Abstract
Immunologic reconstitution following allogeneic hematopoietic cell transplantation (HCT) is a critical component of successful outcome. Umbilical cord blood (UCB) transplantation in adult recipients is associated with slow and often inadequate immune recovery. We characterized the kinetics and extent of immune recovery in 95 adult recipients following a dual UCB (n=29), and matched sibling (MSD) (n=33) or unrelated donor (MUD) (n=33) transplantation. All patients were treated with myeloablative conditioning. There were no differences in the immune recovery profile of MSD and MUD recipients. Significantly lower levels of CD3+, CD4+ and CD8+ T-cells were observed in UCB recipients until 6 months following transplantation. Lower levels of regulatory T-cells persisted until 1 year following transplantation. Thymopoiesis as measured by T-cell receptor rearrangement excision circle (TREC) was comparable among all recipients by 6 months following transplantation. In a subset of patients 1 year following transplantation with similar levels of circulating T-cells and TREC, there was no difference in T-cell receptor diversity. Compared to HLA-identical MSD and MUD adult HCT recipients, quantitative lymphoid recovery in UCB transplant recipients is slower in the first 3 months, but these differences disappeared by 6–12 months following transplantation.
Keywords: adult, dual umbilical cord blood transplantation, matched sibling transplantation, matched unrelated donor transplantation, immune recovery, T-cell receptor excision DNA circles (TRECs), post-thymic T-cell reconstitution
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established cellular therapy for patients with hematologic malignancies, bone marrow failure syndrome, and immune disorders [1]. Following myeloablative allogeneic HCT, host immunity is ablated by the conditioning regimen. Immunologic reconstitution arises from maturation of donor stem cell-derived lymphoid progenitors, and peripheral expansion of mature immune cells included in the donor graft [2, 3]. During the initial neutropenic phase of the HCT, recipients have a high risk of bacterial infection. But a profoundly immunocompromised state continues after neutrophil engraftment due to deficiencies or impairment of cellular immune reconstitution. Initial cellular immune reconstitution following HCT largely depends on thymic-independent peripheral expansion of donor derived memory T cells. This is followed by thymic-dependent maturation of stem cell-derived lymphoid progenitor cells [2, 3]. Because the repertoire of peripherally expanded memory T cells is limited, thymic-dependent maturation is important for diversification of the T-cell repertoire and strengthening host defense against pathogens and even recurrence of malignancy.
Unrelated umbilical cord blood (UCB) has emerged as a viable alternative source of hematopoietic stem cells for adult and pediatric allogeneic HCT [4–6]. In contrast to peripheral blood or bone marrow grafts, UCB grafts contain few, if any antigen-specific memory T cells and a higher proportion of naïve T cells. This limits the potential for thymic-independent immune recovery [7–10], and is felt to be one of the main reasons why adult UCB HCT recipients are more prone to viral infections compared to patients receiving peripheral blood or bone marrow stem cell grafts. The challenge of post-transplant immune reconstitution is further complicated in adult patients as a consequence of thymic atrophy thereby limiting the thymic-dependent pathways of lymphopoiesis [11, 12]. Available data suggest exceedingly slower and less robust immune recovery following adult UCB transplantation than pediatric UCB transplantation [9].
Although immune reconstitution after UCB transplantation has been evaluated by several groups [7–9, 13], there is limited data on how it compares following HCT from other graft sources [14]. We present here results of a comprehensive comparison of immune recovery in adult patients following T-cell-replete myeloablative conditioning and allogeneic dual umbilical cord blood (UCB), matched sibling donor (MSD), or matched unrelated donor (MUD) HCT.
Methods
Patients
Reconstitution of immune cell populations was prospectively characterized in a consecutive cohort of 146 adult patients (≥18 years old) with hematological malignancies undergoing T-cell replete myeloablative HCT, using MSD or MUD peripheral blood stem cells (PBSCs), or dual UCB donor grafts, between April 2006 and December 2010. PBSC recipients were conditioned with either a total body irradiation (TBI)-based regimen (TBI ≥ 12 Gy) or IV busulfan (12.8 mg/kg) (Bu)-based regimen, and UCB transplant recipients were conditioned with a TBI (≥13.2 Gy) and fludarabine (160 mg/m2) (Flu)-based regimen. No patient received in vivo (such as anti-thymocyte globulin) or ex vivo T cell depletion. The algorithm for donor selection was MSD first, followed by a MUD followed by UCB. PBSC grafts were allele-level matched at HLA-A, -B, -C, and -DRB1, whereas dual UCB grafts were at least 4 of 6 HLA-matched with the recipient, and 3 of 6 HLA-matched between grafts (low-resolution for A and B, and high-resolution for DRB1). A total of 34 patients with primary or secondary graft failure (UCB recipients, n = 7; MSD/MUD recipients, n = 1) or those who died or relapsed within 3 months after HCT (UCB recipients, n = 11; MSD recipients, n = 10; MUD recipients, n = 5) were excluded from the analysis of immune reconstitution to focus on comparing immune recovery 3–12 months after transplantation. An additional 17 patients (UCB recipients, n = 3; MSD/MUD recipients, n = 14) were not evaluable due to lack of immune recovery data collection. As a result, 95 patients were included in the analysis of immune reconstitution. In addition to these 95 patients, all consecutive patients (n = 146) were included in the progression-free survival (PFS) outcomes analysis. Standard-risk diseases were defined as acute myelogenous leukemia in 1st or 2nd complete remission, acute lymphoblastic leukemia in 1st or 2nd complete remission, myelodysplastic syndromes; refractory anemia or refractory anemia with excess of blasts-1, Hodgkin or non-Hodgkin lymphoma in any chemotherapy-sensitive remission, chronic myelogenous leukemia in 1st or 2nd chronic phase, and myelofibrosis. High-risk diseases were those other than standard-risk disease. All research samples were collected after obtaining written informed consent for participation in accordance with the Declaration of Helsinki on a protocol approved by Duke University Medical Center Institutional Review Board.
Measurement of immune recovery
Quantification of the following subsets was performed by flow cytometry on fresh peripheral blood at approximately 1 month before transplantation, and then 1.5, 3, 6, and 12 months after transplantation [15, 16]: NK (CD3−, CD16+/CD56+) and NKT (CD3+, CD16+/CD56+) cells, B cells (CD19+, CD3−, CD16−, CD56−), CD3+, CD4+, CD8+, regulatory (CD4+, CD25+, CD62L+), cytotoxic/late memory (CD8+, CD57+, CD28−), and activated (CD8+, HLA-DR+) T cells, naïve CD4+ T cells with L-selectin expression, which is suggestive of recent thymic emigrants (RTE) (CD4+, CD45RA+/CD45RO−, CD62L+), plasmacytoid dendritic cells (CD123+, CD11c−), and myeloid dendritic cells (CD123−, CD11c+).
Quantification of recent thymic immigrants as determined by the presence of T-cell receptor rearrangement excision circles (TREC) was retrospectively performed by real-time quantitative-PCR of DNA collected from an isolated fraction of CD3+ T cells, as previously described [17]. Samples were analyzed in duplicate and expressed as TREC per 10,000 CD3+ T cells. Spectratyping was performed to analyze diversity in the T-cell receptor (TCR) repertoires produced by the rearrangements of the variable region genes [18, 19]. Selected cDNA samples underwent survey-level sequencing for TCRβ repertoire analysis (Adaptive TCR Corporation with ImmunoSeq) [20, 21]. A standard algorithm was used to identify which V, D, and J segments comprised each TCRβ CDR3. Sequence reads from each donor T cell sample were determined to be productive or non-productive, based on CDR3 sequences. CDR3 sequences that could result in a functional TCR were considered to be productive rearrangements. Entropy (i.e. Shannon Entropy), a measure of the uniformity of the frequency distribution of a TCRβ repertoire, was performed for productive clones as follows: Entropy = sum over all clones of −1 * [(frequency of clone) * (log2 frequency of clone)]. Entropy is reported in bits and it ranges from 0 in a sample with only one clone to log2(# of unique clones) for a sample with a uniform distribution of clone frequencies. Monoclonal or oligoclonal samples have low entropy, and polyclonal highly diverse samples have an entropy just under log2(# uniques).
Statistical analysis
The mean ages for the three transplant types were compared using the ANOVA test. The median follow-up periods of survivors were compared using the Krusukal-Wallis test. Pearson’s Chi-square test of proportions was used to compare associations between clinical factors and transplant type. The Wilcoxon rank-sums test was used to compare immune recovery parameters at approximately 1 month before transplantation, and 1.5, 3, 6, and 12 months after transplantation, adjusting P-values for multiple comparison with the Adaptive Holms step-down Bonferroni method [22]. The same method was also used to compare the number of TRECs at 1 month before transplantation, and 3, 6, and 12 months post-transplantation. Acute or chronic graft-versus-host-disease (GVHD) was characterized using standard criteria [23, 24]. CMV reactivation was defined as positive if more than 200 copies of CMV were amplified in peripheral blood by real-time quantitative-PCR. The actual probabilities along with 95% confidence intervals (CI) of acute and chronic GVHD, CMV reactivation and disease, and treatment-related mortality were estimated on the basis of cumulative incidence curves to accommodate the following competing events [25]: death without GVHD for acute and chronic GVHD, death without CMV reactivation/disease for CMV reactivation/disease, and relapse for treatment-related mortality; the groups were compared using Gray’s test [26]. Cumulative corticosteroid usage beginning on the day of transplantation until the 3 months, 6 months, or 1 year time-point following transplantation was determined by area under the curve (AUC) [27]. The central tendency of corticosteroid AUC was compared using the Wilcoxon rank-sums test. T-cell receptor diversity, expressed as an Entropy score, was compared using a student t-test. PFS was defined as period from 3 month after transplantation until disease progression or death, whichever occurred first and censored at time of last follow-up. The probability of PFS was estimated according to the Kaplan-Meier method, and groups were compared using the log-rank test. Cox proportional hazards multivariate regression modeling was used to predict PFS. The following variables were analyzed in a bivariate model adjusted for donor type (MSD/MUD or UCB) as well as in a univariate model in the MSD/MUD group; recipient age (≤41 (median age) or >41), recipient sex, disease (myeloid or lymphoid disease), type of conditioning regimen (TBI- or busulfan-based regimen), GVHD prophylaxis (cyclosporine- or tacrolimus-based, or other), acute GVHD (no and grade I or grade II-IV acute GVHD), and each lymphocyte subset at 3 months after transplantation dichotomized at the median value. Due to few events, a parallel analysis was not performed in the UCB group. All tests were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC) and Stata (Stata Corp., College Station, TX).
Results
Patient characteristics
Patient characteristics are shown in Table 1. The UCB recipients (mean age, 36; range, 19–55 years) were younger than MSD recipients (mean age, 45; range, 24–65 years) and MUD recipients (mean age, 41; range, 20–56 years) (P < 0.01). All UCB recipients received a myeloablative dose of TBI and fludarabine as a part of conditioning regimen, while half of MSD/MUD recipients received a non-TBI, busulfan-based conditioning regimen. Two-thirds of all patients received transplants for acute myelogenous leukemia or myelodysplastic syndromes. Most of the UCB and MUD recipients received tacrolimus-based GVHD prophylaxis, whereas 40% of the MSD recipients received cyclosporine-based GVHD prophylaxis.
Table 1.
Patient characteristics
| Characteristics | Umbilical cord blood | Matched sibling peripheral blood |
Matched unrelated peripheral blood |
P value |
|---|---|---|---|---|
| n = 29 | n = 33 | n = 33 | ||
| Age (years), mean (range) | 36 (19–55) | 45 (24–65) | 41 (20–56) | <0.01 |
| Recipient sex | 0.44 | |||
| Female | 12 (41%) | 16 (48%) | 19 (58%) | |
| Male | 17 (59%) | 17 (52%) | 14 (42%) | |
| Disease | -* | |||
| Myeloid disease | ||||
| AML | 18 (62%) | 16 (48%) | 21 (64%) | |
| CML | 2 (7%) | 3 (9%) | 1 (3%) | |
| MDS | 2 (7%) | 6 (18%) | 3 (9%) | |
| MF | 0 (0%) | 2 (6%) | 0 (0%) | |
| Lymphoid disease | ||||
| ALL | 5 (17%) | 5 (15%) | 5 (15%) | |
| ML | 2 (7%) | 1 (3%) | 3 (9%) | |
| Disease risk | 0.58 | |||
| Standard | 25 (86%) | 25 (76%) | 26 (79%) | |
| High | 4 (14%) | 8 (24%) | 7 (21%) | |
| AML/ALL | ||||
| CR1 | 5 (22%) | 10 (48%) | 14 (54%) | <0.01 |
| CR2 | 16 (70%) | 5 (24%) | 10 (38%) | |
| CR3+ | 2 (9%) | 6 (29%) | 2 (8%) | |
| History of previous chemothearpy (ML) | ||||
| ≤3 courses | 2 | 0 | 2 | 0.60 |
| >4 courses | 0 | 1 | 1 | |
| Conditioning regimen | <0.01 | |||
| TBI-based | 29 (100%) | 14 (42%) | 19 (58%) | |
| TBI + cyclophosphamide | 0 | 10 | 16 | |
| TBI + etoposide | 0 | 3 | 3 | |
| TBI + fludarabine | 20 | 1 | 0 | |
| TBI + fludarabine + cyclophosphamide | 6 | 0 | 0 | |
| TBI + fludarabine + thiotepa | 3 | 0 | 0 | |
| Busulfan-based | 0 (0%) | 19 (58%) | 14 (42%) | |
| Busulfan + cyclophosphamide | 0 | 9 | 12 | |
| Busulfan + fludarabine | 0 | 10 | 2 | |
| GVHD prophylaxis | <0.01 | |||
| Cyclosporin-based | 5 (17%) | 13 (39%) | 0 (0%) | |
| Cyclosporin + methotrexate | 0 | 12 | 0 | |
| Cyclosporin + MMF | 5 | 0 | 0 | |
| Cyclosporin + sirolimus | 0 | 1 | 0 | |
| Tacrolimus-based | 24 (83%) | 20 (61%) | 33 (100%) | |
| Tacrolimus + methotrexate | 0 | 17 | 31 | |
| Tacrolimus + MMF | 24 | 0 | 0 | |
| Tacrolimus + sirolimus | 0 | 3 | 2 | |
| CMV serostatus | 0.52 | |||
| Negative for both recipient and donor | 5 (17%) | 7 (21%) | 3 (9%) | |
| Positive for either recipient or donor | 20 (69%) | 19 (58%) | 21 (64%) | |
| Indeterminate/unknown | 4 (14%) | 7 (21%) | 9 (27%) | |
| Median follow-up period of survivors (range) (months) | 25.3 (3.7–57.5) | 21.1 (4.2–46.3) | 24.5 (3.7–47.9) | 0.91 |
No statistical test is provided due to small sample size.
Abbreviations: AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; MF, myelofibrosis; ALL, acute lymphoblastic leukemia; ML, malignant lymphoma; TBI, total-body irradiation; GVHD, graft-versus-host disease; MMF, Mycophenolate mofetil; cyclosporine-based, cyclosporine with or without other agents; tacrolimus-based, tacrolimus with or without other agents; CMV, cytomegalovirus.
The median combined total nucleated cell dose for the UCB recipients was 4.6 (range 3.3–8.6, n = 29)×107 /kg. Each unit of the UCB graft contained a minimum of 1.5×107 /kg. The median total nucleated cell dose for the MSD and MUD grafts was 13.0 (3.6–31.7, n = 31)×108 /kg and 8.1 (3.2–15.1, n = 32)×108/kg, respectively, and the median CD34+ cell dose for the MSD and MUD grafts was 5.2 (1.9–11, n = 32)×106 /kg and 7.5 (2.4–9.7, n = 32)×106/kg, respectively.
Immune recovery
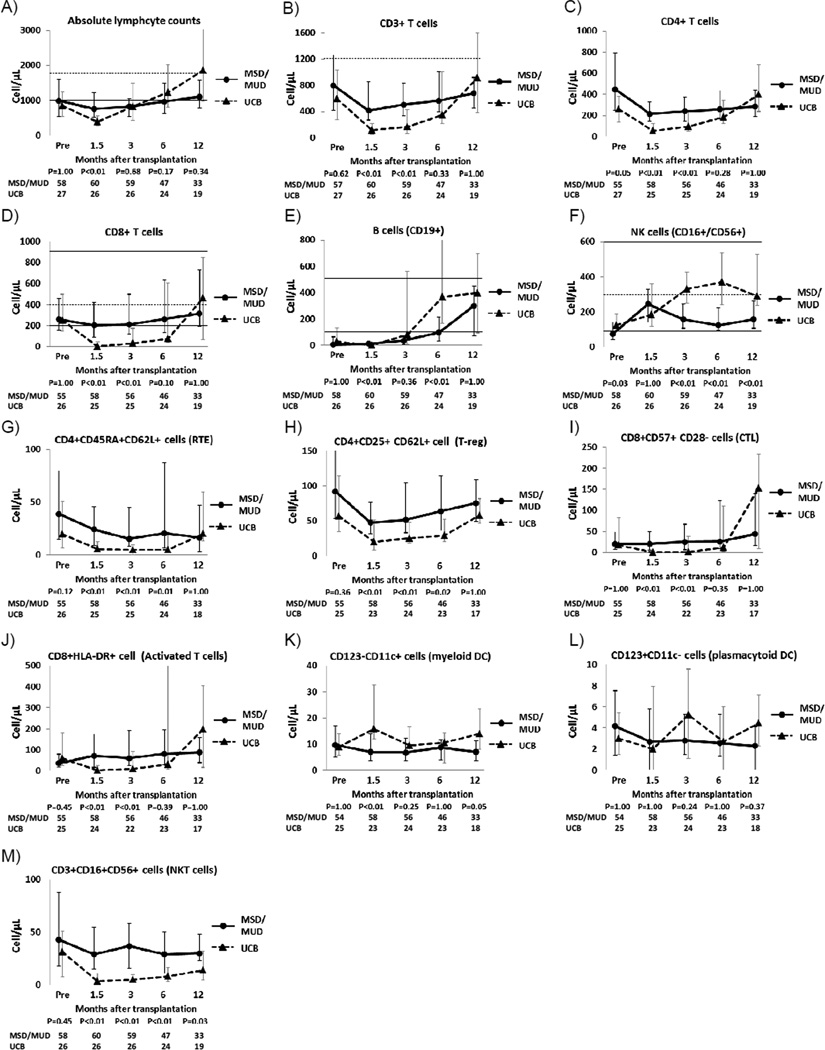
Because there were no significant differences in the kinetics of immune recovery and PFS between MSD and MUD recipients (supplemental figure 1), data on immune recovery of MSD and MUD recipients were combined and then compared with those of UCB recipients (Figure 1). The absolute lymphocyte count was lower in the UCB recipients at 1.5 months after transplantation but reached the same level as MSD/MUD recipients by 3 months after transplantation. The number of CD3+, CD4+, and CD8+ cells was significantly lower in the UCB recipients at 1.5 and 3 months after transplantation. This trend continued at the 6-month time-point, but the difference was no longer statistically significant. At 12 months after transplantation, there was no detectable difference in CD3+, CD4+ and CD8+ T-cell counts in the UCB recipients and the MSD/MUD recipients. Recovery of B-cells was highly variable in the UCB recipients. Overall, B-cell recovery was faster in the UCB recipients than the MSD/MUD recipients at 6 months after transplantation, but this difference was lost by 12 months. The NK cell counts were significantly higher in the UCB recipients at 3, 6, and 12 months after transplantation. Recent thymic emigrant (naïve CD4+ T cells with CD62L expression) and regulatory T-cell populations (CD4+CD25+CD62L+) were significantly lower in the UCB recipient at 1.5, 3, and 6 months after transplantation. Cytotoxic T cell (CD8+CD57+CD28−) and activated T cell (CD8+HLA DR+) counts were lower in the UCB recipients at 1.5 and 3 months after transplantation. There was no significant difference in the plasmacytoid dendritic cell counts, while the myeloid dendritic cell counts were higher in the UCB recipients at 1.5 months after transplantation. The number of NKT cells was significantly lower in UCB recipients throughout the period of observation.
Figure 1.
Sequential changes of immune cell populations after transplantation
Black line shows matched sibling donor/matched unrelated donor (MSD/MUD) recipients and dotted line shows umbilical cord blood (UCB) recipients. Abbreviations; RTE, recent thymic emigrant; T-reg, regulatory T cell; CTL, cytotoxic T cell; DC, dendritic cells. The median values are shown as dots and the ends of the whiskers indicate the 25% and 75% percentile values. Available median and 5%/95% percentiles of healthy adults are shown in dotted and solid horizontal lines.[44]
TREC and TCR β repertoire
To further evaluate thymic-dependent T-cell recovery, we measured TREC levels for 11 UCB and 21 MSD/MUD recipients. TREC levels were lower in the UCB recipients at 3 months following transplantation, but comparable by the 6 month time-point. TREC levels were uniformly low irrespective of donor type, even at 12 months following transplantation (Figure 2). The median TREC level per 105 CD3+ T cells for the UCB and MSD/MUD recipients was 80 (range 0–40, n = 9) and 415 (range 0–4460, n = 20) (P = 0.04) pre-transplantation, 62 (range 0–482, n = 10) and 209 (range 0–4680, n = 20) (P = 0.09) at 3 months post transplantation, 414 (range 0–14740, n = 11) and 232 (range 0–4200, n = 21) (P = 0.86) at 6 months post transplantation, and 517 (range 14–2400, n = 10) and 244 (range 0–6900, n = 19) (P = 0.87) at 12 months post transplantation, respectively. T-cell receptor diversity was assessed via survey-level TCRβ sequencing on a subset of 10 patients (MSD, n = 3; MUD, n = 3; UCB, n = 4) who demonstrated comparable levels of TREC positive cell recovery at 12 months (Table 2). Representative profiles of TCR Vb gene usage in peripheral blood T cells from recipients of MSD, MUD, UCB, and a healthy control are shown in Figure 3. T-cell receptor diversity (Entropy value; healthy control, 11.95) in the UCB recipients was comparable to that of the MSD/MUD recipients (average [standard deviation] of entropy value; UCB, 9.33 ± 3.85; MSD/MUD, 7.71 ± 2.26; P = 0.47).
Figure 2.
Sequential changes in T-cell receptor rearrangement excision DNA circles (TRECs) before and after transplantation
Table 2.
TCR repertoire
| Donor type | TREC (100,000 CD3+ cells) |
Productive Total |
Productive Uniques |
Entropy* | P value | |
|---|---|---|---|---|---|---|
| Value | Ave. (+−SD) | |||||
| MSD 1 | 2140 | 711514 | 10500 | 11.84 | 9.33 (+−3.85) | ref. |
| MSD 2 | 1046 | 485 | 15 | 3.02 | ||
| MSD 3 | 1970 | 6653 | 158 | 6.02 | ||
| MUD 1 | 4080 | 633850 | 7532 | 11.27 | ||
| MUD 2 | 1262 | 493739 | 11957 | 11.90 | ||
| MUD 3 | 1982 | 547860 | 13300 | 11.93 | ||
| UCB 1 | 2400 | 42526 | 467 | 7.49 | 7.71 (+−2.26) | 0.47 |
| UCB 2 | 784 | 519744 | 9128 | 10.61 | ||
| UCB 3 | 436 | 92930 | 1981 | 5.10 | ||
| UCB 4 | 598 | 174762 | 1445 | 7.62 | ||
Entropy is a measure of the uniformity of the frequency distribution of a TCRβ repertoire. Monoclonal or oligoclonal samples have low entropy, and polyclonal highly diverse samples have an entropy just under log2(# uniques). The value of Entropy in a healthy control was 11.95.
Abbreviations: MSD, matched sibling donor; MUD, matched unrelated donor; UCB, umbilical cord blood; Ave., average; SD, standard deviation; ref., reference.
Figure 3.
Representative profiles of TCR Vb gene usage in peripheral blood T cells from recipients of matched sibling donor (A), matched unrelated donor (B), umbilical cord blood transplantation (C), and healthy control (D)
Graft-versus-host disease and corticosteroid exposure
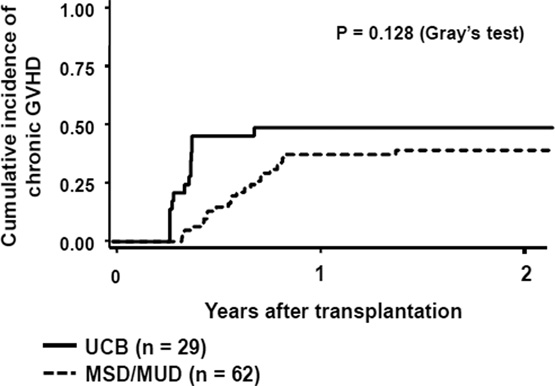
To thoroughly assess the potential confounding effect of GVHD on post-transplantation immune recovery, we compared the cumulative incidence of grade II–IV and grade III-IV acute GVHD and chronic GVHD (Figure 4). The incidence of grade II–IV acute GVHD was significantly higher among the UCB recipients compared to the MSD/MUD recipients (0.66 [95% CI: 0.45–0.80] vs. 0.38 [95% CI: 0.26–0.50, respectively]; Gray’s test, P = 0.006). The incidence of grade III-IV acute GHVD was also higher in the UCB vs. MSD/MUD recipients (0.28 [95% CI: 0.13–0.45] vs. 0.09 [95% CI: 0.04–0.18, respectively]; Gray’s test, P = 0.018). Conversely, there was no significant difference in the incidence of chronic GVHD at 1 year between the UCB and MSD/MUD recipients (0.48 [95% CI: 0.29–0.65] vs. 0.37 [95% CI: 0.25–0.49, respectively]; Gray’s test, P = 0.128). Since the persistence and treatment-responsiveness of GVHD may differ depending on graft source, we assessed cumulative corticosteroid exposure (mg/kg) from 0 to 3, 6, or 12 months following transplantation (Table 3). Consistent with the observed differences in clinical GVHD, the cumulative corticosteroid exposure at 3 months and 6 months following transplantation was significantly higher in the UCB recipients than in the MSD/MUD recipients. However, at 12 months following transplantation, there was no difference in cumulative corticosteroid exposure between the two groups. We also evaluated the cumulative corticosteroid exposure only for those who received corticosteroid treatment for GVHD. The cumulative corticosteroid exposure at 3, 6, or 12 months was not statistically different between the three groups, suggesting treatment response to GVHD was similar regardless of the donor type (Table 3).
Figure 4.
Cumulative incidence of grade II-IV (A) and grade III-IV (B) acute GVHD and chronic GVHD (C) after transplantation
Black line shows umbilical cord blood (UCB) recipients and dotted line shows matched sibling donor/matched unrelated donor (MSD/MUD) recipients
Table 3.
Comparison of cumulative corticosteroid dose
| Corticosteroid exposure | Umbilical cord blood | Matched sibling /unrelated donor |
P value | ||
|---|---|---|---|---|---|
| n | Median AUC (range) | n | Median AUC (range) | ||
| For all patients | |||||
| 0–3 months | 29 | 41.8 (0–247) | 66 | 0 (0–285) | 0.03 |
| 0–6 months | 26 | 59 (0–285) | 49 | 0 (0–286) | <0.01 |
| 0–12 months | 21 | 96 (0–301) | 36 | 74 (0–382) | 0.13 |
| For patients who received corticosteroids | |||||
| 0–3 months | 18 | 60 (24–247) | 25 | 64 (10–285) | 0.65 |
| 0–6 months | 23 | 78 (24–285) | 31 | 78 (10–286) | 0.43 |
| 0–12 months | 23 | 109 (24–301) | 40 | 106 (19–382) | 0.96 |
Abbreviations: AUC, area under the curve.
We then evaluated the impact of grade II-IV and III-IV acute GVHD on immune recovery at 3, 6, 12 months after transplantation. We found that grade III-IV acute GVHD (but not grade II-IV) significantly delayed the recovery of several immune cell populations at 3 months after transplantation (data not shown). Grade II-IV or III-IV acute GVHD did not have any impact on immune recovery at 6 and 12 months after transplantation. Therefore, we performed an additional analysis in which we compared the immune recovery of the UCB group with that of the MSD/MUD group in patients with grade 0-I vs. grade II-IV acute GVHD or patients with grade 0-II vs. grade III-IV acute GVHD (Table 4). Regardless of the presence of grade II-IV acute GVHD, immune cell recovery other than B or NK cells was slower in the UCB group as compared with the MSD/MUD group. Among patients with grade III-IV aGVHD, median values of immune cell populations were lower in the UCB group than in the MSD/MUD group, although it was not significant partly due to small sample size and partly due to the impact of grade III-IV acute GVHD on immune cell recovery.
Table 4.
Recovery of immune cell populations according to the grade of acute GVHD
| MSD/MUD median (range) at 3 months |
UCB median (range) at 3 months |
P value | |
|---|---|---|---|
| Grade 0–I aGVHD | n = 41 | n = 10 | |
| Absolute lymphocyte counts | 832 (35–3359) | 1297 (481–5275) | 0.02 |
| CD3+ T cells | 473 (13–2714) | 306 (25–2986) | 0.30 |
| CD4+ T cells | 233 (82–712) | 134 (21–743) | 0.13 |
| CD8+ T cells | 189 (37–1734) | 33 (2–2240) | 0.05 |
| B cells (CD19+) | 54 (0–496) | 663 (0–1287) | <0.01 |
| NK cells (CD16+/CD56+) | 153 (21–1203) | 373 (223–1049) | <0.01 |
| CD4+CD45RA+CD62L+ cells (RTE) | 16 (4–333) | 1 (0–20) | <0.01 |
| CD4+CD25+CD62L+ cells (T-reg) | 49 (18–197) | 30 (7–89) | <0.01 |
| CD8+CD57+CD28– cells (CTL) | 24 (1–902) | 3 (0–291) | 0.22 |
| CD8+HLA−DR+ cells (Activated T cells) | 53 (5–919) | 11 (0–1702) | 0.28 |
| CD123−CD11c+ cells (myeloid DC) | 4 (0–31) | 8 (2–18) | <0.01 |
| CD123+CD11c− cells (plasmacytoid DC) | 7 (0–39) | 12 (5–24) | 0.05 |
| CD3+CD16+CD56+ cells (NKT cells) | 35 (3–424) | 5 (0–62) | <0.01 |
| Grade 0–II aGVHD | n = 60 | n = 21 | |
| Absolute lymphocyte counts | 837 (35–3359) | 911 (310–5275) | 0.48 |
| CD3+ T cells | 529 (13–2741) | 249 (3–2986) | <0.01 |
| CD4+ T cells | 242 (78–812) | 131 (2–743) | <0.01 |
| CD8+ T cells | 219 (37–2262) | 32 (1–2240) | <0.01 |
| B cells (CD19+) | 35 (0–496) | 104 (0–1287) | 0.01 |
| NK cells (CD16+/CD56+) | 160 (21–1203) | 360 (179–1049) | <0.01 |
| CD4+CD45RA+CD62L+ cells (RTE) | 15 (2–333) | 5 (0–25) | <0.01 |
| CD4+CD25+CD62L+ cells (T-reg) | 53 (11–197) | 28 (0–149) | <0.01 |
| CD8+CD57+CD28− cells (CTL) | 28 (1–902) | 3 (0–291) | 0.01 |
| CD8+HLA−DR+ cells (Activated T cells) | 64 (5–1923) | 13 (0–1702) | 0.02 |
| CD123−CD11c+ cells (myeloid DC) | 3 (0–31) | 6 (0–21) | <0.01 |
| CD123+CD11c− cells (plasmacytoid DC) | 7 (0–39) | 10 (3–25) | 0.06 |
| CD3+CD16+CD56+ cells (NKT cells) | 37 (3–424) | 5 (0–62) | <0.01 |
| Grade II–IV aGVHD | n = 25 | n = 19 | |
| Absolute lymphocyte counts | 817 (108–2879) | 478 (130–6434) | 0.13 |
| CD3+ T cells | 608 (82–2741) | 131 (3–2539) | <0.01 |
| CD4+ T cells | 253 (78–591) | 59 (2–1194) | <0.01 |
| CD8+ T cells | 329 (50–2262) | 32 (1–1291) | <0.01 |
| B cells (CD19+) | 10 (0–240) | 20 (0–781) | 0.77 |
| NK cells (CD16+/CD56+) | 160 (15–422) | 317 (57–2686) | 0.01 |
| CD4+CD45RA+CD62L+ cells (RTE) | 13 (0–170) | 6 (0–25) | 0.09 |
| CD4+CD25+CD62L+ cells (T-reg) | 54 (11–207) | 25 (0–149) | 0.02 |
| CD8+CD57+CD28− cells (CTL) | 33 (4–362) | 1 (0–103) | <0.01 |
| CD8+HLA−DR+ cells (Activated T cells) | 104 (9–1922) | 9 (0–1226) | 0.01 |
| CD123−CD11c+ cells (myeloid DC) | 1 (0–16) | 3 (0–46) | 0.65 |
| CD123+CD11c− cells (plasmacytoid DC) | 5 (0–36) | 9 (0–80) | 0.77 |
| CD3+CD16+CD56+ cells (NKT cells) | 43 (5–157) | 5 (0–124) | <0.01 |
| Grade III–IV aGVHD | n = 6 | n = 8 | |
| Absolute lymphocyte counts | 550 (108–2024) | 348 (130–6434) | 1.00 |
| CD3+ T cells | 305 (82–1518) | 65 (48–2539) | 0.37 |
| CD4+ T cells | 239 (85–591) | 51 (9–1194) | 0.41 |
| CD8+ T cells | 179 (50–852) | 30 (1–1291) | 0.85 |
| B cells (CD19+) | 9 (0–240) | 0 (0–781) | 1.00 |
| NK cells (CD16+/CD56+) | 121 (15–357) | 194 (57–2686) | 1.00 |
| CD4+CD45RA+CD62L+ cells (RTE) | 15 (0–59) | 6 (0–24) | 1.00 |
| CD4+CD25+CD62L+ cells (T-reg) | 47 (27–207) | 21 (0–72) | 0.28 |
| CD8+CD57+CD28− cells (CTL) | 7 (4–40) | 1 (0–77) | 0.84 |
| CD8+HLA−DR+ cells (Activated T cells) | 43 (13–417) | 2 (0–1226) | 1.00 |
| CD123−CD11c+ cells (myeloid DC) | 1 (0–16) | 0 (0–46) | 1.00 |
| CD123+CD11c− cells (plasmacytoid DC) | 5 (3–36) | 6 (0–80) | 1.00 |
| CD3+CD16+CD56+ cells (NKT cells) | 41 (5–101) | 7 (2–124) | 0.99 |
We additionally attempted to identify differences in the number of specific lymphocyte populations in those who did and did not develop chronic GVHD. We did not find any significant difference in these two groups for each immune cell population (data not shown).
CMV reactivation and disease
The cumulative incidence of CMV reactivation among patients at risk of CMV reactivation (serostatus; positive for either recipient or donor) was 0.84 (95% CI: 0.55–0.95) and 0.53 (95% CI: 0.36–0.67) in the UCB and MSD/MUD recipients, respectively (Gray’s test, P = 0.046) (Figure 5), which corresponded with delayed recovery of T cells in the UCB recipients. The cumulative incidence of CMV diseases was 0.21 (0.07–0.41) and 0.03 (0.002–0.11) in the UCB and MSD/MUD recipients, respectively (Gray’s test, P = 0.019). All cases of CMV disease involved the intestinal tract, and none of the patients died of CMV disease. To exclude the effect of acute GVHD on CMV reactivation and disease, we further evaluated the cumulative incidence of CMV in the UCB and MSD/MUD recipients according to the presence of grade II-IV acute GVHD. The incidence of CMV reactivation and disease was consistently higher in the UCB group compared to the MSD/MUD group (CMV reactivation; grade 0-I aGVHD, 57% vs. 36%, P = 0.574; grade II-IV aGVHD, 100% vs. 72%, P = 0.169, CMV disease; grade 0-I aGVHD, 14% vs. 0%, P = 0.076; grade II-IV aGVHD, 25% vs. 6%, P = 0.137), although these differences were not statistically significant due to the small sample size in each stratified category.
Figure 5.
Cumulative incidence of CMV reactivation after transplantation
Black line shows umbilical cord blood (UCB) recipients and dotted line shows matched sibling donor/matched unrelated donor (MSD/MUD) recipients
Progression-free survival
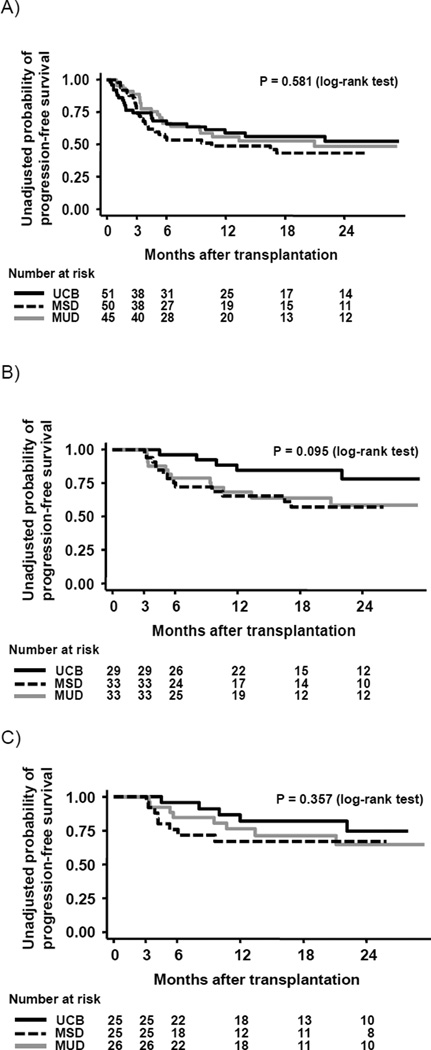
Since the kinetics of post-transplantation immune recovery is expected to significantly impact treatment related mortality and relapse, we compared PFS and TRM by graft type for all consecutive patients (n = 146) who underwent HCT at our center, and met the pre-defined eligibility criteria for this study. PFS at 1 year (95% CI) for UCB, MSD, and MUD recipients was 0.59 (0.44–0.72), 0.49 (0.34–0.62), and 0.56 (0.40–0.69), respectively, without significant difference between the 3 groups (log-rank test, P = 0.581) (Figure 6A). Cumulative incidence of TRM at 1 year (95% CI) for UCB, MSD, and MUD recipients was 0.28 (0.16–0.41), 0.10 (0.04–0.20), and 0.16 (0.07–0.28), respectively (Gray test, P = 0.030). Causes of treatment-related death within 1 year after transplantation are shown in Table 5. We then compared PFS among the patients in whom immune reconstitution was assessed. This select group of patients survived at least 3 months following transplantation in order to be evaluable for immune recovery. There continued to be no statistical difference in PFS in UCB, MSD and MUD recipients when the analysis included both standard-risk and high-risk patients (PFS at 1 year; 0.85 (0.64–0.94), 0.65 (0.46–0.79), and 0.68 (0.49–0.82), respectively; log-rank test, P = 0.095) (n = 95) (Figure 6B) or when the analysis was limited to patients with standard-risk hematological malignancies (PFS at 1 year; 0.81 (0.57–0.92), 0.67 (0.44–0.82), and 0.76 (0.55–0.89), respectively; log-rank test, P = 0.357) (n = 76) (Figure 6C).
Figure 6.
Progression-free survival after transplantation for all consecutive patients undergoing hematopoietic cell transplantation during the study period (A) and patients who survived at least 3 months without death or relapse after transplantation (B; all patients, C; standard-risk patients)
Black line shows umbilical cord blood (UCB) recipients, dotted line shows matched sibling donor (MSD) recipients, and gray line shows matched unrelated donor (MUD) recipients.
Table 5.
Causes of treatment-related death within 1 year after transplantation
| UCB | MSD | MUD | |
|---|---|---|---|
| Graft failure | 1 (7%) | 0 | 0 |
| GVHD | 1 (7%) | 0 | 0 |
| Infection | 8 (57%) | 1 (20%) | 4 (57%) |
| Organ failure | 3 (21%) | 2 (40%) | 3 (43%) |
| Other | 1 (7%) | 2 (40%) | 0 |
| Total | 14 | 5 | 7 |
Abbreviations: UCB, umbilical cord blood; MSD, matched sibling donor; MUD, matched unrelated donor
To assess the impact of recovery of specific lymphocyte subsets on PFS, we tested the various immune cell populations at 3 months after transplantation and other clinical factors on their ability to predict PFS using a 3-month landmark analysis among evaluable 69 patients (irrespective of donor type) with standard-risk hematological malignancies. Patient characteristics were not associated with PFS in the univariate analysis. In the bivariate analysis controlling for donor type, higher numbers of T cells (P = 0.016), Treg (P = 0.014), CTL (P = 0.041), and myeloid DC (P = 0.028) were significantly associated with improved PFS (Table 6). In the MSD/MUD group, the myeloid DC subset was the only significant variable (hazard ratio 4.05, 95% CI, 1.13–14.60, P = 0.032) (Table 6).
Table 6.
Improved progression free survival with recovery of specific lymphocyte subsets among standard-risk patients
| Variables | Comparison | Total (n = 69) | MSD/MUD group (n = 47) | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| T cells (CD3+) | High vs. Low | 3.33 (1.26–8.84) | 0.016 | 1.68 (0.61–4.64) | 0.319 |
| Regulatory T cells (CD4+CD25+CD62L+) |
High vs. Low | 3.84 (1.31–11.30) | 0.014 | 2.72 (0.85–8.71) | 0.093 |
| Cytotoxic T cells (CD8+CD57+CD28−) |
High vs. Low | 3.01 (1.05–8.64) | 0.041 | 2.99 (0.94–9.58) | 0.065 |
| Myloid dendritic cells (CD123−CD11c+) |
High vs. Low | 3.66 (1.15–11.67) | 0.028 | 4.05 (1.13–14.60) | 0.032 |
Standard-risk patients are divided into the high vs. low group according to the median value of each lymphocyte subset. Median value (cells/µL) for T cells, regulatory T cells, cytotoxic T cells, and myeloid dendritic cells are 439, 43.9, 19.0, and 8.8 for all patients, respectively, and 505, 57.8, 23.5, and 7.2 for the matched sibling donor/ matched unrelated donor group, respectively. Only significant variables analyzed among the standard-risk patients were shown.
Abbreviations: HR, hazard ratio; CI, confidence interval; MSD, matched sibling donor; MUD, matched unrelated donor.
Discussion
The past 10 years have brought great strides toward improvement in outcome of UCB transplantation in adult patients [28–31]. Progression-free and overall survival rates now approach that of HCT from matched adult donors [32, 33]. We therefore found it to be an opportune time to perform a comprehensive comparison of immune reconstitution in adult recipients following matched sibling, matched unrelated and umbilical cord blood transplantation. We found that 1) recovery of critical T cell subsets in the UCB recipients was delayed, but reached levels comparable to recipients of MSD/MUD transplantation by 12 months after transplantation, 2) NK and B cell recovery was more rapid in UCB recipients, 3) there was no significant difference in frequency of recent thymic emigrant as measured by CD3+ T-cells containing TRECs, and 4) the T cell repertoire was comparably diversified in the UCB and MSD/MUD recipients at 12 months after transplantation. Finally, we confirm the finding of others that the PFS between recipients of UCB and MSD/MUD HCT is comparable.
The recovery of nearly all the critical T-cell subsets was substantially delayed in the UCB group until 3 to 6 months after transplantation, exposing the patients to an increased risk of viral infection. In fact, the incidence of CMV reactivation reached a plateau at 2 months after MSD/MUD transplantation, while the incidence continuously increased until 6 months after UCB transplantation. This translated into a significant difference in the incidence of CMV reactivation between the 2 groups. This prolonged period of vulnerability in UCB recipients has been described by others [13,14, 34]. However, quantitative differences in T-cell subsets were largely erased by 1 year following transplantation. The kinetics and degree of immune reconstitution observed in our cohort of UCB transplant recipients compares favorably to an earlier report by Komanduri et al who found that the number of CD4+ and CD8+ T-cells was very low at 6 months following transplantation, and remains so even at the 1 year time-point [8]. The likely explanation for this difference is that our UCB recipients did not receive anti-thymocyte globulin as part of the preparative regimen. Our findings are similar to those reported recently by Jacobson and colleagues [14] who compared kinetics of T-cell, B-cell and NK-cell recovery in recipients of dual UCB and MUD transplantation following non-myeloablative conditioning. Our study focuses on hematopoietic cell transplant recipients following myeloablative conditioning, and extends the comparison to include post-transplant thymic function as well as T-cell receptor diversity.
Quantification of TRECs, which are derived from recent thymic emigrants, has been used as a surrogate marker for thymic-dependent T-cell maturation. Early recovery of thymopoietic function following UCB transplantation is a critical determinant of treatment-related morbidity and mortality [13]. Previous studies have described highly variable but generally slow recovery of thymic function as determined by the presence of peripheral blood TREC following UCB transplantation [7–9, 13]. In order to assess impact of graft source on the thymic-dependent pathway of cellular immune reconstitution, we compared TREC recovery in our two cohorts. In contrast to other studies, we assayed TREC frequency from DNA isolated from a purified population of CD3+ T-cells. Although the number of TRECs tended to be lower in the UCB group both prior to and 3 months after transplantation, it was not significantly different at 6 and 12 months after transplantation, mimicking the trend seen in quantitative T-cell subset analysis. One explanation for lower TREC values in the UCB group may be differences in prior cytotoxic therapy resulting in thymic damage. Indeed, the UCB group was more heavily pre-treated with only 22% of acute leukemia patients receiving a transplantation in first remission compared to 51% in the MSD/MUD group. It should be noted that the number of TRECs at 1 year following transplantation remained well below normal irrespective of donor source indicating ongoing impairment of thymus-dependent T-cell recovery, which is consistent with a previous study analyzing mostly pediatric patients [35]. Komanduri et al demonstrate a complete block of thymopoiesis during the first year following UCB transplantation [8,36], which is in stark contrast to what was observed following HCT from autologous or adult matched donor transplantation. However, the observed differences may be related to low total numbers of TREC-containing lymphoid progenitors passively transferred with the stem cell graft or use of anti-thymocyte globulin in the transplant preparative regimen. We also analyzed T-cell receptor diversity in a subset of 10 patients 1 year following HCT with comparable T-cell recovery, as determined by quantitative T-cell subset analysis and TREC output. We were interested to know whether the low but detectable output of recent thymic emigrants in UCB transplant recipients was capable of equalizing T-cell receptor diversity of the adult donor recipients who benefit from homeostatic expansion of passively transferred polyclonal memory T-cells. Despite this advantage, we found that the T-cell receptor diversity in UCB recipients was comparable to that of the matched adult donor recipients.
It is of interest to note that despite the observed delay in quantitative T-cell recovery in recipients of UCB transplantation, we did not observe differences in PFS compared to the recipients of matched adult donor transplantation. This can be explained, in part, by the improvement in supportive care. However it is also possible that more prompt recovery of NK cells and B-cells in UCB recipients may compensate for early T-cell deficits, although rapid recovery of both of these cell types may partly be due to a compensatory response to the profound T-lymphopenia [37, 38]. Tanaka et al. observed a more rapid expansion of NK cells following umbilical cord blood compared to peripheral blood stem cell transplantation [39]. The investigators found that a umbilical cord blood derived mature (CD16+, CD56dim) and immature (CD16−, CD56+) populations of NK cells exert potent cytotoxic activity against tumor cell lines and exhibit decreased expression of inhibitory NKG2A and increased expression of stimulatory NKG2C as compared to NK cells that emerge following matched related donor transplantation.
Patient age [11, 12, 41] incidence and severity of GVHD [40], intensity of the conditioning regimen [7, 41], and T-cell depletion of the donor graft [12] are all significant parameters that affect the pace and quality of immune recovery following HCT. Of all these parameters, patient age and the incidence of acute GVHD differed among the three cohorts analyzed in this study. The younger mean age of UCB recipients is unlikely to be a significant influence on immune recovery since all recipients were over age 19, which is reported to be an age that marks significant decline of thymic function [12]. The higher observed incidence of acute grade II-IV and grade III-IV GVHD in the dual UCB group compared to the MSD/MUD group deserves further discussion. Grade III-IV acute GVHD contributed to delayed immune reconstitution at 3 months after transplantation in this cohort. Therefore, it is possible that the delay in immune reconstitution at 3 months after UCB transplantation was the result of a high incidence of acute GVHD in the UCB group, although a similar pattern of delayed immune recovery in the UCB recipients was observed regardless of how acute GVHD patients were grouped (0-I, 0-II, II-IV or III-IV). The high incidence of acute GVHD in our cohort compared with previous UCB reports may, in part, be due to use of a conditioning regimen that does not contain anti-thymocyte globulin.
Any study comparing immune reconstitution following HCT is subject to a “survivor bias” such that the patients with the most profound impairment in immune recovery die of treatment-related complications and are thus not evaluable for comparison. While a survivor bias cannot be completely excluded from this study, there are two factors that suggest it does not exert a major influence on the findings of this study. First, we did not observe any significant difference in PFS in the three cohorts of patients assessed in this study (Figure 6). Second, the incidence of infection-related deaths was low in both treatment cohorts and therefore unlikely to be a contributing factor to the reported observations (data not shown). An additional limitation of this study arises from the fact that due to technical constraints, we report TREC analysis on a subset of evaluable patients. We cannot rule out the possibility that elimination of these subjects resulted in a biased analysis of this portion of the study.
In conclusion, when compared to recipients of matched sibling and matched unrelated donor HCT recipients, UCB transplant recipients have slower quantitative recovery of T-lineage immune cell populations in the first 6 months, but these differences are erased by 1 year after transplantation. NK and B cell reconstitution is more rapid in UCB recipients.
Acknowledgments
J.K. is a Research Fellow of the JSPS. sjTREC analysis were performed by Jeff Hale in the Duke Human Vaccine Institute Immune Reconstitution and Biomarker Shared Resource Facility.
Funding: This work was supported in part by National Cancer Institute (NIH) 5P01-CA047741-18 (M.H., N.C.) and a Grant-in-Aid for JSPS Fellows (J.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: Authors have no relevant financial relationship to disclose.
Authorship
Contributions: M.H. designed the research and organized the project; J.K., L.C., J.L., and M.H. reviewed data, analyzed data, interpreted data, and wrote the paper; P.S. reviewed data and interpreted data; G.D.S. and J.H. analyzed data, and interpreted data; J.K., G.B. and D.N. performed statistical analysis; N.C. interpreted data. All authors reviewed and approved final manuscript.
A part of this work was presented as an abstract at the 51th Annual Meeting of the American Society of Hematology, Orlando, FL, December 5–8, 2010 [43].
REFERENCES
- 1.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. Jama. 2010;303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 3.Gress RE, Emerson SG, Drobyski WR. Immune reconstitution: how it should work, what's broken, and why it matters. Biol Blood Marrow Transplant. 2010;16:S133–S137. doi: 10.1016/j.bbmt.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. The New England journal of medicine. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 5.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. The New England journal of medicine. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 6.Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 7.Chao NJ, Liu CX, Rooney B, et al. Nonmyeloablative regimen preserves "niches" allowing for peripheral expansion of donor T-cells. Biol Blood Marrow Transplant. 2002;8:249–256. doi: 10.1053/bbmt.2002.v8.pm12064361. [DOI] [PubMed] [Google Scholar]
- 8.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein AK, Patel DD, Gooding ME, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001;7:454–466. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]
- 10.Szabolcs P, Cairo MS. Unrelated umbilical cord blood transplantation and immune reconstitution. Seminars in hematology. 2010;47:22–36. doi: 10.1053/j.seminhematol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castermans E, Hannon M, Dutrieux J, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2010;96:298–306. doi: 10.3324/haematol.2010.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewin SR, Heller G, Zhang L, et al. Direct evidence for new T-cell generation by patients after either T-cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood. 2002;100:2235–2242. [PubMed] [Google Scholar]
- 13.Brown JA, Stevenson K, Kim HT, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010;115:4111–4119. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson CA, Turki AT, McDonough SM, et al. Immune Reconstitution after Double Umbilical Cord Blood Stem Cell Transplantation: Comparison with Unrelated Peripheral Blood Stem Cell Transplantation. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.08.018. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute values of dendritic cell subsets in bone marrow, cord blood, and peripheral blood enumerated by a novel method. Stem cells (Dayton, Ohio) 2003;21:296–303. doi: 10.1634/stemcells.21-3-296. [DOI] [PubMed] [Google Scholar]
- 16.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Experimental hematology. 2003;31:708–714. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 17.Sarzotti-Kelsoe M, Win CM, Parrott RE, et al. Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood. 2009;114:1445–1453. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ria F, van den Elzen P, Madakamutil LT, Miller JE, Maverakis E, Sercarz EE. Molecular characterization of the T cell repertoire using immunoscope analysis and its possible implementation in clinical practice. Current molecular medicine. 2001;1:297–304. doi: 10.2174/1566524013363690. [DOI] [PubMed] [Google Scholar]
- 19.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robins HS, Campregher PV, Srivastava SK, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherwood AM, Desmarais C, Livingston RJ, et al. Deep sequencing of the human TCRgamma and TCRbeta repertoires suggests that TCRbeta rearranges after alphabeta and gammadelta T cell commitment. Science translational medicine. 2011;3:90ra61. doi: 10.1126/scitranslmed.3002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in medicine. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 24.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Seminars in hematology. 1991;28:250–259. [PubMed] [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 27.Purves RD. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC) Journal of pharmacokinetics and biopharmaceutics. 1992;20:211–226. doi: 10.1007/BF01062525. [DOI] [PubMed] [Google Scholar]
- 28.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ooi J, Iseki T, Takahashi S, et al. Unrelated cord blood transplantation for adult patients with de novo acute myeloid leukemia. Blood. 2004;103:489–491. doi: 10.1182/blood-2003-07-2420. [DOI] [PubMed] [Google Scholar]
- 30.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 31.Doan PL, Chao NJ. Umbilical cord blood: biology and transplantation. Expert Rev Hematol. 2009;2:197–208. doi: 10.1586/ehm.09.9. [DOI] [PubMed] [Google Scholar]
- 32.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 33.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. The lancet oncology. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauter C, Abboud M, Jia X, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant. 2011;17:1460–1471. doi: 10.1016/j.bbmt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talvensaari K, Clave E, Douay C, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99:1458–1464. doi: 10.1182/blood.v99.4.1458. [DOI] [PubMed] [Google Scholar]
- 36.Escalon MP, Komanduri KV. Cord blood transplantation: evolving strategies to improve engraftment and immune reconstitution. Current opinion in oncology. 2010;22:122–129. doi: 10.1097/cco.0b013e328335a56e. [DOI] [PubMed] [Google Scholar]
- 37.Malaspina A, Moir S, Ho J, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malaspina A, Moir S, Chaitt DG, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109:2086–2088. doi: 10.1182/blood-2006-06-031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka J, Sugita J, Asanuma S, et al. Increased number of CD16(+)CD56(dim) NK cells in peripheral blood mononuclear cells after allogeneic cord blood transplantation. Human immunology. 2009;70:701–705. doi: 10.1016/j.humimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Clave E, Busson M, Douay C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113:6477–6484. doi: 10.1182/blood-2008-09-176594. [DOI] [PubMed] [Google Scholar]
- 41.Castermans E, Baron F, Willems E, et al. Evidence for neo-generation of T cells by the thymus after non-myeloablative conditioning. Haematologica. 2008;93:240–247. doi: 10.3324/haematol.11708. [DOI] [PubMed] [Google Scholar]
- 42.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiou LW, Kanda J, Szavolcs P, et al. A comprehensive comparison immune recovery In adult patients following allogeneic umbilical cord blood, matched sibling and matched unrelated donor stem cell transplantation. Blood (ASH Annual Meeting Abstracts) 2010 Nov;116 [Google Scholar]
- 44.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. The Journal of pediatrics. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]