyright J. Wiley & Sons.
Synaptic layers of the transplants stain for diverse synaptic proteins (Seiler et al., 2010a) and show diverse synapse types at the electron microscope (EM) level (Seiler and Aramant, 1998; Seiler and Aramant, 2001; Aramant and Seiler, 2002b; Peng et al., 2007; Seiler et al., 2012). The inner plexiform layer of the transplant mostly lacks the lamination found in normal retina (e.g. Figure 8 in Seiler et al., 2008b), and very few, if any, retinal ganglion cells survive (unpublished observations). Recoverin-immunoreactive cone bipolar cells often appear smaller and less numerous than in the host (see Figure 6 B1).
In summary, retinal sheet transplants develop most retinal cell types with the exception of retinal ganglion cells.
4.7. Potential circuitry between transplant and degenerate host retina
In a collaboration with Robert Marc's laboratory (Moran Eye Center, University of Utah), the neurotransmitter expression of retinal sheet transplants has been investigated using computational molecular phenotyping (CMP) (Seiler et al., 2012). CMP combines immunohistochemistry of many different neurochemical markers on adjacent 0.2 μm sections for cell classification and quantitative visualization as tissue theme maps (Marc and Jones, 2002; Jones et al., 2003). The goal of the study was to investigate the potential circuitry of long term retinal fetal sheet transplants that had restored responses in SC of a degenerated host retina. The data indicated that horizontal cells and both glycinergic and GABAergic amacrine cells are involved in a novel circuitry that had generated alternative signal pathways. A normal expression of neurotransmitters of different transplant cell types and areas of neuropil fusion between transplants and host could be seen. Interestingly, amacrine and horizontal cells appeared to be the main cell types involved in connectivity between transplant and host. However, there was an overall reduction of bipolar cells both in host and graft which contradicts previous data about strong PKC alpha expression in the transplants (see section 4.6). The tissue preparation for CMP cannot differentiate between cone and rod bipolar cells. Thus, there was likely a specific loss of cone bipolar cells in the transplant inner nuclear layer which would correspond to unpublished data indicating that there are few recoverin-immunoreactive cone bipolar cells in the transplant (see also Figure 6 B1). This issue needs to be addressed in further studies.
Since transplant photoreceptor signals would have to be transmitted both through transplant and host interneurons before reaching host ganglion cells, “normal” visual processing cannot be expected. However, the data discussed in the sections 5 and 6 indicate that transplants can improve and restore visual function in recipients with retinal degeneration, although with a delay in response time.
5. Transplant photoreceptors are functional and restore visual responses in retinal degeneration models
For our studies, transplants were performed at a stage when most rod photoreceptors had degenerated. The transplants cover a small area of the retina; the initial size of the transplant (in rats) is ca. 1-1.5 mm2, and it doubles in size after full development. This corresponds to ca. 10-20 % of the retina. Thus, we have not been able to distinguish visual responses from transplants from those of host cells by a conventional corneal electroretinogram (ERG) (unpublished observations). This is similar to what has been observed by other researchers (Radner et al., 2001; Gaillard and Sauve, 2007; Pearson et al., 2012). However, it may be possible to show transplant function by more sensitive local ERG methods. Studies investigating transplant rescue effects on remaining photoreceptors have transplanted animals in earlier stages of retinal degeneration for therapeutic intervention, and have been able to find improved ERGs (e.g., Wang et al., 2010b; Tucker et al., 2011).
In addition, even if responses can be recorded from the host retina or retinal ganglion cells, it does not mean that the transplant has any effect on the visual centers in the brain. Therefore, to determine the benefits of transplants for visual function in a recipient with retinal degeneration, transplant effects should be demonstrated not only in the retina, but also in the brain. The transplant should also have an effect on visually guided behavior. It is also important to determine whether beneficial effects remain for a long time after transplantation.
5.1. Light/dark shift of phototransduction proteins in transplant photoreceptors
Photoreceptors in retinal sheet transplants can display a perfect “normal” morphology; but can they respond to light?
S-antigen (arrestin) and rod transducin shift their position within the photoreceptors depending on the light cycle (Whelan and McGinnis, 1988); this process is disturbed in different retinal degeneration models (e.g., Peng et al., 2008). To test whether the phototransduction process is working in transplant photoreceptors, light-damaged rats with retinal sheet transplants were fixed either in light or at the end of dark cycle. The immunoreactivity of transplant photoreceptors for S-antigen (arrestin), α and γ-transducin, and rhodopsin was compared between dark- and light-adapted rats (Seiler et al., 1999).
Rhodopsin distribution was unchanged in light and dark adapted rats as expected. However, S-antigen shifted from outer (in the light) to inner segments (in the dark); α-transducin and γ-transducin shifted from inner (in the light) to outer segments (in the dark). Transducin immunoreactivity of transplant photoreceptors was considerably stronger in laminated than in disorganized transplant areas.
In summary, transplant photoreceptors can perform the phototransduction process similar to normal photoreceptors.
5.2. Transplant effect on visual behavior
Del Cerro et al. (del Cerro et al., 1991) used the indirect “startle reflex” to show that subretinal aggregate transplants had an effect on the vision of light-damaged recipients. This test warns rats with a light flash before exposing them to a loud noise. Rats with vision show less reaction to the noise because they have been pre-warned. Transplanted light-damaged rats showed a 20% less reaction (jumped 20% less) to the noise than non-transplanted light-damaged controls which was interpreted as a 20% restoration of vision. This paper created quite a stir when it was published; however it generated very skeptical responses from other vision scientists because it was only an indirect test to a simple light flash which did not involve any higher aspects of vision.
Kwan et al. used another indirect test of light-dark preference to show that the dark preference is restored after retinal transplantation (Kwan et al., 1999).
Another commonly used test, the pupillary light reflex (PLR), was established by Ray Lund and colleagues to show function of retinal transplants to the brain of newborn (Klassen and Lund, 1987) or adult recipients (Klassen and Lund, 1990; Radel et al., 1995), and was then later used to test photoreceptor sheet transplants (Silverman et al., 1992b), RPE transplants to RCS rats (Klassen et al., 2001) and photoreceptor precursors (MacLaren et al., 2006). In the MacLaren study (MacLaren et al., 2006), rho−/− mice with photoreceptor progenitor transplants showed 50% pupil constriction at about 0.6 log unit lower irradiance (ca. 13.5 log photons per s−1 cm−2) than sham injected rho−/− mice (ca. 14.1 log photons per s–1 cm–2). The increase in sensitivity to the sham-injected eye (max. 0.7 log units) correlated with the number of integrated photoreceptors. To put this into perspective: normal mice had 50% pupil constriction at ca. 10.8 log photons per s−1 cm−2, a 2.7 log difference. However, the PLR is an autonomous reflex and not related to the number of photoreceptors (Kovalevsky et al., 1995) and mediated by intrinsically photosensitive melanopsin-expressing retinal ganglion cells (ipRGCs) in models of retinal degeneration (Semo et al., 2003) which normally receive synaptic inputs from rods and cones (Guler et al., 2008). Since the peak action spectrum of melanopsin ganglion cells is at 380 nm, it would be better to use blue and red light at lower light intensity. The PLR is very variable and depends on transplant location; many responses need to be averaged, and gas anesthesia should be used (Klassen et al., 2001). Thus, PLR testing has to be interpreted with caution and can only give some information about the function of transplants if contributions from melanopsin ganglion cells are eliminated, and appropriate controls are used.
Mice are normally active in the dark. The wheel running activity of normal mice in the dark is completely suppressed by exposure to light. Using this indirect test, Klassen et al. (Klassen et al., 2004b) showed that the running activity of rho−/− mice transplanted with cultured retinal progenitor cells was temporarily suppressed by exposing mice to different light levels at 8 weeks, but not 25 weeks after transplantation. Activity suppression of transplanted mice had a higher threshold than that of normal mice, but a lower threshold than non-transplanted rho−/− mice which only responded to the highest light intensity. This indicated that the transplantation of retinal progenitor cells restored some light sensitivity at 8 weeks after transplantation, but the effect had disappeared at 25 weeks.
The most widely used and accepted test of visual function is the optokinetic nystagmus (OKN) (McGill et al., 1988). Head tracking of experimental animals to moving stripes of varying widths shows thresholds of spatial frequency (discrimination of black and white stripes) and thus provides a measure of visual acuity. It can also be used to test contrast sensitivity (Thomas et al., 2010). It has been systematically used to investigate the progression of retinal degeneration (Thaung et al., 2002; Thomas et al., 2010) and the efficacy of RPE and other cell transplants (Hetherington et al., 2000; McGill et al., 2007; Wang et al., 2008b; Lu et al., 2010; Pearson et al., 2012).
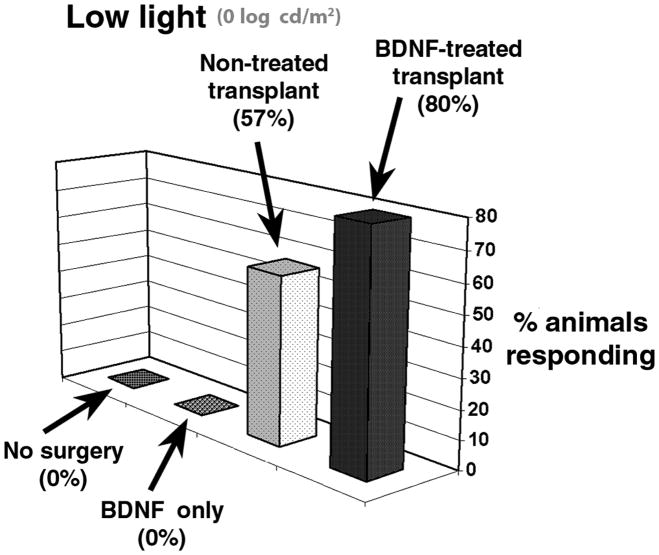
Our group modified the optokinetic drum to stimulate each eye separately and showed that retinal sheet transplants preserved visual acuity as determined by head tracking in S334ter-3 rats (Thomas et al., 2004a) and that BDNF microsphere treatment improves the functional effect of retinal sheet transplants (Seiler et al., 2008a) (see section 5.4).
The water maze test in which rats have to find a hidden platform indicated by visual cues, was used to demonstrate photoreceptor rescue in RCS rats by human fetal RPE transplants (Little et al., 1998), and to characterize the time course of degeneration in the RCS rat (McGill et al., 2004). After transplantation of NRL-GFP+ photoreceptor precursors to Gnat−/− mice that lack functional rods, 4 of 9 transplanted mice were able to find a hidden platform in dim light more than 70% of the time whereas sham surgery controls only found the platform by chance (50%) (Pearson et al., 2012). The performance in the water maze task was correlated to the number of integrated photoreceptors.
In summary, behavioral testing methods show variable reliability. Optokinetic testing is relatively good but not as sensitive to correlate with the quality of the transplant as the electrophysiological recording in the brain, but both methods complement each other.
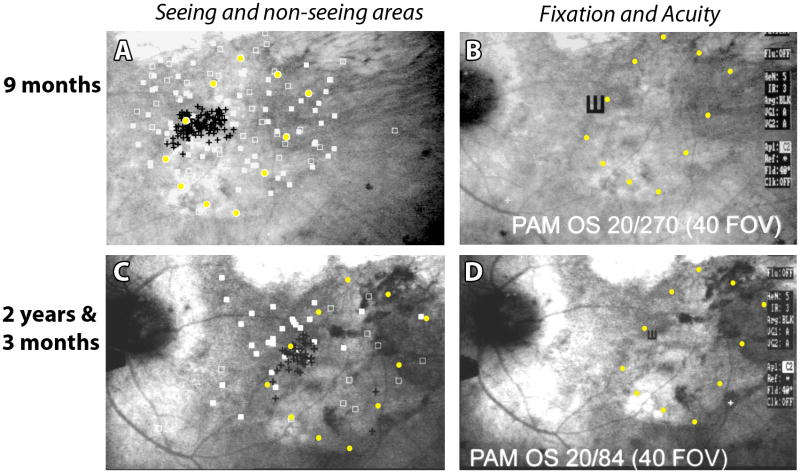
5.3. Visual function of transplants shown by electrophysiology (Figure 3)
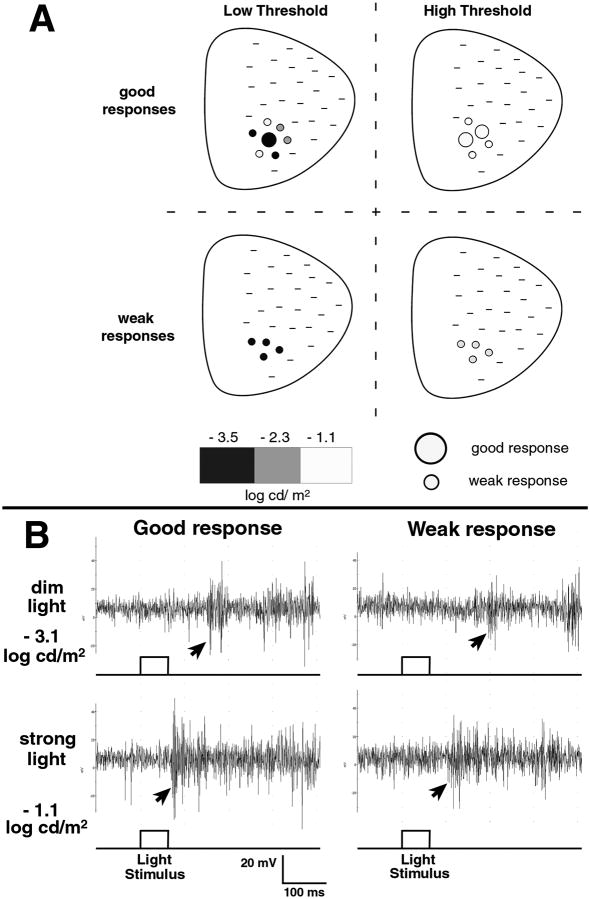
Figure 3. Brain recording after light stimulus to the eye showing the light sensitivity of transplants – electrophysiology in the superior colliculus (SC).
(A) Response characteristics in the SC from transplanted retinal-degenerate S334ter-3 rats using a 60 ms light stimulus (schematic diagram). Modified recording setup for obtaining rod-specific responses (Thomas et al., 2005). Response thresholds were determined by testing at different light intensities (−3.5 to −1 log cd/m2), indicated by black, gray, and white, from an area of the SC corresponding to the transplant location in the eye. Lower thresholds indicate higher light sensitivity and more rod involvement in the response. Examples of 4 different response types are presented.
(B) Recordings from 2 different transplanted rats (left and right column) at two different light intensities (−3.1 and −1.1 log cd/m2) to a 60 ms light stimulus. The onset of the visual response is indicated by arrows. ‘Good-response’ recordings are characterized by robust spike activity in response to increase in stimulus strength, whereas such an increase in spike activity is less apparent for ‘weak responses’. Such responses cannot be found in sham surgery controls at these light intensities. Good responses in dim light indicate restoration of light sensitivity to a higher level than before transplantation.
(A), (B) Reprinted with permission from Seiler et al., 2008. Retinal transplants restore visual responses - Transsynaptic tracing from visually responsive site in the superior colliculus (SC) labels transplant neurons. Eur J. Neurosci, 28:208-220. Copyright Elsevier.
5.3.1. Ganglion cell recordings and electroretinograms
Corneal full-field ERGs can give information about an overall visual response of the retina, but are not sensitive enough in most cases to discriminate whether a change is due to transplant photoreceptor activity directly or to an indirect trophic effect of the transplant on residual host cones. For example, in severely degenerated RP patients, responses to full-field ERGs are often absent although the patients still have measurable visual acuity (e.g., Granse et al., 2004; Wu et al., 2010). RCS rats have essentially a flat ERG at 3 months (Sauve et al., 2004) whereas they still can perform in a water maze task up to 8 months (McGill et al., 2004). In the rd mouse, transplants of retinal progenitors or photoreceptor precursor had no effect on ERGs (Radner et al., 2001; Pearson et al., 2012). On the other hand, trophic transplant effects on ERGs have been shown in several studies (small responses at higher light intensities). After transplantation of hESC-derived photoreceptor precursors to CRX −/− mice (which have no ERG responses), a small ERG response to a strong light flash could be shown. This correlated with the number of integrated photoreceptor precursor cells (Lamba et al., 2009). Similarly, Tucker et al. (2011) showed an increase in the ERG b-wave by 95 μV after transplantation of mouse iPS cell-derived retinal progenitors to rho−/− mice 4 weeks after surgery. Yang et al. showed an increase in photopic ERG b-wave amplitudes in P23H rats 6 months after transplantation of photoreceptor or retinal sheets (Yang et al., 2010b) which correlated with host cone rescue.
Multifocal ERGs can record local retinal responses using a randomized hexagonal stimulus array (Hood, 2000). Our group has also tried multifocal ERGs to determine a transplant-specific response in the retina. Compared to non-surgery rats, an elevated activity was found in the retinal area corresponding to the transplant placement (Thomas et al. unpublished observations). However, the standard setup of the multifocal ERG that we used was specific for stimulating cones (Seeliger et al., 2000). There was still activity of residual host cones in other retinal areas and therefore the transplant-specific rod responses could not be interpreted with certainty. Light-damaged minipigs transplanted with human fetal retina-RPE sheets without immunosuppression showed a small improvement of responses in multifocal ERG (mfERG) up to 8 months post surgery (Li et al., 2009). However, this was also seen in pigs without any histologically verified grafts (but not in sham surgeries).
In theory, it should be possible to pinpoint the exact location of visual responses in the retina to the location of the transplant by in vitro recording from the transplanted retina.
Adolph recorded transient spike potentials and local ERGs in vitro from the surface of subretinal aggregate grafts (isolated from the normal rabbit host retina 45-60 d after transplantation) showing that transplant photoreceptors responded to light (Adolph et al., 1994). These transplants could easily be separated from the host retina and contained photoreceptors in rosettes. Demonstrating visual responses of transplant photoreceptors in vitro did however not tell whether transplants could have any effect on a degenerating host retina.
It is more difficult to maintain a vascularized rodent retina alive for in vitro recording. Radner et al. found ganglion cell responses about 1 month post-transplant in 30% of rd mice that received retinal aggregate transplants to the subretinal space at the early age of 13 days, but not in rd mice transplanted at a later stage of degeneration (8 weeks) (Radner et al., 2001). However, using the same technology, the same group recorded ganglion cell responses to light in non-treated 6 week old rd mice and 3 month old homozygous albino S334ter line-3 rats which had no detectable corneal ERGs (An et al., 2002). Thus, responses to light can be recorded from ganglion cells even in the absence of photoreceptors, likely from melanopsin immunoreactive ganglion cells which are responsible for maintaining the circadian rhythm even after severe retinal degeneration (Semo et al., 2003; Vugler et al., 2008b) (see section 5.2).
The finding of increased ganglion cell responses by in vitro recording after transplantation of photoreceptor precursor cells to rho−/− mice was claimed as visual improvement (MacLaren et al., 2006). This should however be interpreted with caution (Gaillard and Sauve, 2007).
Sum up: ERGs and ganglion cell recordings are not sensitive enough to give reliable information about the function of transplant photoreceptors in the retina. In addition, recording from the retina does not prove that the transplant transmits any signals to the brain.
5.3.2. Recordings from the brain
On the other hand, recording from the SC (Sauvé et al., 1998) will provide information both about topography of ganglion cell responses and whether the transplant can communicate with the host (Gaillard and Sauve, 2007). SC and cortical recordings are established methods to study the effect of RPE transplantation in RCS rats (Sauvé et al., 1998; Girman et al., 2003, 2005). Because RPE transplants affect a larger retinal area in RCS rats than retinal sheet transplants in our rat models, Sauve et al. could establish a correlation between ERGs and SC visual field thresholds (Sauve et al., 2004).
Systematic recording of visual responses from the surface of the SC provides a retinotopic map and gives indirect information about photoreceptor activity in the retina. For our studies, a procedure was developed to expose the surface of the SC (contralateral to the surgery eye) by removing the overlaying cortex. The surgery eye was exposed with a full-field light flash (0.1 ms), in earlier studies with an intensity of 1300 cd/m2 (3.11 log cd/m2) (Woch et al., 2001; Sagdullaev et al., 2003; Thomas et al., 2004b), and multiunit visual responses were recorded. In all our studies, the light intensity of the stimulus was measured as cd/m2, not as cd.s/m2 as standard set by the International Society of Electrophysiology of Vision (ISCEV).
Woch et al. (2001) showed restoration of visual responses in 65% of albino RCS rats after transplantation of fetal (E19-E20) retina-RPE sheets in a small area of the SC that topographically corresponded to the placement of the transplant in the retina. These responses were significantly different (stronger responses, shorter latencies) from visual responses found in 46% of sham surgery rats. Similar results were also demonstrated with E19 retinal sheet transplants in the pigmented S334ter line 3 rat model (Sagdullaev et al., 2003) with the main difference that no responses were found in age-matched sham surgeries. Again, transplant responses were characterized by significantly longer response onset latencies than in normal rats.
The next study, in a model of slow retinal degeneration, the albino S334ter line 5 rat, posed the challenge to distinguish transplant activity from residual host cone activity (Thomas et al., 2004b). One had to wait with recording until a host age of 6.6 to 8.5 months when most of the visual activity in the host SC would have disappeared without treatment. A scotoma started to develop in the caudal SC from 3.6 months of age, and finally covered most of the SC by the age of 8.5 months. The quality of transplant visual responses was correlated with transplant lamination; “good” transplants showed latencies shorter than 90 ms and strong responses corresponding to normal control rats, whereas “poor” disorganized transplants had longer response latencies and weak responses.
On the other hand, there was no correlation between transplant organization and visual responses in the SC with retinal sheet transplants in rd mice (Arai et al., 2004). In this model, the 43% transplanted rd mice with visual responses showed a normal latency response to bright light and increased recoverin immunoreactivity in the host retina. This clearly meant a rescue of host cones which also corroborated results of other groups (Mohand-Said et al., 1997; Mohand-Said et al., 2000). This served a word of caution when comparing different retinal degeneration models.
Since the high intensity of the light flash primarily activates cones; and cones still remain in host retina for a long time, the light stimulus was reduced in intensity (between −1 and −5 log cd/m2) and increased in duration (from 0.1 ms to 40-60 ms) to record more of the rod activity as described in Thomas et al. (2005). This visual stimulation setup was inspired by a study of visual thresholds in the SC of RCS rats without and with RPE grafting (Girman et al., 2005). – In non-surgery S334ter-3 rats older than 3 months, no responses to these light levels (between −1 and −5 log cd/m2) can be recorded. Transplanted S334ter line 3 rats (age 3 months and older) show visual thresholds down to the mesopic range (−3.5 log cd/m2) - significantly better responses than the higher threshold (−0.09 log cd/m2) of young (1.5 month old) non-transplanted rats (Thomas et al., 2006b). This demonstrates that transplants increase the sensitivity to light.
This improved recording setup has been used for a study comparing the effects of BDNF and GDNF microsphere treatment (Yang et al., 2010a) (see section 5.4.) and a trans-synaptic tracing study (Seiler et al., 2008b) (see section 6.2). Figure 3 shows a comparison of response qualities between different transplants using this setup.
In summary, our unique SC recording consists of exposing the SC surface so a more exact systematic mapping is possible in combination with trans-synaptic tracing (see section 6.2). Using this setup, visual responses after transplantation have been demonstrated in different retinal degeneration models up to 10 months post surgery.
5.4 Improvement of transplant effects by growth factor treatment (Figure 4)
Figure 4. BDNF pretreatment of donor tissue improves transplant function – while BDNF treatment alone has no long-term effect in this retinal degenerated model.
S334ter line 3 rats received E19 retinal sheet transplants with or without BDNF microsphere coating in one eye at the age of P24-37. SC recordings to light were done ca. 60 days post-surgery, at the age of 11-14 weeks. Responses were only found in the transplanted groups. A higher percentage of rats with BDNF treated transplants than rats with non-treated transplants responded to low light (1 cd/m2). Rats that received only injection of BDNF microspheres without transplant had no responses.
Reprinted with permission from Seiler et al., 2008: BDNF-Treated Retinal Progenitor Sheets Transplanted to Degenerate Rats - Improved Restoration of Visual Function. Exp Eye Res. 86(1): 92-104 (part of Figure 5). Copyright Elsevier.
In most transplantation experiments, restored visual responses in the SC were apparent only in a restricted area indicating that the area of connectivity between the transplant and the host retina is limited. In order to improve functional outcome and synaptic connections, brain-derived neurotrophic factor (BDNF) and glial cell-line derived neurotrophic factor (GDNF) were introduced which have well-established neuroprotective effects on the retina during development (von Bartheld, 1998; Rohrer et al., 1999; Grishanin et al., 2008) and photoreceptor degeneration (Frasson et al., 1999; McGee Sanftner et al., 2001; Chaum, 2003).
In a first study (Seiler et al., 2008a), the donor tissue was incubated with positively charged BDNF – containing PLGA microspheres before transplantation. Such microspheres stick to the donor tissue and slowly release the factor over a time period of 4 -8 weeks. As a control, rats were injected with BDNF microspheres only. S334ter line 3 transgenic rats received transplants at the age of 3.5 to 5 weeks. Optokinetic head tracking responses of rats with BDNF-treated transplants to a stimulus of 0.25 cd/m2 were significantly improved compared to non-treated transplants. Interestingly, injection of microspheres alone had a transient improvement effect of head tracking seen at the age of 8 weeks which was, however, not sustained at the age of 11 weeks. Visual responses in the SC were recorded to 2 different light stimuli: “low light” (1 cd/m2) and “strong light” (1300 cd/m2) ca. two months after transplantation. BDNF treatment of transplants increased the percentage of rats responding to “low light” in area of the caudal SC corresponding to the transplant placement in the retina (Figure 4). At strong light, rats with BDNF treated transplants showed a larger response area with stronger responses, i.e. significantly shorter response onset latency and larger amplitude. Visual improvement was however not correlated with transplant organization. Control rats injected only with BDNF microspheres showed no responses in the caudal SC and long latency responses in the rostral SC with “strong light”; and no responses anywhere to “low light”.
In a second study (Yang et al., 2010a), BDNF microsphere pretreatment of retinal sheet transplants was compared with GDNF pretreatment. This study used a different, longer duration 40 ms stimulus, and lower light levels to specifically stimulate rods, similar to the stimuli used earlier (Thomas et al., 2005; Seiler et al., 2008b). Retinal transplants with and without treatment of BDNF and GDNF were compared with sham surgery and cortex-transplanted age-matched rats. Responses to a full field light stimulus of −5 to −1 log cd/m2 were recorded in the SC. In this study, transplant lamination was clearly correlated with visual threshold and the size of the visually responsive area in the SC. BDNF-pretreatment improved the threshold of laminated transplants whereas GDNF pretreatment improved the threshold of rosetted (disorganized) transplants. Although GDNF had no effect on visual threshold of laminated transplants, the visually responsive SC area of GDNF-treated laminated transplants was significantly larger than in other experimental groups. However, a difference between GDNF or BDNF treated transplants could not be seen histologically.
In summary, pretreatment of donor retinal sheets with trophic factor-containing microspheres significantly improved the functional effect of retinal transplants. Injection of trophic factors alone had no long-term effect.
5.5. Mechanism of transplant effect
Although fetal-derived retinal transplants can restore visual responses to low light, transplanted rats are not sensitive at the rod-specific scotopic light level, only at the mesopic level corresponding to mixed cone and rod responses (Thomas et al., 2006b). Thus, it has been argued that retinal sheet transplants do not directly transmit light information to the host retina via synapses and only have a rescue effect on host cones (Yang et al., 2010b). In rd mice, host cone rescue has been observed after photoreceptor sheet transplantation (Mohand-Said et al., 1997; Mohand-Said et al., 2000) and retinal sheet transplantation (Arai et al., 2004), as well as after retinal or photoreceptor sheet transplantation to P23H rats, a slow retinal degeneration model (Yang et al., 2010b). However, no cone rescue has been observed in other rat models of retinal degeneration after transplantation of retinal sheets (Woch et al., 2001; Sagdullaev et al., 2003; Thomas et al., 2004b; Seiler et al., 2008a) although a beneficial trophic effect on the host retina is likely also involved because of the fetal donor tissue. After transplantation of BDNF microsphere-pretreated fetal retinal sheets to S334ter-3 rats, visual responses were recorded in the SC in an area topographically corresponding to the transplant placement in the retina (Seiler et al., 2008a). Counts of the few remaining degenerating host red-green cones in the transplant area, and in the central and peripheral retina outside the transplant revealed no correlation of the cone distribution to the location of visual responses in the SC. The residual host cones only consisted of cell bodies with no outer or inner segments. In the BDNF treated group that showed improved SC responses, fewer residual host cones were found in the transplant area than in the host retina outside the transplant – opposite to what would have been expected if rescue of host cones was the origin of the visual responses. This was indirect evidence that the recorded visual responses mainly came from the thousands of fully developed healthy transplanted rod and cone photoreceptors. – In SC recordings from transplanted rd mice, the response latencies were indistinguishable from those of normal mice which clearly indicated host cone rescue (Arai et al., 2004). On the other hand, response latencies were longer in the rat models (Woch et al., 2001; Sagdullaev et al., 2003; Thomas et al., 2004b; Seiler et al., 2010a; Yang et al., 2010a) which provides also indirect evidence that these responses were mediated by graft-host connections because the chain of synapses between transplant photoreceptors and host ganglion cells is likely longer than in the normal retinal circuitry (see section 6).
6. Connectivity
It has been claimed that retinal sheet transplants cannot connect because the outer limiting membrane of the host retina (intermediate junctions between Muller cells and photoreceptors) forms a barrier. However, there are several strong experimental data for transplant-host connectivity.
6.1. Morphological indications for transplant-host connectivity
It was thought that the presence of an outer nuclear layer with an outer limiting membrane in the host presents a barrier for graft-host integration (Zhang et al., 2003). Silverman's studies showed that hardly any integration took place because a barrier was created between transplant and host (Silverman et al., 1992b). Therefore, several groups developed models to disrupt the outer limiting membrane genetically (Kinouchi et al., 2003) or pharmacologically (West et al., 2008). Ghosh et al. even developed a model to induce local photoreceptor degeneration in the recipient by implanting a biodegradeable membrane (Ghosh et al., 2011).
In contrast, our less traumatic transplantation method resulted in apparently “fused” transplants in several retinal degeneration models where even on the EM level, the difference between transplant and host could not be seen (Seiler and Aramant, 1998; Seiler and Aramant, 2001; Seiler et al., 2009) and transplants extended processes into the host (Peng et al., 2007; Seiler et al., 2010a). The glial membrane that sometimes can be seen in the transplant-host interface appears to consist of a disrupted hollow mesh that transplant processes have no problem penetrating. When using alginate as embedding medium, there was often a separation of transplant and host because the gel was not biodegradable (unpublished observations). This issue was absent when using biodegradable matrigel or unembedded donor tissue. Besides the fusion seen at the EM level (Seiler and Aramant, 1998; Seiler and Aramant, 2001), another indication for connectivity was crossing of rod bipolar (PKC alpha immunoreactive) processes (Seiler et al., 2008a; Yang et al., 2010a) (example in Figure 2); extension of transplant processes into host (Seiler et al., 2008a; Seiler et al., 2010a) (Figures 6 and 7); and the presence of synaptic markers at the transplant-host interface (Seiler et al., 2010a). In a recent study, using molecular phenotyping, it was shown that transplant and host neuropil fused in several areas, and that amacrine and horizontal cells were involved in the connectivity (Seiler et al., 2012) (see section 4.7). A more clear demonstration of synaptic connectivity was done in the studies described in sections 6.2 and 6.3.
Figure 7. Transplant processes invading host retina (transplants with visual responses in SC).
The cytoplasm of all transplanted cells, including their processes, is labeled by immunohistochemistry for human placental alkaline phosphatase (hPAP). Arrows indicate a dense donor-derived fiber plexus close to the GCL and close to the INL of the host retina (not so clear in the thin section, B). The two pictures are interesting with their difference in thickness. (A) is a 80 μm thick vibratome section and (B) is a semithin 1-μm section. Both pictures show the distribution of the donor processes all over the host inner plexiform layer and close to the ganglion cells (see also Figure 6 B1 and B2).
(A) In this thick section, a dark dense process layer is seen under the GC and another less dense layer towards the host inner nuclear layer. (A) BDNF-treated transplant, age 3.6 months, 2.4 months after surgery. (B) BDNF-treated transplant, age 6.9 months, 6 months after surgery. Scale bars: 50 μm (A), 20 μm (B).
In another study with a different animal model, the rd mouse, retinal sheet transplants did not integrate well, and there was no correlation between transplant lamination and integration and visual responses (Arai et al., 2004). Similar to this, Gouras et al. showed that host bipolar cells made close contacts, but did not form synapses with transplanted rods in the rd mouse (Gouras and Tanabe, 2003).
Using a more traumatic implantation method, the so-called “full thickness” retinal sheet transplants integrated with the non-vascularized normal rabbit retina only after long survival times when the host photoreceptors had degenerated (Ghosh et al., 1999a), but remained separated from the host in the vascularized pig (Ghosh and Arner, 2002; Ghosh et al., 2004) and cat retina (Bragadottir and Narfstrom, 2003).
In summary, fetal retinal sheet transplants can integrate with the host retina in several retinal degeneration models, with absent transplant-host barriers.
6.2. Trans-synaptic tracing from visually responsive site in the host brain to the transplant in the eye (Figure 5)
Figure 5. Tracing from visually responsive site in brain (superior colliculus, SC) to transplant in the eye - – Indirect evidence of connectivity using pseudorabies virus (PRV).
After electrophysiological recording in the SC, the trans-synaptic tracer PRV was injected into the area that had visual responses. Rats were sacrificed 2 d after virus injection, and PRV was detected in the eye by immunohistochemistry. (A), (B) hPAP (donor cell label) (red) and PRV (green), with blue counterstain of nuclei (DAPI). White arrowheads indicate PRV-labeled transplant cells. The merged image is on top; the 2nd row shows PRV (green); the 3rd row shows hPAP (red). - There is more inner plexiform layer in the transplant in A) (stronger hPAP stain) whereas the transplant area in B) contains more nuclei (less hPAP stain since hPAP is not found in nuclei. (A) Rat age 17.7 weeks at the time of recording, visual threshold −2.9 log cd/m2: Labeled cells in transplant close to the host and in inner nuclear layer (IN). (B) Rat age 16.3 weeks, visual threshold −3.5 log cd/m2. Labeled cells in transplant IN and outer nuclear layer (ON). There is less hPAP stain in the transplant in B) because the transplant area contains more nuclei. Transplant ON close to host IN. Scale bars: 20 μm - Reprinted with permission from Seiler et al., 2008. Retinal transplants restore visual responses - Transsynaptic tracing from visually responsive site in the superior colliculus (SC) labels transplant neurons. Eur J. Neurosci, 28:208-220. Copyright Elsevier.
Since transplant retinal ganglion cells are absent but the transplant contains an inner nuclear layer with different types of retinal interneurons, and the host retina has only few remaining cone photoreceptors, light-evoked responses in the SC suggest that transplant photoreceptors are likely connected to the host ganglion cells via a chain of interneurons. Therefore, we needed to use transsynaptic tracing to demonstrate connections.
Attenuated pseudorabies virus (PRV) (PRV Bartha and derived recombinant strains, such as PRV-Bablu or PRV-152) are established methods to label multi-synaptic circuitry (Card, 1998). These viruses are specifically transferred between neurons at synapses and are only transported in retrograde direction (Card et al., 1993). Virus is only transferred between neurons over functional synapses (Rinaman et al., 2000). Only neurons, not glial cells, can assemble the virus envelope necessary for the transfer of the virus to another cell (Card et al., 1993). Astrocytes enclosing a synapse with their processes will also get infected, but cannot assemble the virus envelope, thus preventing virus spread (Card et al., 1993; Card, 1998). The virus can enter a glial cell but cannot come out. PRV tracing has been used in the visual system to trace the circuitry of melanopsin expressing retinal ganglion cells (Viney et al., 2007). Once the virus has entered a neuron, it will take some time for virus replication and assembly of the virus envelope. The label of a synaptic chain thus depends on the time after virus injection. The timing is different for different neural circuits. However, eventually, the virus will reach important brain centers and kill the animal.
In our first study, PRV labeling patterns were established after injection into the exposed surface of the SC of normal rats and of RCS and S334ter line 5 rats with long-term co-transplants of retina with RPE (2-10 months post surgery) (Seiler et al., 2005).
The optimal tracing time between SC and retina was 2-3 days as determined by PRV injection into normal (non-transplanted) rats. PRV was lethal 5-6 days post-injection. One day after injection, PRV labeled retinal ganglion cells, followed by label of Muller cells at 35 hours, and then label of inner retinal neurons and photoreceptors at 2-3 days. Muller cell label in the retina corresponds to the astrocyte label in the brain. EM analysis of the retina confirmed that enveloped virus was only found in neurons and not in Muller glia. PRV was found in two-thirds of the transplants 2-3 days after virus injection. If the virus injection accidently missed the responsive SC area corresponding to the placement of the transplant in the host retina, no PRV was found in the transplants. In the transplants, because of the timing of sacrifice after virus injection, PRV was mainly found in retinal interneurons, rarely in photoreceptors. The low labeling rate of transplant photoreceptors indicated that there is a chain of several synapses between host ganglion cells and transplant photoreceptors.
This study (Seiler et al., 2005) provided another indirect proof of synaptic connectivity between retinal sheet transplants and degenerated host retina. However, it did not show whether there was any correlation between connectivity and visual responses in the SC.
Therefore, another study was designed to combine recording of visual responses with transsynaptic tracing in S334ter line 3 rats with retinal sheet transplants (Seiler et al., 2008b). This posed an additional level of difficulty because the virus-injected rats needed to be kept alive for two days after several hours of recording with part of their cortex removed. First, the area that responded to light and its visual threshold in the SC had to be determined, and then virus had to be injected into the exact same area. Two days after the virus injection, PRV was found in the host retina overlying the transplant and in many transplant cells. Figure 5 shows an example of virus-labeled cells in the transplant. Rats with “moderate to good” PRV label in the transplants showed significantly lower visual thresholds (−3.25 log cd/m2) in SC recording (i.e., were more sensitive for light) than rats with “poor” label (−2.2 log cd/m2) (see Figure 3 for response characteristics).
Double staining of PRV-labeled cells in selected transplants for different retinal cell markers showed that 25% stained for the markers MAP2 and NeuN and only13% stained for the glial marker glutamine synthetase, i.e. most of the labeled cells were neurons; 8% were PKC-immunoreactive rod bipolar cells. However, although the transplant photoreceptors were strongly immunoreactive for recoverin, few photoreceptors in the transplants labeled for PRV. This indicated that the connectivity with the host was mainly through transplant interneurons, confirming the previous study.
This study (Seiler et al., 2008b) demonstrated that synaptic connections are involved in the restoration of visual responses in the SC by retinal transplants, in addition to trophic effects.
However, it was argued by Yang et al. (Yang et al., 2010b) that “the density of these connections should be higher and cover a larger surface in order to restore a more satisfactory visual function”; they meant that the connections shown in our studies were not sufficient to trigger visual responses and thus were purely due to a paracrine effect. This is a misinterpretation of the experimental design since the virus tracing procedure could only label a small selected area of the SC, and only a small subset of synaptically connected transplant neurons. In the next study, we used the donor cell label hPAP for identification of transplant-host synapses.
6.3 Direct demonstration of synaptic connectivity (Figures 6-8)
Transgenic rats expressing human placental alkaline phosphatase (hPAP) in the cytoplasm (not the nucleus) of all cells (Kisseberth et al., 1999) and consequently also in all processes, were used as donor tissue for transplantation of E19 retinal sheets to transgenic S334ter line 3 rats. The hPAP label can be detected both by histochemistry and immunohistochemistry. Confocal analysis of sections double-stained for hPAP and synaptic markers revealed an extensive outgrowth of transplant processes past remnants of host cones into the host inner nuclear and inner plexiform layers (Seiler et al., 2010a) (Figure 6). Colocalization with the synaptic markers synapsin 1 (Fig. 6A), syntaxin (HPC-1), synaptophysin and mGluR6 indicated that the processes contained both presynaptic and postsynaptic elements. Transplant processes at the border between host inner nuclear layer and inner plexiform layer appeared to be postsynaptic, as seen with staining for the ribbon synapse marker bassoon. Staining for the post-synaptic marker PSD95 showed several presynaptic transplant processes adjacent to PSD95-immunoreactive processes in the host (Fig. 6C). Because of the dense staining of synapses in the host inner plexiform layer with several synaptic markers (synapsin, syntaxin, synaptophysin and bassoon), it could not be determined whether transplant processes in the host inner plexiform layer were presynaptic or postsynaptic.
Vibratome slices were immunostained for hPAP (examples in Figure 7) and processed for electron microscopy. Electron microscopy confirmed the multiple extensions of transplant processes into the host retina and the presence of synapses between donor processes and unlabeled (presumably host) cells in the inner plexiform layer of the host (Figure 8).
However, the study was limited in that the hPAP antibody only penetrated 10 μm of the surface of the 80-100 μm vibratome sections so the processes could not be followed continuously. Thus, it was very difficult to quantify the data.
In summary, this was the first study to unequivocally demonstrate synaptic connectivity of retinal sheet transplants on the EM level (Seiler et al., 2010a). Combining the different connectivity studies and the electrophysiological confirmation of the anatomical connectivity demonstrated in several different ways a strong proof of synaptic communication between transplant and host and invasion of transplant processes with ample opportunities for appropriate connections.
7. Clinical application
7.1. RPE and IPE transplantation
In the 1990s, the success of RPE transplants in RCS rats led to clinical trials of fetal allogeneic RPE transplants in ARMD patients by a team in Sweden in collaboration with Columbia University, NY (Algvere et al., 1999) and in the U.S. (Weisz et al., 1999). Another group transplanted allografts of adult RPE to ARMD patients (Del Priore et al., 2001). The results were mixed. Slow rejection was observed depending on the stage of retinal degeneration, the presence of an intact blood brain barrier, and immunosuppression (see Binder et al., 2007 for review).
To overcome the rejection problems with allogeneic RPE, different groups have performed autologous transplants of adult RPE cells (Binder et al., 2007; Krebs et al., 2008; Ma et al., 2009) and iris pigment epithelial (IPE) cells (Abe et al., 2000b; Thumann et al., 2000) (review Abe et al., 2007), mostly to patients with “wet” age-related macular degeneration (ARMD). Improvements in visual acuity (subjective) and reading speed were reported.
In summary, these early clinical trials with RPE and IPE cell suspensions resulted in cell rejection. Autologous cell transplants had only temporary effects. Because the autologous cells still carry the same gene defects as the patient, results should be taken with caution, and their usefulness could be limited.
7.2 RPE/choroid translocation
Later, techniques were developed to translocate in a patient's eye a patch of RPE with choroid (Heussen et al., 2008; Maaijwee et al., 2008; Chen et al., 2009b; Caramoy et al., 2010; Cereda et al., 2010) or a patch of iris epithelium (Aisenbrey et al., 2006) to the subfoveal space, mainly in patients with exudative (“wet”) ARMD. Some patients improved, but the visual outcome was mixed. These procedures might give a positive outcome, but presently the techniques need to improve because the procedures are very traumatic and have frequent side effects like retinal detachment (Chen et al., 2009a). In a comparative study of 12 patients in each group, visual acuity of ARMD patients was maintained after 3 years with macular translocation, but not with the choroid/RPE patch graft (Chen et al., 2009a).
A recent study of 130 patients that received RPE/choroid patch grafts after subretinal membrane removal between 2001 and 2006, concluded that “RPE-choroid graft transplantation may maintain macular function for up to 7 years after surgery, with relatively low complication and recurrence rates” (van Zeeburg et al., 2012). The study was difficult to interpret because the number of patients dropped with years follow-up, from 130 eyes after 1 year to 46 eyes at 4 years, and 9 eyes at 7 years. There was no control group. 10% of the patients developed proliferative vitreoretinopathy or recurrent neovascularization. Preoperatively, 40% of the patients had a best corrected visual acuity (BCVA) better than 20/200 which dropped to 36% at one year, and ca. 24% 4 years after surgery. One patient maintained a BCVA of 20/32 for 7 years. Fixation on the graft was confirmed by microperimetry in a subgroup of patients. The authors suggested that the functional outcome may become better with recent surgery improvements.
7.3 Clinical trials with stem-cell derived RPE
In January 2011, Advanced Cell Technology (ACT; Santa Monica, CA) received FDA permission for a Phase 1 clinical trial to transplant dissociated hESC-derived RPE cells to patients with ARMD based on the studies of Ray Lund's group (Lu et al., 2009). They had previously received permission for a similar trial in patients with Stargardt's disease. The trials have now started (Schwartz et al., 2012), but it is too early to report about the long-term outcome (the study published 4-month results on 2 patients). Coffey's group in United Kingdom is close to starting clinical trials transplanting sheets of hESC-derived RPE to patients with RPE tears (Lee and Maclaren, 2011). Humayun's group in California is following a similar approach (Lu et al., 2012).
However, although hESC-derived RPE express all RPE markers, there are still differences to fetal RPE in terms of growth factor expression and attachment to aged Bruch's membrane (Sugino et al., 2011). Considering this knowledge, the expected success appears limited to delaying retinal degeneration in early disease stages.
7.4 Early clinical trials with retinal cell and photoreceptor sheet transplantation
Fourteen RP patients in India (Das et al., 1999), and eight patients with RP and one patient with ARMD in the U.S. (Humayun et al., 2000) received aggregate fetal retinal transplants. Ten patients received adult photoreceptor sheet transplants (Kaplan et al., 1997; Berger et al., 2003). These experiments have shown no clinical signs of rejection (all patients received no immunosuppression), but also no improvement in vision. However, the presence of a slow, difficult to detect graft rejection cannot be excluded.
7.5. Phase I and II clinical trials with intact fetal retinal sheet transplants together with its RPE
Limited clinical trials of retinal sheet transplantation by Dr. Radtke's group (Louisville, KY) have been underway since 1998, first with retina-only transplants to 4 RP patients with light perception only. The studies were approved and reviewed by the local Hospital IRB, initially by the IRB of the University of Louisville and, after 2004, by the Western IRB. Donor eyes from dead fetuses of 10-16 weeks gestational age were obtained by informed consent. There was a clear separation between tissue donors and recipients. There was no incentive provided for abortion; in contrary, donors had to consent to provide their medical history and to blood tests to exclude any risks of contamination by sexually transmitted diseases, HIV and hepatitis. No immunosuppression was used. Both donors and recipients were tissue-typed. Although there was no match, no clinical signs of rejection were observed.
After FDA approval (BB-IND number #8354, Principal Investigator Norman D. Radtke), co-grafted sheets of fetal retina together with its RPE were transplanted to 5 RP patients with light perception or no light perception between 1998 and 2001 as a phase I clinical trial. This was done to demonstrate the safety of implanting freshly harvested sheets of fetal retina together with its RPE (Radtke et al., 2002). No adverse effect, but also no vision improvement was noted. After these 5 patients, the FDA was satisfied with the safety of the procedure, and allowed transplanting patients with vision of 20/800 or worse in one eye in a Phase II study. This limit was later changed to 20/200.
A Phase II trial was conducted in a group of 10 patients (six RP and four ARMD) with transplant surgeries between 2002 and 2005 (Radtke et al., 2008). Patient testing was performed 3 times pre-operatively, and then at 1, 3, 6, 9 and 12 months after surgery. EDTRS tests were also performed by an independent ophthalmologist. Some patients were followed up for longer time, up to 6 years. All patients had their cataracts removed and intraocular lenses implanted before starting the preoperative testing. Vision improvement started about 6 months after surgery, corresponding to the time expected for the fetal cells to develop functional expression of photoreceptor markers. This indicates that the transplant effect depends on maturation, and was not a short-term trophic effect. At one year post-transplantation, seven of the patients (4 ARMD, 3 RP) showed visual improvements in their EDTRS visual acuity scores. Vision in one RP patient remained the same, while vision in two RP patients decreased.
The best results were achieved with an RP patient (transplanted in February 2002) with pre-operative vision of 20/800. Her vision improved to 20/160 at 1 year post-op and remained stable at 20/200 for 5 years post-op. Her non-surgery eye improved from 20/400 to 20/320 at one year post surgery, and then reverted back to 20/400 (at 5 years post surgery). At the six-year examination, the patient's vision was still at 20/320 in the surgery eye while the non-surgery eye had deteriorated to hand motion vision (Radtke et al., 2008).
This RP patient's improvements were also confirmed independently in another eye hospital by a scanning laser ophthalmoscope (SLO) microperimetry test, from 20/270 at 9 months to 20/84 at 2 years and 3 months after surgery (Radtke et al., 2004) (see Figure 9). This meant that the patient, among other activities, was able to read, handle e-mail, play games from a computer screen and completely transform her quality of life.
Figure 9. Visual Improvement in Retinitis Pigmentosa Patient as shown by SLO microperimetry.
The transplant area is outlined by yellow dots. A),C) Seeing and non-seeing areas. Seeing areas: filled white squares, non-seeing areas: open white squares; fixation points: black crosses. B), D) Fixation. (A) At 9 months post surgery, fixation was not stable, and sometimes involved retina over the transplant area as well as retina adjacent to the transplant. (B) The patient fixated a large size horizontal black “E” outside of the area of the transplant, at the nasal edge of the transplant. Acuity is 20/270. (C) At 2 years 3 months post surgery, the fixation is now more concentrated, apparently inside the transplant at the nasal edge. (D) The patient has improved acuity of 20/84 and fixated a small horizontal black “E”, appears inside the nasal edge of the transplant. A) B) Modified and reprinted with permission from Figure 3 of Radtke et al. Arch Ophthalmol. 2004;122:1159-1165. Copyright American Medical Association.
The trials will need to be repeated at larger surgical center(s) to systematically use currently available, state-of-the-art objective testing and imaging methods, e.g., SLO in combination with high-resolution OCT, multifocal ERG and microperimetry etc. In addition, combination of transplants with growth factors should also be tested clinically. Visual stimulation, challenging the patient to use the transplant for different visual tasks will likely also improve the outcome. Further trials are in preparation outside the United States.
In summary, the results of a Phase II clinical trial of transplantation of fetal retina with its RPE was very positive and a good beginning to develop it to a viable therapy to restore eyesight. Especially encouraging backup was the corroboration of the many hopeful preclinical studies.
7.6. Clinical trials with stem-cell derived retinal progenitors?
Several groups have developed procedures to differentiate retinal and photoreceptor progenitors from embryonic stem cells and iPS cells (Lamba et al., 2006; Lamba et al., 2009; Meyer et al., 2009; Lamba et al., 2010; Yue et al., 2010; Parameswaran et al., 2010; Meyer et al., 2011; Hambright et al., 2012) (see section 3.3). Clinical trials with stem-cell derived retinal progenitors are still a long way off. Although transplants have shown visual effects in retinal degeneration models such as the rho−/− mouse (Lamba et al., 2009; Lamba et al., 2010; Tucker et al., 2011), fundamental questions must be asked regarding long-term survival, possibility of tumor formation, and the fate of cells not integrating into the host retina remaining in the subretinal space (see section 3.3.).
Several laboratories have achieved to differentiate both ES and iPS stem cells into optic vesicle- and even optic cup-like structures that developed some degree of lamination (Eiraku et al., 2011; Meyer et al., 2011; Phillips et al., 2012; Nakano et al. 2012). Our laboratory is also working on the approach to develop stem cells into retinal progenitor sheets with RPE (Nistor et al., 2010), to make a transplantable retina for clinical trials. Thus, it may be possible in the future to apply the sheet transplantation method to stem-cell-derived retinal progenitor sheets.
8. Important issues to consider
Although availability of fetal tissue will remain limited, much could be learned to aid complementary approaches. Fetal retinal sheet transplants could set a standard for what an optimal replacement therapy could do, provided they are used while observing important ethical restrictions, such as informed consent, no incentive to abortions, strict separation of donor and recipient, and prevention of transmission of disease.
Retinal transplantation started seriously in the 1980s with the aim of finding remedies for incurable blinding diseases. Unfortunately, some disregard valuable research produced in the 1990s and later. For example, a recent article stated “for many years there was no consensus as to what types of cells would provide the best source for cell replacement therapy for retinal disease” (Gust and Reh, 2011). Fetal cells have proven to be the most effective for transplantation in CNS for two decades but their use has been blocked by ignorance and ethical and political reasons. When we learn how to introduce donor properties of fetal intact retinal sheets to stem-cell derived tissue, the concern about a glial barrier between host and graft will likely be resolved and immunosuppression may become obsolete.
Many published studies are limited because 2-3 weeks survival after transplantation is too short to assess functional and adverse effects. One needs at least 2-3 months and longer survival to extract productive information in a rodent model, and much longer time for a larger animal. Transplants to normal retina can only be a first step that gives limited information because the integration of transplants is different than in a degenerated retina; and need to be repeated in retinal degeneration models.
Overselling vision restoration results gives false hope to people suffering from retinal diseases. It creates skepticism towards science and has negative effects on vision research. By example, the editorial (Reh, 2006) about “Retinal repair by photoreceptor precursors” (MacLaren et al., 2006) stated “These results provide the best evidence so far that cell-replacement therapy may be possible.” and “Unfortunately, both the intact embryonic retinal sheets and the microaggregates of photoreceptors keep to themselves, without interacting or integrating very effectively with the host retinal neurons” – thus ignoring previous peer-reviewed publications. Here, less than 0.2 % of transplanted cells had integrated into the host retina which was called “retinal repair”. The in vitro and in vivo testing methods (at only 3 weeks post-transplantation) showed only a very small increase in visual function (see sections 5.2. and 5.3.1), and it was not clear whether the cells would have survived long-term (see Mansergh et al., 2010). Their 2012 paper used more reliable and thorough testing methods (Pearson et al., 2012) and was more cautious in their conclusions.
9. Future Directions
The string of positive research results with fetal sheet transplants that culminated in clinical improvement has given real hope that some incurable eye diseases can be reversed. As expressed by The TransEuro Project “much can be learned from the optimal cells for transplantation - fetal cells - while waiting for other opportunities like stem cells to be clinically developed.” During the interim, many patients suffering from retinal diseases might be helped. As discussed in the review, retinal sheet transplantation may be possible with stem cell-derived retinal tissues as several recent studies have shown that pluripotent stem cells can be differentiated into three-dimensional optic vesicle- or cup-like structures that develop lamination in culture. This research might create transplantable retinal sheets together with RPE.
Clinical trials need to continue
(a) for systematic documentation of visual effects in patients, using all available testing and imaging procedures; (b) to test different trophic factors which already have shown improvement in animal models; and (c) to activate patients with visual stimulation that might improve the appropriate connections of the transplant with the host retina.
(d) In addition, much research remains to elucidate the mechanisms of vision restoration, to show which cells participate in transplant-host connectivity, and to study the role of Muller cells. To know which retinal cell types need to be included will be important for developing retinal progenitor sheets together with RPE derived from stem cells for transplantation, as an alternative to fetal retinal sheets.
(e) A newly developed immunodeficient retinal degeneration rat strain will aid to transplant human cell sheets that can be tested without immunosuppression.
(f) A special project needs to be established to analyze why fetal sheet transplants and not dissociated cells are immunologically tolerated within the same species.
(h) Other questions to be studied are stimulation of synapse formation and development of “appropriate connections”.
Acknowledgments
This work was supported recently by the Lincy Foundation, the International Stem Cell Corporation, and the Beckman Initiative for Macular Research. RBA has been supported by NEI SBIR grants 1 R44 EY015584, and 1R43EY020787. MJS and RBA have proprietary interests in the transplantation instrument and procedure (Ocular Transplantation LLC).
Part of the work described here was performed at the Doheny Eye Institute, University of Southern California, Dept. of Ophthalmology (2002-2006), and at the University of Louisville, Departments of Ophthalmology & Visual Sciences, Anatomical Sciences & Neurobiology (1993 – 2002). Previous support: at Doheny Eye Institute: Foundation Fighting Blindness, Foundation for Retinal Research, Michael Panitch Fund for Retinal Research, NIH EY03040, NIH EY054375; at University of Louisville: NIH EY08519 (RBA), Foundation Fighting Blindness, private funds.
The authors want to thank Hans S. Keirstead (UC Irvine, Depts. Anatomy & Neurobiology and Neurological Surgery), for his advice and support; Leonard Kitzes (UC Irvine, Dept. Anatomy & Neurobiology) for advice with the electrophysiology and critical reading of the manuscript; Biju B. Thomas (Doheny Eye Institute, USC, Los Angeles) and Pamela B. Yang (UC Irvine) for the electrophysiological recordings of rats, and Zhenhai Chen (Doheny Eye Institute, USC, Los Angeles); Fengrong Yan, Ilse Sears-Kraxberger, Lakshmi Patil, Eric Katayama, Melissa Jones, Dave Ferguson, Sabhya Rana, and Josh Almodovar (UC Irvine) for technical assistance. We thank Sharon Valiquette (Albany, NY) for critical reading and editing of the manuscript.
For space reasons, only the most important collaborators involved can be mentioned. Zhongping Chen (UC Irvine), Robert E. Marc, Bryan Jones (Moran Eye Center, University of Utah), Melissa Mahoney (University of Colorado); Srinivas Sadda (Doheny Eye Institute, Los Angeles, CA); Shinichi Arai (now Ophthalmologist in Niigata, Japan); Qing Peng (now Shanghai JiaoTong Univ., School of Medicine, Shanghai, PR China); Botir T. Sagdullaev (now Cornell University, NY); Norman D. Radtke, Heywood M. Petry, Maureen A. McCall; Peng Yang (University of Louisville); Gustaw Woch (Reading Hospital, Reading, PA); and Sherry L. Ball (Cleveland University, Cleveland, OH) contributed to the work presented in this paper. We thank Lynn W. Enquist (Princeton University, NJ) and J. Patrick Card (Pittsburgh University, PA), for their generous gifts of PRV strains and the PRV antiserum. We thank Matthew M. LaVail, UCSF, for the founder breeding pairs of transgenic S334ter rats, and Eric Sandgren, University of Wisconsin, for founder breeders of transgenic hPAP rats. We thank Marco Zarbin (Ophthalmology & Visual Science, UMDNJ-New Jersey Medical School) for the SLO testing in the phase II clinical trial.
List of Abbreviations used in figures and text
- ARMD
age-related macular degeneration
- BCVA
best corrected visual acuity
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- CRX
cone-rod homeobox gene (cone and rod photoreceptor progenitor marker)
- CRALBP
cellular retinaldehyde binding protein
- DAPI
4′6-diamidino-2-phenylindole dihydrochloride
- EM
electron microscope
- EDTRS
Early Treatment Diabetic Retinopathy Study visual acuity testing
- ERG
electroretinography
- GC
ganglion cell
- GDNF
glial cell-line derived neurotrophic factor
- GS
glutamine synthetase
- H
host
- H-E
Hematoxylin-Eosin
- hESC
human embryonic stem cell
- hPAP
human placental alkaline phosphatase
- HPC-1
syntaxin 1
- IN
inner nuclear layer
- IP
inner plexiform layer
- IPE
iris pigment epithelium
- iPSC
induced pluripotent stem cell
- ms
milliseconds
- NRL
neural retina leucine zipper transcription factor (rod progenitor marker)
- OCT
Ocular coherence tomography
- OLM
outer limiting membrane
- ON
outer nuclear layer
- OP
outer plexiform layer
- OS
outer segments
- PKC
protein kinase C (rod bipolar cell marker)
- PRV
pseudorabies virus (transsynaptic tracer)
- PSD95
post-synaptic density protein 95
- RD
retinal degenerate or retinal degeneration
- RG opsin
red-green opsin
- rho
rhodopsin
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- SC
superior colliculus
- SLO
Scanning Laser Ophthalmoscope
- T
transplant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Tomita H, Kano T, Yoshida M, Ohashi T, Nakamura Y, Nishikawa S, Tamai M. Autologous iris pigment epithelial cell transplantation in monkey subretinal region. Curr Eye Res. 2000a;20:268–275. [PubMed] [Google Scholar]
- Abe T, Yoshida M, Tomita H, Kano T, Sato M, Wada Y, Fuse N, Yamada T, Tamai M. Auto iris pigment epithelial cell transplantation in patients with age-related macular degeneration: short-term results. Tohoku J Exp Med. 2000b;191:7–20. doi: 10.1620/tjem.191.7. [DOI] [PubMed] [Google Scholar]
- Abe T, Yoshida M, Yoshioka Y, Wakusawa R, Tokita-Ishikawa Y, Seto H, Tamai M, Nishida K. Iris pigment epithelial cell transplantation for degenerative retinal diseases. Prog Retin Eye Res. 2007;26:302–321. doi: 10.1016/j.preteyeres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Adolph AR, Zucker CL, Ehinger B, Bergstrom A. Function and structure in retinal transplants. J Neural Transplant Plast. 1994;5:147–161. doi: 10.1155/NP.1994.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenbrey S, Lafaut BA, Szurman P, Hilgers RD, Esser P, Walter P, Bartz-Schmidt KU, Thumann G. Iris pigment epithelial translocation in the treatment of exudative macular degeneration: a 3-year follow-up. Arch Ophthalmol. 2006;124:183–188. doi: 10.1001/archopht.124.2.183. [DOI] [PubMed] [Google Scholar]
- Akimoto M, Cheng H, Zhu D, Brzezinski JA, Khanna R, Filippova E, Oh EC, Jing Y, Linares JL, Brooks M, Zareparsi S, Mears AJ, Hero A, Glaser T, Swaroop A. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci U S A. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algvere PV, Gouras P, Dafgard Kopp E. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol. 1999;9:217–230. doi: 10.1177/112067219900900310. [DOI] [PubMed] [Google Scholar]
- An GJ, Asayama N, Humayun MS, Weiland J, Cao J, Kim SY, Grebe R, de Juan E, Jr, Sadda S. Ganglion cell responses to retinal light stimulation in the absence of photoreceptor outer segments from retinal degenerate rodents. Curr Eye Res. 2002;24:26–32. doi: 10.1076/ceyr.24.1.26.5432. [DOI] [PubMed] [Google Scholar]
- Arai S, Thomas BB, Seiler MJ, Aramant RB, Qiu G, Mui C, de Juan E, Sadda SR. Restoration of visual responses following transplantation of intact retinal sheets in rd mice. Exp Eye Res. 2004;79:331–341. doi: 10.1016/j.exer.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Aramant R, Seiler M, Ehinger B, Bergstrom A, Adolph AR, Turner JE. Neuronal markers in rat retinal grafts. Brain Res Dev Brain Res. 1990a;53:47–61. doi: 10.1016/0165-3806(90)90123-g. [DOI] [PubMed] [Google Scholar]
- Aramant R, Seiler M, Turner JE. Donor age influences on the success of retinal grafts to adult rat retina. Invest Ophthalmol Vis Sci. 1988;29:498–503. [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Cryopreservation and transplantation of rat embryonic retina into adult rat retina. Dev Brain Res. 1991;61:151–159. doi: 10.1016/0165-3806(91)90126-4. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Human embryonic retinal cell transplants in athymic immunodeficient rat hosts. Cell Transplantation. 1994;3:461–474. doi: 10.1177/096368979400300603. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Fiber and synaptic connections between embryonic retinal transplants and host retina. Exp Neurol. 1995;133:244–255. doi: 10.1006/exnr.1995.1027. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Retinal Transplantation - Advantages of Intact Fetal Sheets. Prog Retin Eye Res. 2002a;21:57–73. doi: 10.1016/s1350-9462(01)00020-9. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Transplanted sheets of human retina and retinal pigment epithelium develop normally in nude rats. Exp Eye Res. 2002b;75:115–125. doi: 10.1006/exer.2002.2001. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog Retin Eye Res. 2004;23:475–494. doi: 10.1016/j.preteyeres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ, Ball SL. Successful cotransplantation of intact sheets of fetal retinal pigment epithelium with retina. Invest Ophthalmol Vis Sci. 1999;40:1557–1564. [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ, Ehinger B, Bergström A, Gustavii B, Brundin P, Adolph AR. Transplantation of human embryonic retina to adult rat retina. Restor Neurol Neurosci. 1990b;2:9–22. doi: 10.3233/RNN-1990-2102. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Hollander H, Streit W, Stone J. The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci. 1989;2:437–448. doi: 10.1017/s0952523800012335. [DOI] [PubMed] [Google Scholar]
- Baehr W, Frederick JM. Naturally Occurring Animal Models with Outer Retina Phenotypes. Vision Res. 2009;49:2636–2652. doi: 10.1016/j.visres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Bartsch U, Oriyakhel W, Kenna PF, Linke S, Richard G, Petrowitz B, Humphries P, Farrar GJ, Ader M. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86:691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Berger AS, Tezel TH, Del Priore LV, Kaplan HJ. Photoreceptor transplantation in retinitis pigmentosa: short-term follow-up. Ophthalmology. 2003;110:383–391. doi: 10.1016/S0161-6420(02)01738-4. [DOI] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Moser A, Brockhurst RJ, Hayes KC, Johnson CA, Anderson EJ, Gaudio AR, Willett WC, Schaefer EJ. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch Ophthalmol. 2004;122:1297–1305. doi: 10.1001/archopht.122.9.1297. [DOI] [PubMed] [Google Scholar]
- Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Bragadottir R, Narfstrom K. Lens sparing pars plana vitrectomy and retinal transplantation in cats. Vet Ophthalmol. 2003;6:135–139. doi: 10.1046/j.1463-5224.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Brundin P, Barbin G, Strecker RE, Isacson O, Prochiantz A, Bjorklund A. Survival and function of dissociated rat dopamine neurones grafted at different developmental stages or after being cultured in vitro. Brain Res. 1988;467:233–243. doi: 10.1016/0165-3806(88)90027-2. [DOI] [PubMed] [Google Scholar]
- Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- Busskamp V, Roska B. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr Opin Neurobiol. 2011;21:942–946. doi: 10.1016/j.conb.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Caramoy A, Liakopoulos S, Menrath E, Kirchhof B. Autologous translocation of choroid and retinal pigment epithelium in geographic atrophy: long-term functional and anatomical outcome. Br J Ophthalmol. 2010;94:1040–1044. doi: 10.1136/bjo.2009.161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehav Rev. 1998;22:685–694. doi: 10.1016/s0149-7634(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, Enquist LW. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Schwaber JS, Miselis RR, Whealy ME, Robbins AK, Enquist LW. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanheira P, Torquetti LT, Magalhas DR, Nehemy MB, Goes AM. DAPI diffusion after intravitreal injection of mesenchymal stem cells in the injured retina of rats. Cell Transplant. 2009;18:423–431. doi: 10.3727/096368909788809811. [DOI] [PubMed] [Google Scholar]
- Cereda MG, Parolini B, Bellesini E, Pertile G. Surgery for CNV and autologous choroidal RPE patch transplantation: exposing the submacular space. Graefes Arch Clin Exp Ophthalmol. 2010;248:37–47. doi: 10.1007/s00417-009-1201-8. [DOI] [PubMed] [Google Scholar]
- Chacko DM, Das AV, Zhao X, James J, Bhattacharya S, Ahmad I. Transplantation of ocular stem cells: the role of injury in incorporation and differentiation of grafted cells in the retina. Vision Res. 2003;43:937–946. doi: 10.1016/s0042-6989(02)00688-0. [DOI] [PubMed] [Google Scholar]
- Chacko DM, Rogers JA, Turner JE, Ahmad I. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem Biophys Res Commun. 2000;268:842–846. doi: 10.1006/bbrc.2000.2153. [DOI] [PubMed] [Google Scholar]
- Chan F, Bradley A, Wensel TG, Wilson JH. Knock-in human rhodopsin-GFP fusions as mouse models for human disease and targets for gene therapy. Proc Natl Acad Sci U S A. 2004;101:9109–9114. doi: 10.1073/pnas.0403149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Wang J, Howell D, Davisson MT, Roderick TH, Nusinowitz S, Heckenlively JR. Mouse models of ocular diseases. Vis Neurosci. 2005;22:587–593. doi: 10.1017/S0952523805225075. [DOI] [PubMed] [Google Scholar]
- Chaum E. Retinal neuroprotection by growth factors: a mechanistic perspective. J Cell Biochem. 2003;88:57–75. doi: 10.1002/jcb.10354. [DOI] [PubMed] [Google Scholar]
- Chen FK, Patel PJ, Uppal GS, Rubin GS, Coffey PJ, Aylward GW, Da Cruz L. A comparison of macular translocation with patch graft in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009a;50:1848–1855. doi: 10.1167/iovs.08-2845. [DOI] [PubMed] [Google Scholar]
- Chen FK, Uppal GS, MacLaren RE, Coffey PJ, Rubin GS, Tufail A, Aylward GW, Da Cruz L. Long-term visual and microperimetry outcomes following autologous retinal pigment epithelium choroid graft for neovascular age-related macular degeneration. Clin Experiment Ophthalmol. 2009b;37:275–285. doi: 10.1111/j.1442-9071.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LM, Baker SJ, Sorrentino BP, Dyer MA. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A. 2009;106:6685–6690. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Ballios BG, van der Kooy D. Generation and clonal isolation of retinal stem cells from human embryonic stem cells. Eur J Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08123.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Das T, del Cerro M, Jalali S, Rao VS, Gullapalli VK, Little C, Loreto DA, Sharma S, Sreedharan A, del Cerro C, Rao GN. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: results of a long-term safety study. Exp Neurol. 1999;157:58–68. doi: 10.1006/exnr.1998.6992. [DOI] [PubMed] [Google Scholar]
- del Cerro M, Gash DM, Rao GN, Notter MF, Wiegand SJ, Gupta M. Intraocular retinal transplants. Invest Ophthalmol Vis Sci. 1985;26:1182–1185. [PubMed] [Google Scholar]
- del Cerro M, Ison JR, Bowen GP, Lazar E, del Cerro C. Intraretinal grafting restores visual function in light-blinded rats. NeuroReport. 1991;2:529–532. doi: 10.1097/00001756-199109000-00008. [DOI] [PubMed] [Google Scholar]
- del Cerro M, Yeh HH, Marrero-Rodriguez A, Lazar E, del Cerro C. Intraocular transplantation of cell layers derived from neonatal rat retina. Brain Res. 1990;535:25–32. doi: 10.1016/0006-8993(90)91819-3. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Kaplan HJ, Tezel TH, Hayashi N, Berger AS, Green WR. Retinal pigment epithelial cell transplantation after subfoveal membranectomy in age-related macular degeneration: clinicopathologic correlation. Am J Ophthalmol. 2001;131:472–480. doi: 10.1016/s0002-9394(00)00850-3. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Tezel TH, Kaplan HJ. Survival of allogeneic porcine retinal pigment epithelial sheets after subretinal transplantation. Invest Ophthalmol Vis Sci. 2004;45:985–992. doi: 10.1167/iovs.03-0662. [DOI] [PubMed] [Google Scholar]
- Di Polo A, Cheng L, Bray GM, Aguayo AJ. Colocalization of TrkB and brain-derived neurotrophic factor proteins in green-red-sensitive cone outer segments. Invest Ophthalmol Vis Sci. 2000;41:4014–4021. [PubMed] [Google Scholar]
- Diaz-Araya CM, Provis JM, Penfold PL. Ontogeny and cellular expression of MHC and leucocyte antigens in human retina. Glia. 1995;15:458–470. doi: 10.1002/glia.440150409. [DOI] [PubMed] [Google Scholar]
- DiLoreto D, Jr, del Cerro C, del Cerro M. Cyclosporine treatment promotes survival of human fetal neural retina transplanted to the subretinal space of the light-damaged Fischer 344 rat. Exp Neurol. 1996;140:37–42. doi: 10.1006/exnr.1996.0112. [DOI] [PubMed] [Google Scholar]
- Djojosubroto MW, Arsenijevic Y. Retinal stem cells: promising candidates for retina transplantation. Cell Tissue Res. 2008;331:347–357. doi: 10.1007/s00441-007-0501-8. [DOI] [PubMed] [Google Scholar]
- Du J, Gouras P, Kjeldbye H, Kwun R, Lopez R. Monitoring photoreceptor transplants with nuclear and cytoplasmic markers. Exp Neurol. 1992;115:79–86. doi: 10.1016/0014-4886(92)90226-g. [DOI] [PubMed] [Google Scholar]
- Eberle D, Schubert S, Postel K, Corbeil D, Ader M. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest Ophthalmol Vis Sci. 2011;52:6462–6471. doi: 10.1167/iovs.11-7399. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Engelsberg K, Ghosh F. Human Retinal Development in an in situ Whole Eye Culture System. Dev Neurosci. 2011;33:110–117. doi: 10.1159/000328170. [DOI] [PubMed] [Google Scholar]
- Frasson M, Picaud S, Leveillard T, Simonutti M, Mohand-Said S, Dreyfus H, Hicks D, Sabel J. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci. 1999;40:2724–2734. [PubMed] [Google Scholar]
- Gaillard F, Sauve Y. Cell-based therapy for retina degeneration: the promise of a cure. Vision Res. 2007;47:2815–2824. doi: 10.1016/j.visres.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Arner K. Transplantation of full-thickness retina in the normal porcine eye: surgical and morphologic aspects. Retina. 2002;22:478–486. doi: 10.1097/00006982-200208000-00013. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Arnér K, Ehinger B. Transplant of full-thickness embryonic rabbit retina using pars plana vitrectomy. Retina. 1998;18:136–142. doi: 10.1097/00006982-199818020-00007. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Arner K, Engelsberg K. Isolation of photoreceptors in the cultured full-thickness fetal rat retina. Invest Ophthalmol Vis Sci. 2009;50:826–835. doi: 10.1167/iovs.08-2389. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Bruun A, Ehinger B. Graft-host connections in long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999a;40:126–132. [PubMed] [Google Scholar]
- Ghosh F, Johansson K, Ehinger B. Long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999b;40:133–142. [PubMed] [Google Scholar]
- Ghosh F, Juliusson B, Arner K, Ehinger B. Partial and full-thickness neuroretinal transplants. Exp Eye Res. 1999c;68:67–74. doi: 10.1006/exer.1998.0582. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Neeley WL, Arner K, Langer R. Selective removal of photoreceptor cells in vivo using the biodegradable elastomer poly(glycerol sebacate) Tissue Eng Part A. 2011;17:1675–1682. doi: 10.1089/ten.tea.2008.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh F, Rauer O, Arner K. Neuroretinal xenotransplantation to immunocompetent hosts in a discordant species combination. Neuroscience. 2008;152:526–533. doi: 10.1016/j.neuroscience.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Wong F, Johansson K, Bruun A, Petters RM. Transplantation of full-thickness retina in the rhodopsin transgenic pig. Retina. 2004;24:98–109. doi: 10.1097/00006982-200402000-00014. [DOI] [PubMed] [Google Scholar]
- Gias C, Jones M, Keegan D, Adamson P, Greenwood J, Lund R, Martindale J, Johnston D, Berwick J, Mayhew J, Coffey P. Preservation of visual cortical function following retinal pigment epithelium transplantation in the RCS rat using optical imaging techniques. Eur J Neurosci. 2007;25:1940–1948. doi: 10.1111/j.1460-9568.2007.05459.x. [DOI] [PubMed] [Google Scholar]
- Girman SV, Wang S, Lund RD. Cortical visual functions can be preserved by subretinal RPE cell grafting in RCS rats. Vision Res. 2003;43:1817–1827. doi: 10.1016/s0042-6989(03)00276-1. [DOI] [PubMed] [Google Scholar]
- Girman SV, Wang S, Lund RD. Time course of deterioration of rod and cone function in RCS rat and the effects of subretinal cell grafting: a light- and dark-adaptation study. Vision Res. 2005;45:343–354. doi: 10.1016/j.visres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Gouras P, Du J, Gelanze M, Kwun R, Kjeldbye H, Lopez R. Transplantation of photoreceptors labeled with tritiated thymidine into RCS rats. Invest Ophthalmol Vis Sci. 1991a;32:1704–1707. [PubMed] [Google Scholar]
- Gouras P, Du J, Gelanze M, Lopez R, Kwun R, Kjeldbye H, Krebs W. Survival and synapse formation of transplanted rat rods. J Neural Transplant Plast. 1991b;2:91–100. doi: 10.1155/NP.1991.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Du J, Kjeldbye H, Yamamoto S, Zack DJ. Reconstruction of degenerate rd mouse retina by transplantation of transgenic photoreceptors. Invest Ophthalmol Vis Sci. 1992;33:2579–2586. [PubMed] [Google Scholar]
- Gouras P, Du J, Kjeldbye H, Yamamoto S, Zack DJ. Long-term photoreceptor transplants in dystrophic and normal mouse retina. Invest Ophthalmol Vis Sci. 1994;35:3145–3153. [PubMed] [Google Scholar]
- Gouras P, Flood MT, Kjeldbye H. Transplantation of cultured human retinal cells to monkey retina. An Acad Bras Cienc. 1984;56:431–443. [PubMed] [Google Scholar]
- Gouras P, Tanabe T. Survival and integration of neural retinal transplants in rd mice. Graefes Arch Clin Exp Ophthalmol. 2003;241:403–409. doi: 10.1007/s00417-003-0648-2. [DOI] [PubMed] [Google Scholar]
- Granse L, Ponjavic V, Andreasson S. Full-field ERG, multifocal ERG and multifocal VEP in patients with retinitis pigmentosa and residual central visual fields. Acta Ophthalmol Scand. 2004;82:701–706. doi: 10.1111/j.1600-0420.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Grishanin RN, Yang H, Liu X, Donohue-Rolfe K, Nune GC, Zang K, Xu B, Duncan JL, LaVail MM, Copenhagen DR, Reichardt LF. Retinal TrkB receptors regulate neural development in the inner, but not outer, retina. Mol Cell Neurosci. 2008;38:431–443. doi: 10.1016/j.mcn.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdoni S, Baron M, Lakowski J, Decembrini S, Smith AJ, Pearson RA, Ali RR, Sowden JC. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. Stem Cells. 2010;28:1048–1059. doi: 10.1002/stem.423. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullapalli VK, Sugino IK, Van Patten Y, Shah S, Zarbin MA. Retinal pigment epithelium resurfacing of aged submacular human Bruch's membrane. Trans Am Ophthalmol Soc. 2004;102:123–137. discussion 137-128. [PMC free article] [PubMed] [Google Scholar]
- Gust J, Reh TA. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci. 2011;52:5266–5272. doi: 10.1167/iovs.10-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjantonakis AK, Macmaster S, Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. doi: 10.1186/1472-6750-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–936. [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Kohsaka S, Wada E, Yoshida K, Ohno S, Mamada H, Tanaka K, Parada LF, Wada K. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkind P, Gartner S. The relationship between retinal pigment epithelium and the choriocapillaris. Trans Ophthalmol Soc U K. 1983;103(Pt 4):444–447. [PubMed] [Google Scholar]
- Hetherington L, Benn M, Coffey PJ, Lund RD. Sensory capacity of the Royal College of Surgeons rat. Invest Ophthalmol Vis Sci. 2000;41:3979–3983. [PubMed] [Google Scholar]
- Heussen FM, Fawzy NF, Joeres S, Lux A, Maaijwee K, Meurs JC, Kirchhof B, Joussen AM. Autologous translocation of the choroid and RPE in age-related macular degeneration: 1-year follow-up in 30 patients and recommendations for patient selection. Eye (Lond) 2008;22:799–807. doi: 10.1038/sj.eye.6702823. [DOI] [PubMed] [Google Scholar]
- Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000;19:607–646. doi: 10.1016/s1350-9462(00)00013-6. [DOI] [PubMed] [Google Scholar]
- Huang JC, Ishida M, Hersh P, Sugino IK, Zarbin MA. Preparation and transplantation of photoreceptor sheets. Curr Eye Res. 1998;17:573–585. [PubMed] [Google Scholar]
- Humayun MS, de Juan E, del Cerro M, Dagnelie G, Radner W, Sadda SR, del Cerro C. Human neural retinal transplantation. Invest Ophthalmol Vis Sci. 2000;41:3100–3106. [PubMed] [Google Scholar]
- Humayun MS, Prince M, de Juan E, Jr, Barron Y, Moskowitz M, Klock IB, Milam AH. Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40:143–148. [PubMed] [Google Scholar]
- Inoue T, Coles BL, Dorval K, Bremner R, Bessho Y, Kageyama R, Hino S, Matsuoka M, Craft CM, McInnes RR, Tremblay F, Prusky GT, van der Kooy D. Maximizing functional photoreceptor differentiation from adult human retinal stem cells. Stem Cells. 2010;28:489–500. doi: 10.1002/stem.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ivert L, Gouras P, Naeser P, Narfstrom K. Photoreceptor allografts in a feline model of retinal degeneration. Graefes Arch Clin Exp Ophthalmol. 1998;236:844–852. doi: 10.1007/s004170050169. [DOI] [PubMed] [Google Scholar]
- Jensen RJ, Rizzo JF., 3rd Activation of ganglion cells in wild-type and rd1 mouse retinas with monophasic and biphasic current pulses. J Neural Eng. 2009;6:035004. doi: 10.1088/1741-2560/6/3/035004. [DOI] [PubMed] [Google Scholar]
- Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, LaVail MM, Marc RE. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol. 2003;464:1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- Juliusson B, Bergstrom A, van Veen T, Ehinger B. Cellular organization in retinal transplants using cell suspensions or fragments of embryonic retinal tissue. Cell Transplant. 1993;2:411–418. doi: 10.1177/096368979300200509. [DOI] [PubMed] [Google Scholar]
- Kaplan HJ, Tezel TH, Berger AS, Wolf ML, Del Priore LV. Human photoreceptor transplantation in retinitis pigmentosa. A safety study. Arch Ophthalmol. 1997;115:1168–1172. doi: 10.1001/archopht.1997.01100160338012. [DOI] [PubMed] [Google Scholar]
- Kinouchi R, Takeda M, Yang L, Wilhelmsson U, Lundkvist A, Pekny M, Chen DF. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003;6:863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Klassen H, Lund RD. Retinal transplants can drive a pupillary reflex in host rat brains. Proc Natl Acad Sci U S A. 1987;84:6958–6960. doi: 10.1073/pnas.84.19.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H, Lund RD. Retinal graft-mediated pupillary responses in rats: restoration of a reflex function in the mature mammalian brain. J Neurosci. 1990;10:578–587. doi: 10.1523/JNEUROSCI.10-02-00578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H, Warfvinge K, Schwartz PH, Kiilgaard JF, Shamie N, Jiang C, Samuel M, Scherfig E, Prather RS, Young MJ. Isolation of progenitor cells from GFP-transgenic pigs and transplantation to the retina of allorecipients. Cloning Stem Cells. 2008;10:391–402. doi: 10.1089/clo.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H, Whiteley SJ, Young MJ, Lund RD. Graft location affects functional rescue following RPE cell transplantation in the RCS rat. Exp Neurol. 2001;169:114–121. doi: 10.1006/exnr.2000.7617. [DOI] [PubMed] [Google Scholar]
- Klassen H, Ziaeian B, Kirov II, Young MJ, Schwartz PH. Isolation of retinal progenitor cells from post-mortem human tissue and comparison with autologous brain progenitors. J Neurosci Res. 2004a;77:334–343. doi: 10.1002/jnr.20183. [DOI] [PubMed] [Google Scholar]
- Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, Young MJ. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004b;45:4167–4173. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011;29:825–835. doi: 10.1002/stem.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomiets B, Dubus E, Simonutti M, Rosolen S, Sahel JA, Picaud S. Late histological and functional changes in the P23H rat retina after photoreceptor loss. Neurobiol Dis. 2010;38:47–58. doi: 10.1016/j.nbd.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, Usukura J, Fujikado T, Tano Y, Terasaki H. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:1371–1377. doi: 10.1167/iovs.08-2863. [DOI] [PubMed] [Google Scholar]
- Kovalevsky G, DiLoreto D, Jr, Wyatt J, del Cerro C, Cox C, del Cerro M. The intensity of the pupillary light reflex does not correlate with the number of retinal photoreceptor cells. Exp Neurol. 1995;133:43–49. doi: 10.1006/exnr.1995.1006. [DOI] [PubMed] [Google Scholar]
- Krebs I, Binder S, Stolba U, Kellner L, Glittenberg C, Goll A. Subretinal surgery and transplantation of autologous pigment epithelial cells in retinal angiomatous proliferation. Acta Ophthalmol. 2008;86:504–509. doi: 10.1111/j.1600-0420.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- Kwan AS, Wang S, Lund RD. Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Exp Neurol. 1999;159:21–33. doi: 10.1006/exnr.1999.7157. [DOI] [PubMed] [Google Scholar]
- Lakowski J, Baron M, Bainbridge J, Barber AC, Pearson RA, Ali RR, Sowden JC. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet. 2010;19:4545–4559. doi: 10.1093/hmg/ddq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J, Han YT, Pearson RA, Gonzalez-Cordero A, West EL, Gualdoni S, Barber AC, Hubank M, Ali RR, Sowden JC. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011;29:1391–1404. doi: 10.1002/stem.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Survival factors for treatment of retinal degenerative disorders: preclinical gains and issues for translation into clinical studies. Retina. 2005;25:S25–S26. doi: 10.1097/00006982-200512001-00009. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Sauve Y, Keegan DJ, Coffey PJ, Hetherington L, Girman S, Whiteley SJ, Kwan AS, Pheby T, Lund RD. Schwann cell grafting into the retina of the dystrophic RCS rat limits functional deterioration. Invest Ophthalmol Vis Sci. 2000;41:518–528. [PubMed] [Google Scholar]
- Lee E, Maclaren RE. Sources of retinal pigment epithelium (RPE) for replacement therapy. Br J Ophthalmol. 2011;95:445–449. doi: 10.1136/bjo.2009.171918. [DOI] [PubMed] [Google Scholar]
- Leveillard T, Mohand-Said S, Lorentz O, Hicks D, Fintz AC, Clerin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, Dolle P, Poch O, Lambrou G, Sahel JA. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- Li L, Turner JE. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47:911–917. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Li SY, Yin ZQ, Chen SJ, Chen LF, Liu Y. Rescue from light-induced retinal degeneration by human fetal retinal transplantation in minipigs. Curr Eye Res. 2009;34:523–535. doi: 10.1080/02713680902936148. [DOI] [PubMed] [Google Scholar]
- Li ZY, Wong F, Chang JH, Possin DE, Hao Y, Petters RM, Milam AH. Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1998;39:808–819. [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. Cell therapy in Parkinson's disease. NeuroRx. 2004;1:382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. Cell therapeutics in Parkinson's disease. Neurotherapeutics. 2011;8:539–548. doi: 10.1007/s13311-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Rehncrona S, Gustavii B, Brundin P, Astedt B, Widner H, Lindholm T, Bjorklund A, Leenders KL, Rothwell JC, Frackowiak R, Marsden CD, Johnels B, Steg G, Freedman R, Hoffer BJ, Seiger L, Stromberg I, Bygdeman M, Olson L. Fetal dopamine-rich mesencephalic grafts in Parkinson's disease. Lancet. 1988;2:1483–1484. doi: 10.1016/s0140-6736(88)90950-6. [DOI] [PubMed] [Google Scholar]
- Little CW, Cox C, Wyatt J, del Cerro C, del Cerro M. Correlates of photoreceptor rescue by transplantation of human fetal RPE in the RCS rat. Exp Neurol. 1998;149:151–160. doi: 10.1006/exnr.1997.6642. [DOI] [PubMed] [Google Scholar]
- Liu MM, Tuo J, Chan CC. Gene therapy for ocular diseases. Br J Ophthalmol. 2011;95:604–612. doi: 10.1136/bjo.2009.174912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R, Gouras P, Kjeldbye H, Sullivan B, Reppucci V, Brittis M, Wapner F, Goluboff E. Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Invest Ophthalmol Vis Sci. 1989;30:586–588. [PubMed] [Google Scholar]
- Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- Lu B, Wang S, Girman S, McGill T, Ragaglia V, Lund R. Human adult bone marrow-derived somatic cells rescue vision in a rodent model of retinal degeneration. Exp Eye Res. 2010;91:449–455. doi: 10.1016/j.exer.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Lu B, Zhu D, Hinton D, Humayun MS, Tai YC. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed Microdevices. 2012 doi: 10.1007/s10544-012-9645-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lu L, Garcia CA, Mikos AG. Retinal pigment epithelium cell culture on thin biodegradable poly(DL-lactic-co-glycolic acid) films. J Biomater Sci Polym Ed. 1998;9:1187–1205. doi: 10.1163/156856298x00721. [DOI] [PubMed] [Google Scholar]
- Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, Girman S, Bischoff N, Sauve Y, Lanza R. Human Embryonic Stem Cell-Derived Cells Rescue Visual Function in Dystrophic RCS Rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- Ma N, Streilein JW. Contribution of microglia as passenger leukocytes to the fate of intraocular neuronal retinal grafts. Invest Ophthalmol Vis Sci. 1998;39:2384–2393. [PubMed] [Google Scholar]
- Ma Z, Han L, Wang C, Dou H, Hu Y, Feng X, Xu Y, Wang Z, Yin Z, Liu Y. Autologous transplantation of retinal pigment epithelium-Bruch's membrane complex for hemorrhagic age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2975–2981. doi: 10.1167/iovs.08-2573. [DOI] [PubMed] [Google Scholar]
- Maaijwee K, Missotten T, Mulder P, van Meurs JC. Influence of intraoperative course on visual outcome after an RPE-choroid translocation. Invest Ophthalmol Vis Sci. 2008;49:758–761. doi: 10.1167/iovs.07-0510. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Mansergh FC, Vawda R, Millington-Ward S, Kenna PF, Haas J, Gallagher C, Wilson JH, Humphries P, Ader M, Farrar GJ. Loss of photoreceptor potential from retinal progenitor cell cultures, despite improvements in survival. Exp Eye Res. 2010;91:500–512. doi: 10.1016/j.exer.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Marc RE. Injury and Repair: Retinal Remodeling. In: Besharse J, Bok D, editors. Encyclopedia of the Eye. Elsevier; 2010. pp. 414–420. [Google Scholar]
- Marc RE, Jones BW. Molecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22:413–427. doi: 10.1523/JNEUROSCI.22-02-00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Newkirk G, Euler T, Detwiler PB. Functional stability of retinal ganglion cells after degeneration-induced changes in synaptic input. J Neurosci. 2008;28:6526–6536. doi: 10.1523/JNEUROSCI.1533-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Navarrete G, Seiler MJ, Aramant RB, Fernandez-Sanchez L, Pinilla I, Cuenca N. Retinal degeneration in two lines of transgenic S334ter rats. Exp Eye Res. 2011;92:227–237. doi: 10.1016/j.exer.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei M, Zeitz O, Keseru M, Wagenfeld L, Hornig R, Post N, Richard G. Progress in the development of vision prostheses. Ophthalmologica. 2011;225:187–192. doi: 10.1159/000318042. [DOI] [PubMed] [Google Scholar]
- McGee Sanftner LH, Abel H, Hauswirth WW, Flannery JG. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- McGill MG, Schreiner LA, Hansen RM, Fulton AB. Optokinetic nystagmus thresholds of dark-adapted RCS rats. Vision Res. 1988;28:761–764. doi: 10.1016/0042-6989(88)90022-3. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Douglas RM, Lund RD, Prusky GT. Quantification of spatial vision in the Royal College of Surgeons rat. Invest Ophthalmol Vis Sci. 2004;45:932–936. doi: 10.1167/iovs.03-0964. [DOI] [PubMed] [Google Scholar]
- McGill TJ, Lund RD, Douglas RM, Wang S, Lu B, Silver BD, Secretan MR, Arthur JN, Prusky GT. Syngeneic Schwann cell transplantation preserves vision in RCS rat without immunosuppression. Invest Ophthalmol Vis Sci. 2007;48:1906–1912. doi: 10.1167/iovs.06-1117. [DOI] [PubMed] [Google Scholar]
- McGinnis JF, Stepanik PL, Jariangprasert S, Lerious V. Functional significance of recoverin localization in multiple retina cell types. J Neurosci Res. 1997;50:487–495. doi: 10.1002/(SICI)1097-4547(19971101)50:3<487::AID-JNR15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- McMenamin PG, Loeffler KU. Cells resembling intraventricular macrophages are present in the subretinal space of human foetal eyes. Anat Rec. 1990;227:245–253. doi: 10.1002/ar.1092270213. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, Pattnaik B, Thomson JA, Gamm DM. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118:807–811. doi: 10.1001/archopht.118.6.807. [DOI] [PubMed] [Google Scholar]
- Mohand-Said S, Hicks D, Simonutti M, Tran-Minh D, Deudon-Combe A, Dreyfus H, Silverman MS, Ogilvie JM, Tenkova T, Sahel J. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29:290–297. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
- Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Holland Deckman K, Menotti-Raymond M. The domestic cat as a large animal model for characterization of disease and therapeutic intervention in hereditary retinal blindness. J Ophthalmol. 2011;2011:906943. doi: 10.1155/2011/906943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- Nistor G, Seiler MJ, Yan F, Ferguson D, Keirstead HS. Three-dimensional early retinal progenitor 3D tissue constructs derived from human embryonic stem cells. J Neurosci Methods. 2010;190:63–70. doi: 10.1016/j.jneumeth.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Ong JM, Cruz LD. The bionic eye: a review. Clin Experiment Ophthalmol. 2011;40:6–17. doi: 10.1111/j.1442-9071.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29:113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran S, Balasubramanian S, Babai N, Qiu F, Eudy JD, Thoreson WB, Ahmad I. Induced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: therapeutic implications in degenerative changes in glaucoma and age-related macular degeneration. Stem Cells. 2010;28:695–703. doi: 10.1002/stem.320. [DOI] [PubMed] [Google Scholar]
- Park KW, Kuhholzer B, Lai L, Machaty Z, Sun QY, Day BN, Prather RS. Development and expression of the green fluorescent protein in porcine embryos derived from nuclear transfer of transgenic granulosa-derived cells. Anim Reprod Sci. 2001;68:111–120. doi: 10.1016/s0378-4320(01)00138-5. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, Luhmann UF, Benucci A, Sung CH, Bainbridge JW, Carandini M, Yau KW, Sowden JC, Ali RR. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Thomas BB, Aramant RB, Chen Z, Sadda SR, Seiler MJ. Structure and function of embryonic rat retinal sheet transplants. Curr Eye Res. 2007;32:781–789. doi: 10.1080/02713680701530597. [DOI] [PubMed] [Google Scholar]
- Peng YW, Zallocchi M, Meehan DT, Delimont D, Chang B, Hawes N, Wang W, Cosgrove D. Progressive morphological and functional defects in retinas from alpha1 integrin-null mice. Invest Ophthalmol Vis Sci. 2008;49:4647–4654. doi: 10.1167/iovs.08-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Nishikawa S, Matthes MT, Yasumura D, LaVail MM. The relationship of photoreceptor degeneration to retinal vascular development and loss in mutant rhodopsin transgenic and RCS rats. Exp Eye Res. 2008;87:561–570. doi: 10.1016/j.exer.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven A, Martin JM, Wright L, Shen W, Capowski EE, Percin EF, Perez ET, Zhong X, Canto-Soler MV, Gamm DM. Blood-derived Human iPS Cells Generate Optic Vesicle-like Structures with the Capacity to Form Retinal Laminae and Develop Synapses. Invest Ophthalmol Vis Sci. 2012;53:2007–2019. doi: 10.1167/iovs.11-9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis JM, Leech J, Diaz CM, Penfold PL, Stone J, Keshet E. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res. 1997;65:555–568. doi: 10.1006/exer.1997.0365. [DOI] [PubMed] [Google Scholar]
- Qiu G, Seiler MJ, Arai S, Aramant RB, Sadda SR. Alternative culture conditions for isolation and expansion of retinal progenitor cells. Curr Eye Res. 2004;28:327–336. doi: 10.1076/ceyr.28.5.327.28679. [DOI] [PubMed] [Google Scholar]
- Qiu G, Seiler MJ, Mui C, Arai S, Aramant RB, de Juan E, Jr, Sadda S. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res. 2005;80:515–525. doi: 10.1016/j.exer.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Radel JD, Kustra DJ, Das S, Elton S, Lund RD. The pupillary light response: assessment of function mediated by intracranial retinal transplants. Neuroscience. 1995;68:909–924. doi: 10.1016/0306-4522(95)00192-l. [DOI] [PubMed] [Google Scholar]
- Radner W, Sadda SR, Humayun MS, Suzuki S, Melia M, Weiland J, de Juan E., Jr Light-driven retinal ganglion cell responses in blind rd mice after neural retinal transplantation. Invest Ophthalmol Vis Sci. 2001;42:1057–1065. [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision Improvement in Retinal Degeneration Patients by Implantation of Retina Together with Retinal Pigment Epithelium. Am J Ophthalmol. 2008;146:172–182. doi: 10.1016/j.ajo.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Seiler MJ, Petry HM, Pidwell DJ. Vision change after sheet transplant of fetal retina with RPE to a Retinitis Pigmentosa patient. Arch Ophthalmol. 2004;122:1159–1165. doi: 10.1001/archopht.122.8.1159. [DOI] [PubMed] [Google Scholar]
- Radtke ND, Seiler MJ, Aramant RB, Petry HM, Pidwell DJ. Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. Am J Ophthalmol. 2002;133:544–550. doi: 10.1016/s0002-9394(02)01322-3. [DOI] [PubMed] [Google Scholar]
- Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H, Langer R, Young MJ. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30:3405–3414. doi: 10.1016/j.biomaterials.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA. Neurobiology: right timing for retina repair. Nature. 2006;444:156–157. doi: 10.1038/444156a. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Levitt P, Card JP. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. J Neurosci. 2000;20:2731–2741. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919–8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JW, de Castro JP, Zhao J, Samuel M, Walters E, Rios C, Bray-Ward P, Jones BW, Marc RE, Wang W, Zhou L, Noel JM, McCall MA, Demarco PJ, Prather RS, Kaplan HJ. Generation of an inbred miniature pig model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012;53:501–507. doi: 10.1167/iovs.11-8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo PE, Quay WB. Retinal transplantation from fetal to maternal mammalian eye. Growth. 1959;23:313–336. [PubMed] [Google Scholar]
- Sagdullaev BT, Aramant RB, Seiler MJ, Woch G, McCall MA. Retinal transplantation-induced recovery of retinotectal visual function in a rodent model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:1686–1695. doi: 10.1167/iovs.02-0615. [DOI] [PubMed] [Google Scholar]
- Sauvé Y, Klassen H, Whiteley SJ, Lund RD. Visual field loss in RCS rats and the effect of RPE cell transplantation. Exp Neurol. 1998;152:243–250. doi: 10.1006/exnr.1998.6849. [DOI] [PubMed] [Google Scholar]
- Sauve Y, Lu B, Lund RD. The relationship between full field electroretinogram and perimetry-like visual thresholds in RCS rats during photoreceptor degeneration and rescue by cell transplants. Vision Res. 2004;44:9–18. doi: 10.1016/j.visres.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Schraermeyer U, Kayatz P, Thumann G, Luther TT, Szurman P, Kociok N, Bartz-Schmidt KU. Transplantation of iris pigment epithelium into the choroid slows down the degeneration of photoreceptors in the RCS rat. Graefes Arch Clin Exp Ophthalmol. 2000;238:979–984. doi: 10.1007/s004170000194. [DOI] [PubMed] [Google Scholar]
- Schraermeyer U, Thumann G, Luther T, Kociok N, Armhold S, Kruttwig K, Andressen C, Addicks K, Bartz-Schmidt KU. Subretinally transplanted embryonic stem cells rescue photoreceptor cells from degeneration in the RCS rats. Cell Transplant. 2001;10:673–680. [PubMed] [Google Scholar]
- Schuschereba ST, Silverman MS. Retinal cell and photoreceptor transplantation between adult New Zealand red rabbit retinas. Exp Neurol. 1992;115:95–99. doi: 10.1016/0014-4886(92)90228-i. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Seeliger MW, Narfstrom K, Reinhard J, Zrenner E, Sutter E. Continuous monitoring of the stimulated area in multifocal ERG. Doc Ophthalmol. 2000;100:167–184. [PubMed] [Google Scholar]
- Seiler M, Turner JE. The activities of host and graft glial cells following retinal transplantation into the lesioned adult rat eye: developmental expression of glial markers. Brain Res. 1988;471:111–122. doi: 10.1016/0165-3806(88)90156-3. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. Transplantation of embryonic retinal donor cells labeled with BrdU or carrying a genetic marker to adult retina. Exp Brain Res. 1995;105:59–66. doi: 10.1007/BF00242182. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest Ophthalmol Vis Sci. 1998;39:2121–2131. [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. Intact-sheet fetal retinal transplants can repair degenerated retinas. In: Anderson RE, LaVail MM, Hollyfield JG, editors. New Insights into retinal degenerative diseases. Kluwer Academic/Plenum; New York: 2001. pp. 163–173. [Google Scholar]
- Seiler MJ, Aramant RB, Ball SL. Photoreceptor function of retinal transplants implicated by light-dark shift of S-antigen and rod transducin. Vision Res. 1999;39:2589–2596. doi: 10.1016/s0042-6989(98)00326-5. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, Seeliger MW, Bragadottir R, Mahoney M, Narfstrom K. Functional and structural assessment of retinal sheet allograft transplantation in feline hereditary retinal degeneration. Vet Ophthalmol. 2009;12:158–169. doi: 10.1111/j.1463-5224.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, Thomas BB, Peng Q, Sadda SR, Keirstead HS. Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets. Eur J Neurosci. 2010a;31:508–520. doi: 10.1111/j.1460-9568.2010.07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Jones BW, Aramant RB, Yang PB, Keirstead HS, Marc RE. Computational molecular phenotyping of retinal sheet transplants to rats with retinal degeneration. Eur J Neurosci. 2012;35:1692–1704. doi: 10.1111/j.1460-9568.2012.08078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Liu OL, Cooper NG, Callahan TL, Petry HM, Aramant RB. Selective photoreceptor damage in albino rats using continuous blue light. A protocol useful for retinal degeneration and transplantation research. Graefes Arch Clin Exp Ophthalmol. 2000;238:599–607. doi: 10.1007/s004170000143. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Rao B, Aramant RB, Yu L, Wang Q, Kitayama E, Pham S, Yan F, Chen Z, Keirstead HS. Three-dimensional optical coherence tomography imaging of retinal sheet implants in live rats. J Neurosci Methods. 2010b;188:250–257. doi: 10.1016/j.jneumeth.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Sagdullaev BT, Woch G, Thomas BB, Aramant RB. Transsynaptic virus tracing from host brain to subretinal transplants. Eur J Neurosci. 2005;21:161–172. doi: 10.1111/j.1460-9568.2004.03851.x. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Thomas BB, Chen Z, Arai S, Chadalavada S, Mahoney MJ, Sadda SR, Aramant RB. BDNF-treated retinal progenitor sheets transplanted to degenerate rats: improved restoration of visual function. Exp Eye Res. 2008a;86:92–104. doi: 10.1016/j.exer.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Thomas BB, Chen Z, Wu R, Sadda SR, Aramant RB. Retinal transplants restore visual responses - Trans-synaptic tracing from visually responsive sites labels transplant neurons. Eur J Neurosci. 2008b;28:208–220. doi: 10.1111/j.1460-9568.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- Semo M, Peirson S, Lupi D, Lucas RJ, Jeffery G, Foster RG. Melanopsin retinal ganglion cells and the maintenance of circadian and pupillary responses to light in aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;17:1793–1801. doi: 10.1046/j.1460-9568.2003.02616.x. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Gouras P, Cao H, Berglin L, Kjeldbye H, Lopez R, Rosskothen H. Patch transplants of human fetal retinal pigment epithelium in rabbit and monkey retina. Invest Ophthalmol Vis Sci. 1995;36:381–390. [PubMed] [Google Scholar]
- Silverman MS, Hughes SE. Transplantation of photoreceptors to light-damaged retina. Invest Ophthalmol Vis Sci. 1989;30:1684–1690. [PubMed] [Google Scholar]
- Silverman MS, Hughes SE, Valentino T, Liu Y. Photoreceptor transplantation: Anatomic, electrophysiologic, and behavioral evidence for the functional reconstruction of retinas lacking photoreceptors. Exp Neurol. 1992;115:87–94. doi: 10.1016/0014-4886(92)90227-h. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Flannery JG, Naash M, Oh P, Matthes MT, Yasumura D, Lau-Villacorta C, Chen J, LaVail MM. Transgenic rat models of inherited retinal degeneration caused by mutant opsin genes [ARVO abstract] Invest Ophthalmol Vis Sci. 1996;37:S698. [Google Scholar]
- Streilein JW, Ma N, Wenkel H, Ng TF, Zamiri P. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002;42:487–495. doi: 10.1016/s0042-6989(01)00185-7. [DOI] [PubMed] [Google Scholar]
- Sugino IK, Sun Q, Wang J, Nunes CF, Cheewatrakoolpong N, Rapista A, Johnson AC, Malcuit C, Klimanskaya I, Lanza R, Zarbin MA. Comparison of fRPE and human embryonic stem cell-derived RPE behavior on aged human Bruch's membrane. Invest Ophthalmol Vis Sci. 2011;52:4979–4997. doi: 10.1167/iovs.10-5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Cai H, Tezel TH, Paik D, Gaillard ER, Del Priore LV. Bruch's membrane aging decreases phagocytosis of outer segments by retinal pigment epithelium. Mol Vis. 2007;13:2310–2319. [PubMed] [Google Scholar]
- Talcott KE, Ratnam K, Sundquist SM, Lucero AS, Lujan BJ, Tao W, Porco TC, Roorda A, Duncan JL. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest Ophthalmol Vis Sci. 2011;52:2219–2226. doi: 10.1167/iovs.10-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Srinivasan B, Wordinger RJ, Roque RS. Glutamate stimulates neurotrophin expression in cultured Muller cells. Brain Res Mol Brain Res. 2003;111:189–197. doi: 10.1016/s0169-328x(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Tezel TH, Kaplan HJ. Harvest and storage of adult human photoreceptor cells: the vibratome compared to the excimer laser. Curr Eye Res. 1998;17:748–756. [PubMed] [Google Scholar]
- Thaung C, Arnold K, Jackson IJ, Coffey PJ. Presence of visual head tracking differentiates normal sighted from retinal degenerate mice. Neurosci Lett. 2002;325:21–24. doi: 10.1016/s0304-3940(02)00223-9. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Arai S, Ikai Y, Qiu G, Chen Z, Aramant RB, Sadda SR, Seiler MJ. Retinal transplants evaluated by optical coherence tomography in photoreceptor degenerate rats. J Neurosci Methods. 2006a;151:186–193. doi: 10.1016/j.jneumeth.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Aramant RB, Sadda SR, Seiler MJ. Light response differences in the superior colliculus of albino and pigmented rats. Neurosci Lett. 2005;385:143–147. doi: 10.1016/j.neulet.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Aramant RB, Sadda SR, Seiler MJ. Retinal transplantation - A treatment strategy for retinal degenerative diseases. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Retinal Degenerative Diseases. Springer; New York, NY: 2006b. pp. 367–376. [PubMed] [Google Scholar]
- Thomas BB, Seiler M, Sadda SR, Coffey PJ, Aramant RB. Optokinetic test to evaluate visual acuity of each eye independently. J Neurosci Methods. 2004a;138:7–13. doi: 10.1016/j.jneumeth.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Seiler MJ, Aramant RB, Samant D, Qiu G, Vyas N, Arai S, Chen Z, Sadda SR. Visual functional effects of constant blue light in a retinal degenerate rat model. Photochem Photobiol. 2007;83:759–765. doi: 10.1562/2006-09-19-RA-1044. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Seiler MJ, Sadda SR, Aramant RB. Superior colliculus responses to light - preserved by transplantation in a slow degeneration rat model. Exp Eye Res. 2004b;79:29–39. doi: 10.1016/j.exer.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Shi D, Khine K, Kim LA, Sadda SR. Modulatory influence of stimulus parameters on optokinetic head-tracking response. Neurosci Lett. 2010;479:92–96. doi: 10.1016/j.neulet.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumann G, Aisenbrey S, Schraermeyer U, Lafaut B, Esser P, Walter P, Bartz-Schmidt KU. Transplantation of autologous iris pigment epithelium after removal of choroidal neovascular membranes. Arch Ophthalmol. 2000;118:1350–1355. doi: 10.1001/archopht.118.10.1350. [DOI] [PubMed] [Google Scholar]
- Thumann G, Schraermeyer U, Bartz-Schmidt KU, Heimann K. Descemet's membrane as membranous support in RPE/IPE transplantation. Curr Eye Res. 1997;16:1236–1238. doi: 10.1076/ceyr.16.12.1236.5031. [DOI] [PubMed] [Google Scholar]
- Tian C, Weng CC, Yin ZQ. BDNF Improves the Efficacy ERG Amplitude Maintenance by Transplantation of Retinal Stem Cells in RCS Rats. Adv Exp Med Biol. 2010;664:375–384. doi: 10.1007/978-1-4419-1399-9_43. [DOI] [PubMed] [Google Scholar]
- Tomita H, Sugano E, Isago H, Tamai M. Channelrhodopsins provide a breakthrough insight into strategies for curing blindness. J Genet. 2009;88:409–415. doi: 10.1007/s12041-009-0062-6. [DOI] [PubMed] [Google Scholar]
- Treharne AJ, Grossel MC, Lotery AJ, Thomson HA. The chemistry of retinal transplantation: the influence of polymer scaffold properties on retinal cell adhesion and control. Br J Ophthalmol. 2011;95:768–773. doi: 10.1136/bjo.2010.184002. [DOI] [PubMed] [Google Scholar]
- Tsai KL, Clark LA, Murphy KE. Understanding hereditary diseases using the dog and human as companion model systems. Mamm Genome. 2007;18:444–451. doi: 10.1007/s00335-007-9037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubura A, Lai YC, Miki H, Sasaki T, Uehara N, Yuri T, Yoshizawa K. Review: Animal models of N-Methyl-N-nitrosourea-induced mammary cancer and retinal degeneration with special emphasis on therapeutic trials. In Vivo. 2011;25:11–22. [PubMed] [Google Scholar]
- Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Redenti SM, Jiang C, Swift JS, Klassen HJ, Smith ME, Wnek GE, Young MJ. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials. 2010;31:9–19. doi: 10.1016/j.biomaterials.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Turner JE, Blair JR. Newborn rat retinal cells transplanted into a retinal lesion site in adult host eyes. Dev Brain Res. 1986;26:91–104. doi: 10.1016/0165-3806(86)90011-8. [DOI] [PubMed] [Google Scholar]
- van Zeeburg EJ, Maaijwee KJ, Missotten TO, Heimann H, van Meurs JC. A free retinal pigment epithelium-choroid graft in patients with exudative age-related macular degeneration: results up to 7 years. Am J Ophthalmol. 2012;153:120–127. e122. doi: 10.1016/j.ajo.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local Retinal Circuits of Melanopsin- Containing Ganglion Cells Identified by Transsynaptic Viral Tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Neurotrophins in the developing and regenerating visual system. Histol Histopathol. 1998;13:437–459. doi: 10.14670/HH-13.437. [DOI] [PubMed] [Google Scholar]
- Vugler A, Carr AJ, Lawrence J, Chen LL, Burrell K, Wright A, Lundh P, Semo M, Ahmado A, Gias C, da Cruz L, Moore H, Andrews P, Walsh J, Coffey P. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008a;214:347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Vugler AA, Semo M, Joseph A, Jeffery G. Survival and remodeling of melanopsin cells during retinal dystrophy. Vis Neurosci. 2008b;25:125–138. doi: 10.1017/S0952523808080309. [DOI] [PubMed] [Google Scholar]
- Wang HC, Brown J, Alayon H, Stuck BE. Transplantation of quantum dot-labelled bone marrow-derived stem cells into the vitreous of mice with laser-induced retinal injury: survival, integration and differentiation. Vision Res. 2010a;50:665–673. doi: 10.1016/j.visres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Wang NK, Tosi J, Kasanuki JM, Chou CL, Kong J, Parmalee N, Wert KJ, Allikmets R, Lai CC, Chien CL, Nagasaki T, Lin CS, Tsang SH. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation. 2010b;89:911–919. doi: 10.1097/TP.0b013e3181d45a61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Girman S, Lu B, Bischoff N, Holmes T, Shearer R, Wright LS, Svendsen CN, Gamm DM, Lund RD. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008a;49:3201–3206. doi: 10.1167/iovs.08-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lu B, Girman S, Holmes T, Bischoff N, Lund RD. Morphological and functional rescue in RCS rats after RPE cell line transplantation at a later stage of degeneration. Invest Ophthalmol Vis Sci. 2008b;49:416–421. doi: 10.1167/iovs.07-0992. [DOI] [PubMed] [Google Scholar]
- Wasselius J, Ghosh F. Adult rabbit retinal transplants. Invest Ophthalmol Vis Sci. 2001;42:2632–2638. [PubMed] [Google Scholar]
- Weisz JM, Humayun MS, De Juan E, Jr, Del Cerro M, Sunness JS, Dagnelie G, Soylu M, Rizzo L, Nussenblatt RB. Allogenic fetal retinal pigment epithelial cell transplant in a patient with geographic atrophy. Retina. 1999;19:540–545. doi: 10.1097/00006982-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012;31:136–151. doi: 10.1016/j.preteyeres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenkel H, Streilein JW. Evidence that retinal pigment epithelium functions as an immune- privileged tissue. Invest Ophthalmol Vis Sci. 2000;41:3467–3473. [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- West EL, Pearson RA, Barker SE, Luhmann UF, Maclaren RE, Barber AC, Duran Y, Smith AJ, Sowden JC, Ali RR. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28:1997–2007. doi: 10.1002/stem.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Pearson RA, Tschernutter M, Sowden JC, Maclaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86:601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan JP, McGinnis JF. Light dependent subcellular movement of photoreceptor proteins. J Neurosci Res. 1988;20:263–270. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- Woch G, Aramant RB, Seiler MJ, Sagdullaev BT, McCall MA. Retinal transplants restore visually evoked responses in rats with photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:1669–1676. [PubMed] [Google Scholar]
- Wu DM, Khanna H, Atmaca-Sonmez P, Sieving PA, Branham K, Othman M, Swaroop A, Daiger SP, Heckenlively JR. Long-term follow-up of a family with dominant X-linked retinitis pigmentosa. Eye (Lond) 2010;24:764–774. doi: 10.1038/eye.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Du J, Gouras P, Kjeldbye H. Retinal pigment epithelial transplants and retinal function in RCS rats. Invest Ophthalmol Vis Sci. 1993;34:3068–3075. [PubMed] [Google Scholar]
- Yang P, Seiler MJ, Aramant RB, Whittemore SR. Differential lineage restriction of rat retinal progenitor cells in vitro and in vivo. J Neurosci Res. 2002;69:466–476. doi: 10.1002/jnr.10320. [DOI] [PubMed] [Google Scholar]
- Yang PB, Seiler MJ, Aramant RB, Yan F, Mahoney MJ, Kitzes LM, Keirstead HS. Trophic Factors GDNF and BDNF Improve Function of Retinal Sheet Transplants. Exp Eye Res. 2010a;91:727–738. doi: 10.1016/j.exer.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mohand-Said S, Leveillard T, Fontaine V, Simonutti M, Sahel JA. Transplantation of photoreceptor and total neural retina preserves cone function in P23H rhodopsin transgenic rat. PLoS ONE. 2010b;5:e13469. doi: 10.1371/journal.pone.0013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F, Johkura K, Shirasawa S, Yokoyama T, Inoue Y, Tomotsune D, Sasaki K. Differentiation of primate ES cells into retinal cells induced by ES cell-derived pigmented cells. Biochem Biophys Res Commun. 2010;394:877–883. doi: 10.1016/j.bbrc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, Brown DM, Jaffe GJ, Tao W, Williams GA. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:6241–6245. doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Arner K, Ehinger B, Perez MT. Limitation of anatomical integration between subretinal transplants and the host retina. Invest Ophthalmol Vis Sci. 2003;44:324–331. doi: 10.1167/iovs.02-0132. [DOI] [PubMed] [Google Scholar]
- Zhu D, Deng X, Spee C, Sonoda S, Hsieh CL, Barron E, Pera M, Hinton DR. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest Ophthalmol Vis Sci. 2011;52:1573–1585. doi: 10.1167/iovs.10-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]