Abstract
Background
Although the hypothalamic orexin system is known to regulate appetitive behaviors and promote wakefulness and arousal (Sakurai, 2007), this system may also be important in adaptive and pathological anxiety/stress responses (Suzuki et al., 2005). In a recent study, we demonstrated that CSF orexin levels were significantly higher in patients experiencing panic attacks compared to non-panicking depressed subjects (Johnson et al., 2010). Furthermore, genetically silencing orexin synthesis or blocking orexin 1 receptors attenuated lactate-induced panic in an animal model of panic disorder. Therefore, in the present study, we tested if orexin (ORX) modulates the panic responses and brain pathways activated by two different panicogenic drugs.
Methods
We conducted a series of pharmacological, behavioral, physiological and immunohistochemical experiments to study the modulation by the orexinergic inputs of anxiety behaviors, autonomic responses, and activation of brain pathways elicited by systemic injections of anxiogenic/panicogenic drugs in rats.
Results
We show that systemic injections of two different anxiogenic/panicogenic drugs (FG-7142, an inverse agonist at the benzodiazepine site of the GABAA receptor, and caffeine, a nonselective competitive adenosine receptor antagonist) increased c-Fos induction in a specific subset of orexin neurons located in the dorsomedial/ perifornical (DMH/PeF) but not the lateral hypothalamus. Pre-treating rats with an orexin 1 receptor antagonist attenuated the FG-7142-induced anxiety-like behaviors, increased heart rate, and neuronal activation in key panic pathways, including subregions of the central nucleus of the amygdala, bed nucleus of the stria terminalis, periaqueductal gray and in the rostroventrolateral medulla.
Conclusion
Overall, the data here suggest that the ORX neurons in the DMH/PeF region are critical to eliciting a coordinated panic responses and that ORX1 receptor antagonists constitute a potential novel treatment strategy for panic and related anxiety disorders. The neural pathways through which ORX1 receptor antagonists attenuate panic responses involve the extended amygdala, periaqueductal gray, and medullary autonomic centers.
1. Introduction
Discovered in 1998 [1, 2], the orexin (ORX: also known as hypocretin) neuropeptide system is unique due to the ORX neurons being exclusive to a small region of the hypothalamus that encompasses the dorsomedial/perifornical (DMH/PeF) and adjacent lateral (LH) hypothalamus. There are two active forms of ORXs, which are ORXA and ORXB that are produced from a common prepro-ORX precursor that are endogenous ligands for the G-protein-coupled ORX1 and ORX2 receptors. The ORX1 receptor has greater affinity for ORXA than for ORXB, and the ORX2 receptor has similar affinity for both ORXA and ORXB [2]. The orexin system has robust projections to brain regions implicated in arousal and emotional responses such as the locus coeruleus (LC), dorsal raphe nucleus (DRN), bed nucleus of the stria terminalis (BNST), central amygdala (CeA) and periaqueductal grey [3]. Since 1998, the ORX system has been known mainly for its role in promoting wakefulness and arousal but also coordinating energy homeostasis and reward [see review (Sakurai, 2007)]. The location of the ORX neurons, the associated neural networks, and critical role ORX plays in arousal and wakefulness suggest that ORX may also play a role in acute and/or pathological anxiety states.
Consistent with this hypothesis, intracerebroventricular injections of ORX in rats mobilizes an integrated stress response evidenced by 1) increases in anxiety-associated behavior as measured in the elevated plus-maze (EPM) and light-dark exploration [4] tests, 2) mobilization of the hypothalamic-pituitary-adrenal (HPA) axis [5], and 3) mobilization of sympathetic responses (i.e., tachycardia, hypertension, and increases in renal sympathetic activity and plasma concentrations of norepinephrine and epinephrine [6, 7]. In addition, pretreating rats with an ORX1 receptor antagonist attenuates anxiety-like responses to CO2 inhalation, a well known panicogenic stimuli [8] and a hyperactive ORX system recently has been linked to pathological anxiety and panic states in a rat model of panic vulnerability and in humans with elevated anxiety and panic symptoms [9].
In order to further elucidate the role of ORX in mobilizing panic and anxiety responses, the present studies: 1) determined the effects of two different anxiogenic/panicogenic drugs, FG-7142, a partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor, [10, 11]; or caffeine, a nonselective competitive adenosine receptor antagonist, [12, 13] on ORX neuronal responses using dual immunohistochemistry for the protein product of the immediate-early gene c-fos and ORX-A; and 2) evaluated the effects of systemically blocking the ORX1 receptors on FG-7142-induced anxiety behavior and FG-7142-induced changes in cellular responses in different brain regions that are key regions implicated in panic and anxiety responses, are efferent targets of the ORX system and have been shown to have heightened cellular responses to these two anxiogenic drugs [14].
2. Methods and Materials
2.1 Experiment 1: Effects of anxiogenic drugs on c-Fos induction in ORX neurons
2.1.1 Animals
Adult male Wistar rats (250–300 g; B&K Universal, Hull, UK) were acclimatized to the animal facility for 1 week in group housing (four/cage), then single-housed on a 14:10-h light/dark cycle (lights on at 05:00 h) and habituated to the experimental room (36–48 h) before the experiment. Food and water were provided ad libitum. Injections were performed using a completely randomized experimental design utilizing 20 rats each day on 2 separate days (during the rats’ inactive phase). Time-matched groups of rats were injected between 09:00 and 17:00 h. All UK experiments were approved by the University of Bristol Ethical Review Group, were consistent with the regulations and guidelines of the UK Home Office, and were carried out according to the UK Animals (Scientific Procedures) Act 1986. In addition, all UK studies were consistent with the NIH Guide for the Care and Use of Laboratory Animals (N.I.H. Publication No. 85-23) and were covered by Animal Welfare Assurance A505-01. Retrograde and anterograde tracing studies were conducted under the authority of the Animal Core Facility of the Panum Institute School of Medicine, University of Copenhagen, in accordance with and approved by The Animal Experiments Inspectorate, Ministry of Justice, Denmark. In all cases particular care was taken in order to minimize the number of animals used and their suffering.
2.1.2 Drug treatment and video recording of behavior
Rats were injected i.p. with either saline vehicle (n=8), the adenosine receptor antagonist caffeine (Fluka, Dorset, UK; 50 mg/kg; n = 6), or the partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor FG-7142 (Tocris, Avonmouth, UK; 7.5 mg/kg; n = 6). Caffeine was dissolved in 0.9% saline while FG-7142 was dissolved in 0.9% saline/40% 2-hydroxypropyl-cyclodextrin (Tocris) to increase solubility as in previous studies [14, 15]. Ninety min post-injection, rats were anesthetized i.p. with sodium pentobarbital (65 mg/kg; Sagatal) and transcardially perfused with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB). All buffers used were pH 7.4. Brains were removed, post-fixed in the same fixative for 12 h, rinsed twice in PB (12 h), and placed in 0.1 M PB containing 30% sucrose for 12 h. Brain tissues were blocked using a standard adult rat brain matrix (model RBM-4000C, ASI Instruments, Warren, MI, USA) and frozen using liquid isopentane cooled by liquid nitrogen.
2.1.3 Double immunohistochemistry for c-Fos and ORX-A
Double immunostaining for c-Fos protein and ORX-A was accomplished with sequential immunohistochemical procedures using 1) primary antibodies directed against c-Fos (rabbit anti-c-Fos polyclonal antibody, Cat. no. sc-52, Ab-5, Santa Cruz Biotech., Santa Cruz, CA, USA; diluted 1:10,000) then 2) primary antibodies directed against ORX-A (rabbit anti-ORX-A-polyclonal, affinity-purified antibody, Cat. no. H-003-30, Phoenix Pharmaceuticals, Burlingame, CA, USA; diluted 1:10,000). Western blot analysis suggests that this anti-c-Fos antibody recognizes the 55 kilodalton (kDa) c-Fos protein (information provided by the supplier), and omission of the c-Fos antibody from the immunohistochemistry protocol results in elimination of nuclear immunostaining (data not shown). Omission of the ORX-A antibody from the immunohistochemistry protocol results in elimination of cytoplasmic immunostaining in the DMH/PeF and LH (data not shown), and within the rat brain the distribution of the ORX-A immunoreactivity occurs in only soma of neurons in the DMH/PeF and LH in a distribution similar to that of the preproORX mRNA [1]. All brain sections were immunostained in a single immunohistochemical run, rather than in batches, with large volume incubations to limit variability in the quality of immunohistochemical staining among brain sections.
Free-floating sections were washed in 0.05 M PBS for 30 min, then incubated in 1% H2O2 in PBS for 20 min. Sections were then washed 10 min in PBS and 20 min in PBS with 0.3% Triton X-100 (PBST). Sections were then incubated 12-16 hr in PBST with primary antibody solution at room temperature. After a 30 min wash in PBST, sections were incubated in biotinylated goat anti-rabbit IgG (c-Fos, ORX-A; Cat.no. BA-1000; Vector Laboratories, Burlingame, CA, USA; diluted 1:500). Sections were washed again for 30 min in PBST then incubated 1.5 hr in an avidin-biotin complex provided in a standard Vector Elite kit (c-Fos, ORX-A, Cat no. PK-6100, Vector Laboratories; diluted 1:500). Substrates for chromogen reactions were SG (c-Fos; SK-4700, Vector Laboratories) or 0.01% 3,3’-diaminobenzidine tetrahydrochloride (ORX-A; DAB) (Cat. no. D-5637, Sigma-Aldrich, Poole, UK) in PBS containing 0.003% H2O2, pH 7.4. Substrate reactions were run for 20 min for c-Fos and 10 min for ORX-A. All sections were mounted on clean glass slides, dried overnight, dehydrated and mounted with cover slips using DPX mounting medium (BDH Laboratory Supplies, Poole, U.K.). All washes and incubations were done in 12-well polystyrene plates with low frequency shaking on an orbital shaker.
2.1.4 Counting of ORX-A- and c-Fos-ir neurons
Selection of anatomical levels for analysis of c-Fos/ORX-A-immunostained cells was conducted with reference to illustrations from a rat brain stereotaxic atlas [16]. Selection of anatomical levels was also done in reference to major anatomical landmarks including white matter tracts and the ventricular systems. Specifically, darkfield contrast [i.e., using a 1.6X Leica phase contrast Plan objective and Leica binocular microscope (model DMLB, Leica Mikroskopie and Systeme GmbH, Wetzler, Germany) with a darkfield condenser] was used to visualize white matter tracts (e.g., the fornix and optic tracts) and ventricular systems (e.g., lateral, 3rd ventricles) that aided in selection of appropriate coronal levels with reference to illustrations in a standard stereotaxic atlas of the rat brain [16]. The numbers of c-Fos/ORX-A-ir neurons were counted in the entire field of view at 400X magnification (i.e., 10X eyepiece and 40X Plan objective) for each brain region. The area of the DMH/PeF where single ORX-A-ir neurons and double c-Fos/ORX-ir neurons was counted was roughly square in dimension with the corners being the mammillothalamic tract, the fornix, the top of the 3rd ventricle and a point located halfway down the 3rd ventricle (immediately medial from the fornix). The DMH/PeF, as described, is particularly sensitive to bicuculline methiodide (BMI: a GABAA receptor antagonist) -induced cardioexcitatory response (Samuels et al., 2004). All single ORX-A-ir neurons and double c-Fos/ORX-ir neurons counted that were lateral to the DMH/PeF area were considered to be in the LH region. All cell counts were done by an observer (PLJ) that was blind to the experimental treatment of each animal.
2.2 Experiment 2: Effects of an ORX1 receptor antagonist on FG-7142-induced anxiety and brain responses
2.2.1 Animals and housing conditions
All experiments were conducted on adult male Wistar rats (250-300 g) purchased from Harlan Laboratories (Indianapolis, IN, USA) and were housed individually in plastic cages under standard environmental conditions (22 °C; 12/12 light/dark cycle; lights on at 7:00 A.M.) for 7-10 days prior to the surgical manipulations. Food and water were provided ad libitum. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication no. 80-23) revised 1996 and the guidelines of the IUPUI Institutional Animal Care and Use Committee.
In this experiment, rats were split into 3 drug treatment groups (n = 8/group), where the rats each received two injections (i.p.), 30 min apart, as follows: group 1) vehicle injection (0.2 ml dimethyl sulfoxide (DMSO) in 100 g volume dH2O) followed by another vehicle (0.2 ml DMSO/0.1 ml TWEEN80 in 100 g volume dH2O); group 2) vehicle injection followed by FG-7142 (7.5 mg/kg, Sigma-Aldrich); group 3) an orexin 1 receptor antagonist (SB334867, 30 mg/kg, Tocris) followed by FG-7142. Fifteen min after the FG-7142 injection, all rats were tested for anxiety-related behavior using a 5 min open-field test (OFT) and a 5 min social interaction (SI) test immediately following. Ninety min following FG-7142 injections, rats were anesthetized and then the brains were removed and processed for immunohistochemistry as described in detail below.
2.2.2 Open-field test (OFT)
The open-field arena covered an area of 90 cm × 90 cm, with 40 cm-high walls under red light. The open-field arena was divided into a 6 × 6 grid of equally-sized squares using black tape (36 total squares) with 4 squares forming the center; 12 squares forming the middle perimeter; and 20 squares forming the outer perimeter. The test started by placing a rat in the center. The behavior of each rat in the open-field arena was recorded on video and scored afterwards by an observer (PLJ) blind to the experimental treatment of each rat. Time spent in each region of the open-field was recorded. In addition, locomotor activity was assessed by counting the number of times the rat’s entire body (excluding tail) completely crossed into another square.
2.2.3 Social interaction test (SI)
A modified version of the social interaction (SI) test (File, 1980) was utilized to measure anxiety-like responses. Immediately following the OFT, the experimental rat was placed in an open-field (0.9 m long × 0.9 m wide with walls 0.3 m high) with an untreated novel partner rat under red light conditions. A video camera was fixed above the box, and all behavioral tests were videotaped. During the 5 min test the total amount of time the treated rat initiates interaction with the partner rat is recorded (sniffing, grooming etc.) as described previously (Shekhar and Keim, 1997). Videotaped sessions were scored at a later time by an investigator SDF (blind to treatments) and a decrease in total interaction time was taken as an increase in “anxiety”-like behavior.
2.2.4 Perfusion
Ninety min following the initiation of treatment, rats were anesthetized with an overdose of sodium pentobarbital (40 mg, i.p.) then perfused transcardially with 0.05 M phosphate buffered saline (PBS; 250 ml), followed by 0.1 M sodium phosphate buffer (PB; 250 ml) containing 4% paraformaldehyde (PFA) and 3% sucrose. Brains were removed and post-fixed for 24 h in the same fixative, rinsed for 24 h in 0.1 M PB, then placed in cryoprotectant (30% sucrose in 0.1 M PB) for an additional 4-5 days. To maintain a consistent plane for coronal sections, brains were placed in a rat brain matrix (ASI instruments, Model No. RBM-4000C, Warren MI, USA) and cut with a razor blade at the caudal border of the mammillary bodies. Brains were frozen in cooled liquid isopentane made by immersing a plastic vessel containing isopentane into a dewar flask containing liquid nitrogen. Serial coronal sections (30 μm) were cut using a cryostat and were immediately placed in cryoprotectant consisting of 27% ethylene glycol and 16% glycerol in 0.05 M PB to yield six alternate sets of sections. Sections were stored at −20 °C until immunohistochemical processing. All solutions had a pH of 7.4.
2.2.5 Immunohistochemistry for c-Fos
All brain sections were immunostained with the c-Fos primary antibody in a single immunohistochemical run, rather than in batches, with large volume incubations to limit variability in the quality of immunohistochemical staining among brain sections. However, the forebrain was immunostained for c-Fos in one run and the brainstem (i.e., midbrain, pons and medulla) in another. Immunostaining for c-Fos protein was accomplished using an affinity-purified primary antibody directed at c-Fos (rabbit anti-human c-Fos polyclonal affinity-purified antibody, Cat. no. sc-52, Santa Cruz Biotechnology, San Diego, CA; diluted 1:10,000). Free-floating sections were washed in 0.1 M PBS for 30 min, then incubated in 1% H2O2 in PBS for 20 min. Sections were then washed 10 min in PBS and 20 min in PBS with 0.3% Triton X-100 (PBST). Sections were then incubated 12-16 h in PBST with primary antibody solution at room temperature. After a 30 min wash in PBST, sections were incubated for 2 hr in biotinylated goat anti-rabbit IgG (Cat no. BA-1000, Vector Laboratories, Burlinghame, CA; diluted 1:500). Sections were washed again for 30 min in PBST then incubated 1.5 hr in an avidin-biotin-peroxidase complex provided in a standard Vector Elite kit (Cat no. PK-6100, Vector Laboratories, diluted 1:200). The peroxidase substrate for the chromogen reaction was Vector SG, which was prepared as recommended by the manufacturer (Cat. no. SK-4700, Vector Laboratories). The substrate reaction was run for 20 min for the forebrain. All sections were mounted on clean glass slides, dried overnight, dehydrated and mounted with coverslips using DPX mounting medium (BDH Laboratory Supplies, Poole, UK). All washes and incubations were done at room temperature in 12-well polystyrene plates with low frequency shaking on an orbital shaker.
2.2.6 Counting single c-Fos-ir cells
Selection of anatomical levels for analysis of c-Fos-immunostained cells was conducted with reference to illustrations from a rat brain stereotaxic atlas [16]. Selection of anatomical levels was also done in reference to major anatomical landmarks including white matter tracts and the ventricular systems. Specifically, darkfield contrast [i.e., using a 1.6X Leica phase contrast Plan objective and Leica binocular microscope (model DMLB, Leica Mikroskopie and Systeme GmbH, Wetzler, Germany) with a darkfield condenser] was used to visualize white matter tracts (e.g., the fornix and optic tracts) and ventricular systems (e.g., lateral, 3rd and 4th ventricles) that aided in selection of appropriate coronal levels with reference to illustrations in a standard stereotaxic atlas of the rat brain [16]. As stated in the introduction, our hypothesis is that the FG-7142 drug used here increases unconditioned, stress-related behavioral responses in a similar pattern as is seen in a previous article that gave the same dose of the FG-7142 drug and assessed c-Fos induction in forebrain regions implicated in anxiety behavior [14]. Therefore, the Singewald article was referenced in selecting regions to assess c-Fos responses. The regions selected for analysis were as follows: prelimbic cortex (PRL) and infralimbic cortex (IL) at +2.70 mm bregma; bed nucleus of the stria terminalis (BNST) divisions that included the anterior part of BNST (aBNST) at +0.20 mm bregma; the intermediate (LSI) and ventral (LSV) parts of the lateral septum at +0.20 and −0.30 mm bregma; the hypothalamic paraventricular nucleus (PVN) at −1.80 mm bregma; the amygdala subdivisions [central amygdaloid nucleus (CeA); basolateral amygdaloid nucleus, anterior part (BLA); lateral amygdaloid nucleus (LA); and the medial amygdaloid nucleus (MeA)] at −2.56 mm bregma; the dorsomedial (DMH) and perifornical (PeF) hypothalamus at −3.14 mm bregma; the dorsal (DPAG), dorsolateral (DLPAG) and ventrolateral VLPAG) periaqueductal gray and dorsal raphe nucleus (DRN) at −7.80 mm bregma;, the lateral (lPBN) and medial (mPBN) parts of the parabrachial nucleus at −9.30 mm bregma; the locus coeruleus (LC) at −10.04 mm bregma; and rostroventrolateral medulla (RVLM) at −11.60 mm bregma. The numbers of c-Fos-ir cells were counted in the entire field of view at 400X (for the PrL, IL, LSI, LSV, aBNST, CeA, BLA, LA, MeA and subdivisions of the PAG) or 600X (for the PVN, DMH, PeF, lPBN, mPBN, LC and RVLM) magnification (i.e., 10X eyepiece + 40X or 60X Plan objective) for each brain region. All cell counts were done by an observer (PLJ) that was blind to the experimental treatment of each animal.
2.3 Experiment 3: Effects of an ORX1 receptor antagonist on FG-7142-induced cardiovascular and general motor responses
In order to assess cardiovascular activity rats were anesthetized with a nose cone connected to an isoflurane system (MGX Research Machine; Vetamic, Rossville IN, USA) during the surgery, then fitted with femoral arterial catheters for measurement of mean arterial blood pressure (MAP) and heart rate (HR) as previously described [17]. Briefly, cardiovascular responses were measured by a femoral arterial line connected to a telemetric probe that contained a pressure transducer [Cat. no. C50-PXT, Data Sciences International (DSI), St. Paul, MN, USA]. DSI dataquest software was used to monitor and record MAP, HR, and general motor activity by turning on the telemetry probe and placing the homecage onto the receiver plates. For the duration of each experiment, MAP and HR were recorded continuously in freely moving conscious rats. Data were analyzed during the period 5 min prior to and 60 min following the vehicle or FG-7142 injections. The data reported are changes in MAP and HR, expressed in 1 min bins, relative to the average of the baseline measurement (t -5 min to t -1 min) from each rat.
In experiment 3a, rats received either a vehicle injection (0.2 ml DMSO in 100 g volume dH2O, n = 12) or FG-7142 (7.5 mg/kg i.p., Sigma-Aldrich, n=12) in a crossover design where each rat received either treatment with 48 hours between treatments. In experiment 3b, each rat from experiment 3a received injections, again given in a crossover design, either a vehicle injection (0.2 ml DMSO in 100 g volume dH2O, n=6) or a centrally acting orexin 1 receptor antagonist (SB334867, 30 mg/kg i.p., Tocris, n=6[18] 30 min prior to all rats receiving FG-7142 (7.5 mg/kg i.p., Sigma-Aldrich).
2.4 Statistical analyses
2.4.1 Statistical analyses of cell counts in experiment 1 and 2
The dependent variables for cell counts (experiment 1: c-Fos-ir/ORX-ir neurons, or the total number of ORX-ir neurons; experiment 2: single c-Fos-immunoreactive cells) were each analyzed using a one-way ANOVA. In the presence of significant main effects post-hoc pairwise comparisons were using Fisher’s Protected Least Significant Difference (LSD) tests. Statistical significance was accepted at p < 0.05.
2.4.2 Analyses of open-field and social interaction behavior in experiment 2
The dependent variables for behaviors (e.g, duration of time in area or duration of social interaction) were analyzed using a one-way ANOVA. In the presence of significant main effects post-hoc pairwise comparisons were conducted using Fisher’s LSD tests. Statistical significance was accepted at p < 0.05.
2.4.3 Analyses of cardiovascular and locomotor responses in experiment 3a-b
Dependent variables for analyses of cardiovascular (HR, MAP), and locomotor activity were analyzed using a one-way ANOVA with repeated measures, using drug treatment as the between-subjects factor and time as a within-subjects factor. In the presence of significant main effects or main effect × time interactions, paired t-tests were used for post-hoc pairwise comparisons. Within-subjects comparisons were also made on the cardiovascular and locomotor measures using a Dunnett’s test for multiple comparisons with a single control using the 5 min baseline measurement as the control. The alpha level was set at 0.05 in all cases.
All statistical analyses were carried out using SYSTAT 5.02 (SYSTAT Inc., San Jose, CA) and SPSS 14.0 (SPSS Inc., Chicago, IL), and all graphs were generated using SigmaPlot 2001 (SPSS Inc.) and an illustration program (CorelDraw 11.633 for Windows, Viglen Ltd., Alperton, Middlesex, UK).
2.5 Photography
Photomicrographs were obtained using a brightfield microscope using N Plan 5x, 10x, 40x and 63x objective lenses (Leica binocular microscope, model DMLB), an Insight digital camera (Diagnostics Instruments Inc., Sterling Heights, Michigan, USA) and SPOT 3.5.5 for Windows digital imaging software (Silicon Graphics, Mountain View, California, USA). Photographic plates were prepared in CorelDraw 11.633 for Windows.
3 Results
3.1 Experiment 1: Effects of anxiogenic drugs on c-Fos induction in ORX neurons
Rats injected with either caffeine or FG-7142 had greater numbers of c-Fos/ORX-A-ir neurons in the DMH/PeF, but not LH, as compared to vehicle-injected rats (DMH/PeF: −2.94 mm bregma: F(2,18) = 9.2, p = 0.002 with Fisher’s LSD posthoc test Fig. 1a,b; LH (−2.94 mm bregma: F(2,18) = 3.4, p = 0.058 Fig. 1a). There were no significant differences in the total number of ORX-ir neurons between treatment groups in the DMH/PeF (F(2,18)=0.5, p=0.634) or LH (F(2,18)=1.7, p=0.215, see gray-lined bars in Fig. 1a).
Figure 1.
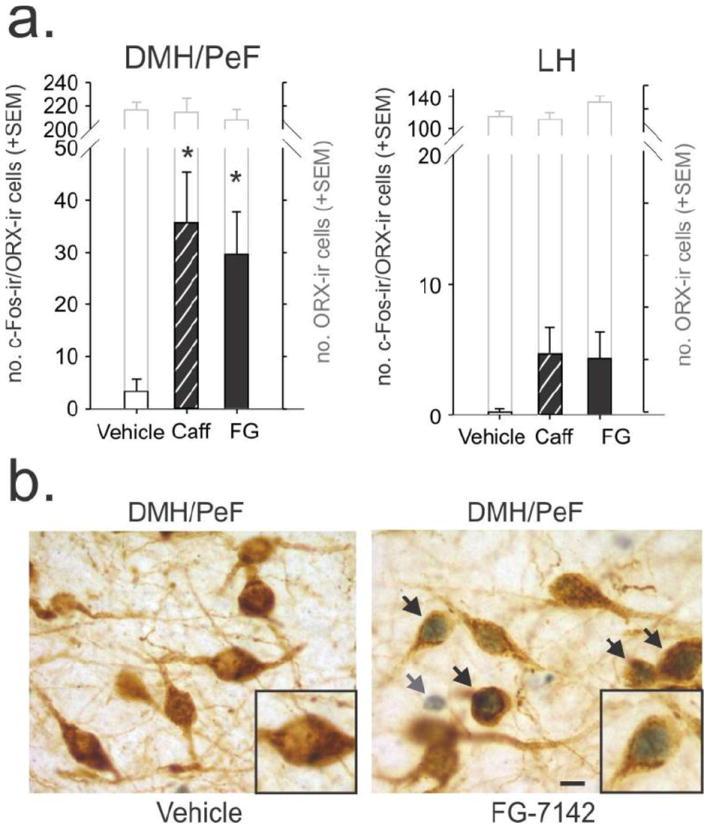
Effects of anxiogenic drugs on c-Fos expression in orexin (ORX) neurons. a) Graph illustrating the effects of caffeine (Caff) and FG-7142 (FG) on c-Fos expression in the dorsomedial/perifornical hypothalamic (DMH/PeF) region (left) and lateral hypothalamus (right). Black-lined bars in graph illustrate the numbers of ORX neurons from each treatment group that expressed c-Fos and gray-lined bars represent the total number of ORX neurons present. Error bars indicate the standard error of the mean (SEM) and * indicates significance compared to the vehicle group (Fisher’s LSD test). b) photomicrographs of a coronal section of the DMH/PeF region depicting ORX-immunoreactive (ir) neurons (brown cytoplasmic stain) with or without nuclear blue/black c-Fos-immunostained nuclei. Black arrows in Fig. 1b indicate double labelling whereas gray arrow indicates a c-Fos-ir/ORX-immunonegative cell. Scale bar, 10 μm.
3.2 Experiment 2: Effects of an ORX1 receptor antagonist on FG-7142-induced behavioral and brain responses
3.2.1 Open-field test
Although approaching significance for time spent in center region, an overall ANOVA did not detect a significant difference between treatment groups for time spent in any of the regions of the open-field test [center region duration: F(2,22)= 2.8, p = 0.083; middle area duration F(2,22) = 2.5, p = 0.109; or outer region duration F(2,22) = 2.9, p = 0.077, (Fig. 2)].
Figure 2.
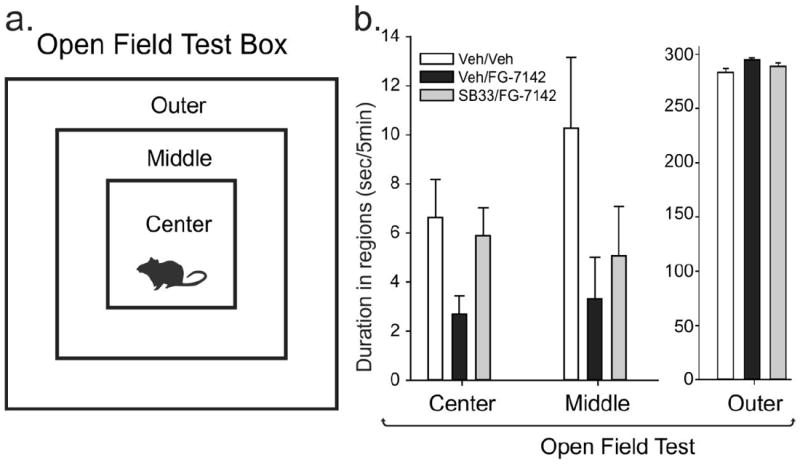
Effects of FG-7142 and the ORX 1 receptor antagonist SB334867 (SB33) on behavior in the open-field test. a) Illustration of an open-field test box with outer, middle and center regions. A decrease in time spent exploring the center and middle regions indicates an increase in anxiety-associated behavior. b) Bar graphs illustrate the duration of time each treatment group spent in regions of the open-field test (i.e., from left to right the graphs indicate the time spent in the outer and middle perimeter and center of the open-field during the 5-min test). Treatment groups were as follows: vehicle pre-treatment + vehicle; vehicle pre-treatment + FG-7142; SB334867 pre-treatment + FG-7142. Bars represent the means and error bars represent the SEMs. Abbreviations: Veh, vehicle; SB33, SB334667 (orexin 1 receptor antagonist).
3.2.2 Social interaction test
An overall ANOVA with Fisher’s LSD posthoc test detected a significant difference among treatment groups for time spent initiating social contact with a control partner rat (F(3,46) = 16.8, p < 0.001). Subsequent post-hoc tests determined that the vehicle/FG-7142 group was significantly different than all other groups (Fig. 3).
Figure 3.
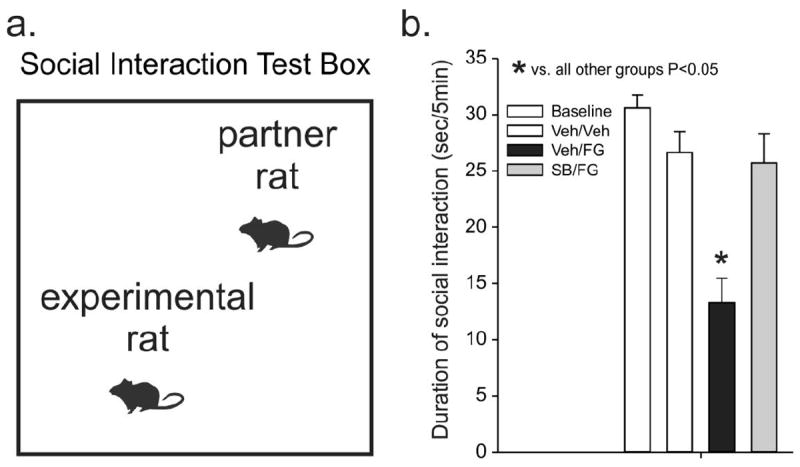
Effects of FG-7142 and the ORX 1 receptor antagonist SB334867 (SB33) on behavior in the social interaction (SI) test. a) Illustration of an SI test box where a decrease in the time spent initiating social contacts with a partner rat indicates an increase in anxiety-associated behavior. b) Bar graph illustrates the duration of time each treatment group spent initiating social contacts during a 5-min test. The baseline bar indicates the duration of social interaction from all rats at 5 days prior to treatments. Treatment groups were as follows: vehicle pre-treatment + vehicle; vehicle pre-treatment + FG-7142; SB334867 pre-treatment + FG-7142. Bars represent the means and error bars represent the SEMs. * p < 0.05, different from all other treatment groups (Fisher’s SD test). Abbreviations: Veh, vehicle; SB33, SB334667 (orexin 1 receptor antagonist).
3.2.3 Ex vivo analyses of numbers of c-Fos-ir cells in different brain regions
Brain regions affected by FG-7142 only
Within the hypothalamus, treating rats with FG-7142 increased the number of c-Fos-ir cells in the paraventricular hypothalamic nucleus (PVN: F(2,23)= 6.0, p = 0.009 with Fisher’s LSD posthoc test, Fig. 3a,b). The ANOVA for the DMH did not pass a normality test (p=0.007), so a nonparametric Mann- Whitney Rank Sum Test was performed that found an increase in the number of c-Fos-ir cells in the vehicle/FG-7142 group relative to vehicle/vehicle controls (p = 0.040, Fig. 3a,b).
Brain regions affected by FG-7142 and the ORX1 receptor antagonist
The following brain regions had increases in c-Fos-ir cells in the vehicle/FG-7142 group compared to the vehicle/vehicle group, as well as a significant reduction in the number of c-Fos-ir cells in the SB334867/FG-7142 group compared to the vehicle/FG-7142 group. Those brain regions include the central amygdala (CeA: F(2,23) = 5.3, p = 0.014); anterior part of the bed nucleus of the stria terminalis (aBNST: F(2,23) = 4.6, p = 0.022); dorsal periaqueductal gray (DPAG: F(2,22) = 4.9, p = 0.018); dorsolateral PAG (DLPAG: F(2,22) = 5.9, p = 0.010); ventrolateral PAG (VLPAG: F(2,22) = 8.5, p = 0.002); and rostroventrolateral medulla (RVLM: F(2,23) = 4.3, p = 0.028). All posthoc compisons were done with a Fisher’s LSD posthoc test. See Fig. 3a,b for details.
The table in Fig. 3c indicates brain regions that had no significant differences in the numbers of c-Fos-ir cells among treatment groups. See also Fig. 3a for illustration of regions counted.
3.3 Experiment 3: Effects of an ORX1 receptor antagonist on FG-7142-induced cardiovascular and general motor responses
3.3.1 In experiment 3a
In this experiment systemically injecting freely moving conscious rats with a vehicle alone caused an increase in HR (F(12,132) = 7.1, p < 0.001) and locomotor activity (F(12,132) = 3.5, p < 0.001). This also occurred in the FG-7142 injected group, but the effect on HR was prolonged [FG-7142 × time effect (F(12,264) = 4.2, p < 0.001), Fig. 4a] and FG-7142 elicited a greater increase in locomotor activity [overall effect of FG-7142 effect (F(1,22) = 9.2, p = 0.006), but no FG-7142 × time effect (F(12,264) = 1.0, p = 0.449) Fig. 4b]. Both vehicle and FG-7142, had an increase in MAP post injection [vehicle: Shapiro-Wilk normalily test failed, p < 0.050, Chi-square(13)= 33.3, p = 0.002, but a Dunnett’s posthoc test did not detect differences over time from baseline; FG-7142, Shapiro-Wilk normality test failed p < 0.050), Chi-square(13)= 51.0, p = <0.001 with t10 and t15 higher than the 5 min baseline pre injection]. However, there was no difference in MAP between group comparisons of vehicle to FG-7142 [overall FG-7142 effect (F(1,22) = 0.05, p=0.832, or a FG-7142 × time effect (F(12,264) = 0.6, p=0.830) data not shown]. No significant differences in baseline HR, MAP or activity (over 5 min prior to drug injections) were noted between treatment groups.
Figure 4.
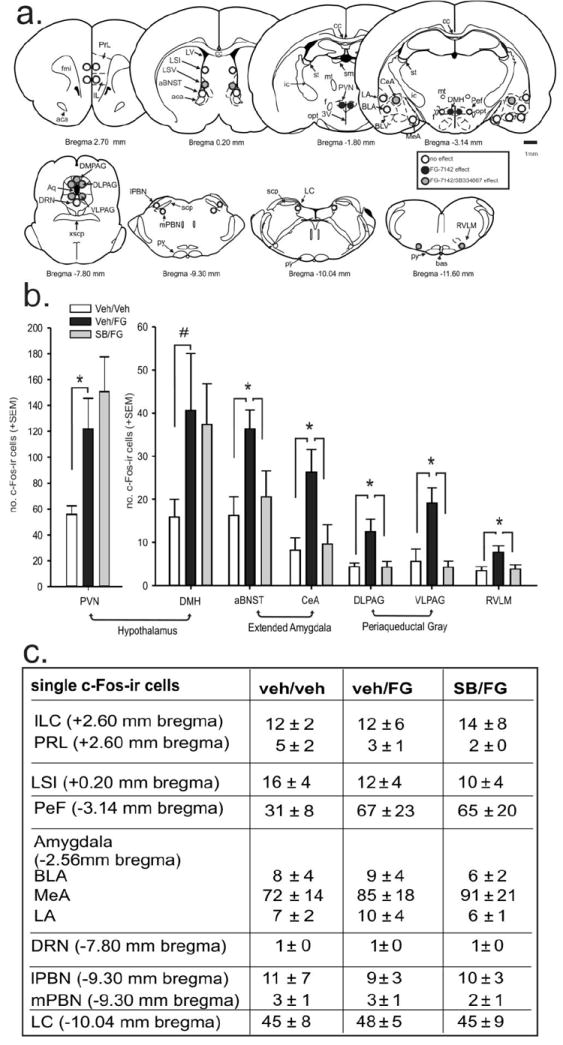
Effects of FG-7142 and the ORX 1 receptor antagonist SB334867 (SB) on c-Fos expression in anxiety- and stress-related brain regions. a) Diagrammatic illustrations of brain areas where subregional analyses of the numbers of c-Fos-ir cells were conducted in coronal brain sections as illustrated in a standard stereotaxic atlas of the rat brain [16]. The anterior-posterior coordinate (in mm from bregma) is indicated below each section. Studies by Singewald and colleagues where they observed significant increases in c-Fos-ir cells in specific brain regions following FG-7142 treatment was referenced in selecting regions to assess c-Fos responses [14, 15]. Open circles indicate region with no significant differences between groups; black-filled circles indicate regions where only a FG-7142 effect was observed; and gray-filled circles indicate regions where there was an effect with FG-7142 that was attenuated with the orexin 1 receptor antagonist (i.e., SB334867). Small circles indicate regions counted at 600X magnification and large circles indicate regions counted at 400× magnification. A 1 mm scale bar is located on the right side of the figure. Abbreviations: prelimbic cortex (PRL) and infralimbic cortex (IL) at +2.70 mm bregma; bed nucleus of the stria terminalis (BNST) divisions that included the anterior part of BNST (aBNST) at +0.20 mm bregma; the intermediate (LSI) and ventral (LSV) part of lateral septum at +0.20 and −0.30 mm bregma; the hypothalamic paraventricular nucleus (PVN) at −1.80 mm bregma; the amygdala subdivisions [central amygdaloid nucleus (CeA); basolateral amygdaloid nucleus, anterior part (BLA); lateral amygdaloid nucleus (LA); and the medial amygdaloid nucleus (MeA)] at −2.56 mm bregma; the dorsomedial (DMH) and perifornical (PeF) hypothalamus at −3.14 mm bregma; the dorsal (DPAG), dorsolateral (DLPAG) and ventrolateral VLPAG) periaqueductal gray and dorsal raphe nucleus (DRN) at −7.80 mm bregma;, the lateral (lPBN) and medial (mPBN) part of the parabrachial nucleus at −9.30 mm bregma; the locus coeruleus (LC) at −10.04 mm bregma; and rostroventrolateral medulla (RVLM) at −11.60 mm bregma. b) Graphs illustrating the effects of SB334867 pretreatment on the FG-7142-induced increaseas in numbers of c-Fos-immunoreactive cells in specific forebrain and brainstem regions. Treatment groups were as follows: vehicle pre-treatment + vehicle; vehicle pre-treatment + FG-7142; SB334867 pre-treatment + FG-7142. Bars represent means and errors bars indicated S.E.M.s *, p < 0.05 (Fisher’s Protected LSD). c) The first column in this table indicates brain regions where there was no significant effect of either FG-7142 or SB334867 interaction on the no. of c-Fos-ir cells. Subsequent columns indicate the mean of the no. of c-Fos-ir cells +/- SEM from each treatment group in each brain region. Abbreviations: Veh, vehicle, FG, FG-7142; SB33, SB334667 (orexin 1 receptor antagonist).
3.3.2 In experiment 3b
In this experiment rats injected with SB334867 30 min prior to FG-7142 had an overall lower heart rate response to FG-7142 than rats pretreated with vehicle (SB334867 effect F(1,10) = 5.7, p = 0.039). There was no SB334867 × time effect noted (F(12,120) = 1.0, p = 0.462), which was most likely due to vehicle/FG-7142 treatment not eliciting as potent a tachycardia as in experiment 3a (i.e.,. rats treated with vehicle/FG-7142 did not increase heart rate over time (F(12,60) = 0.9, p = 0.509) Fig. 4c]. Both the vehicle/FG-7142 (F(12,60) = 18.0, p < 0.001) and SB334867/FG-7142 (F(12,60) = 8.0, p < 0.001) groups had increased locomotor activity that was not altered by pretreatment with SB334867 [SB334867 × time effect F(12,120) = 0.7, p = 0.676)(Fig. 4d). For experiments 3a and 3b, which used the same rats with 48 hours between experiments, the locomotor responses to FG-7142 were fairly similar, but the tachycardia responses following FG-7142 were lower (~5-10 BPM) in the experiment 3b, compared to experiment 3a, which may reflect drug desensitization or the pretreatment with the vehicle which did not occur prior to the FG-7142 in experiment 3a. In vehicle pretreated rats, the FG-7142 did increase MAP over time [F(12,60) = 6.6, p<0.001]. In SB334867 pretreated rats, the MAP over time failed a Normality Test (Shapiro-Wilk, P < 0.050) therefore a Friedman Repeated Measures Analysis of Variance on Ranks was done which did not detect a significant difference over time [Chi-square(12)=18.5, p = 0.101]. There was a difference in MAP between group comparisons of rats pretreated with vehicle+FG-7142 and rats treated with SB334867+FG-7142 [a SB334867 × time effect (F(12,120) = 1.9, p=0.035) where the MAP was lower in the SB334867 group 10 min post injection of FG-7142 (compared with a paired t-test), data not shown]. Since there was no difference in MAP between vehicle and FG-7142 treated rats in experiment 3a, the SB33486 appears to be attenuating and increase in MAP associated with the handling and injection. No significant differences in baseline HR, MAP or activity (over 5 min prior to drug injections) were noted between treatment groups.
4. Discussion
In the present studies we provide evidence that the ORX neurons within the DMH/PeF are an important substrate in the ability of an anxiogenic/panicogenic drug to mobilize a coordinated response. This is also is the first report that we are aware of which elucidates potential postsynaptic target sites where ORX1 receptor antagonist attenuates anxiogenic drug-induced increases in anxiety-related behavior and cardioexcitatory responses. Both FG-7142 and caffeine increased c-Fos responses in ORX neurons in the DMH/PeF, but not in the adjacent lateral hypothalamus (LH), which did approach significance (see results in section in 3.1). The lack of significant activation of the ORX neurons in the LH is a pattern that has also been observed following other stressors such as footshock [see review [19]], hypercapnia [8], and also in an animal model of panic following panic provocation [9]. In contrast, increases in c-Fos occur in the LH, but not DMH/PeF, in response to food, morphine or cocaine-related cues [see review [19]]. Overall, these data suggest that there may be some functional differentiation between ORX neurons predominantly located in the DMH/PeF versus ORX neurons in the LH.
Pre-treating rats with a centrally active ORX 1 receptor antagonist, attenuated FG-7142 induced anxiety-related behavior and cardioexcitation (i.e., tachycardia). Although the SB334867 did not significantly attenuate FG-7142 induced locomotor activity [as we have seen before [9]], there did appear to be a lower trend in the SB334867 group. This lack of effect could also be attributed to the weak effects of FG-7142 on locomotor activity. The DMH/PeF, which contains the ORX neurons that were activated by the FG-7142 drug collectively comprises the classical “hypothalamic defense system” identified long ago by electrical stimulation studies done in cats by Hess and colleagues (Hess & Brugger, 1943). Subsequent pharmacological manipulations in the DMH/PeF of rats supported the hypothesis that this region is the classical “hypothalamic defense system” [see review [20]]. For instance, disinhibiting the DMH/PeF region of rats elicits increases in heart rate (HR), mean arterial blood pressure (MAP), respiratory rate (RR), flight behaviors, and experimental anxiety [21-23]. This led to the hypothesis that the DMH/PeF is a critical anatomical substrate for coordinating an integrated panic response (Shekhar, 1994). Yet, the specific neurochemical systems in the DMH/PeF that mobilize anxiety-related behavior and cardioexcitation following local disinhibition have remained elusive until recently. In a recent report, we utilized a rat model of panic vulnerability and showed that silencing the ORX precursor gene expression in the DMH/PeF region, or systemically pretreating rats with the same ORX1 receptor antagonist used here, blocked intravenous sodium lactate-induced anxiety-related behavior and cardioexcitation [9]. The studies conducted here collectively provide further evidence that the ORX neuronal system acting via the ORX1 receptor may be the critical neurochemical system in the DMH/PeF for coordinating an integrative panic-like response.
Ex vivo analyses of cellular responses (i.e., c-Fos induction) in efferent targets of the ORX system and where FG-7142 increases cellular responses in previous studies [14], revealed that pretreating rats with the ORX 1 receptor antagonist blocked FG-7142-induced increases in c-Fos responses in 1) subregions of the extended amygdala (i.e., central amygdala and bed nucleus of the stria terminalis) involved in conditioned and unconditioned aversive responses, 2) the dorsal and ventral divisions of the periaqueductal gray involved in active and passive defensive behavioral responses to threat, respectively [for reviews, see [24, 25]]; and 3) a cardioexcitatory region of the medulla (rostroventrolateral medulla). All of these brain regions that were active following FG-7142 treatment are important postsynaptic targets of the DMH/PeF [26] and receive direct orexinergic projections [3]. Among the forebrain limbic structures, the BNST and CeA were selectively activated following FG-7142 treatment, and this c-Fos activation was blocked in rats pretreated with the ORX 1 receptor antagonist.
The BNST is suggested to be a critical substrate in eliciting unconditioned anxiety responses [27-31] and may be critical in ORX-mediated increases in anxiety seen here. The BNST is part of the extended amygdala [32, 33] and has extensive connections with the different nuclei of the amygdala, and the hypothalamus [32, 34-38]. Hammack et al. [39] found that the typical freezing and increase in escape latency associated with uncontrollable or inescapable shock were also blocked following BNST lesioning, while Sullivan et al. [40] found that lesions of this nucleus did not disrupt specific-cue related fear responses, but a more general contextual cue-mediated anxiety. The BNST is densely innervated by ORX neurons [3], and expresses both ORX 1 and ORX 2 receptors [41]. We have also determined that injecting ORX A into the BNST increases anxiety-associated behaviors [42]. Finally, in a model of panic vulnerability involving disinhibition of the ORX hypothalamic region, we were able to block anxiety provocation by injecting an ORX 1 receptor antagonist directly into the BNST [9].
The CeA, which is known to play a role in unconditioned [43] and conditioned [44] fear and anxiety responses, is also densely innervated by ORX neurons [3], and expresses ORX 1 receptors [41]. Orexin depolarizes many CeA neurons in vitro [45]. In the present study, an ORX 1 receptor antagonist attenuated FG-7142-induced increases in c-Fos-ir cells in the CeA. The presence of ORX 1 receptors in the CeA suggests that this attenuation is a result of direct antagonism of the ORX 1 receptor in the CeA.
Central ORX release mobilizes centrally mediated sympathetic responses and induces tachycardia. For instance, i.c.v. injections of ORX produce tachycardia (ORX A and B), hypertension (ORX A and B) and increases in renal sympathetic activity (ORX A only) and plasma concentrations of norepinephrine and epinephrine (ORX A only) [6, 7]. Furthermore, mice lacking pre-pro-ORX have attenuated cardioexcitatory responses following disinhibition of the DMH [46], and rats with specific lesions of ORX neurons (utilizing an ORX-saporin technique) have a 75% reduction in foot shock-induced tachycardia and hypertension [47]. A putative site for FG-7142 induced cardioexcitation is a direct projection of ORX neurons in the DMH/PeF to the rostroventrolateral medulla (RVLM), which is a site where FG-7142-induced increases in c-Fos were attenuated with the ORX 1 receptor antagonist. The RVLM plays a critical role in cardiovascular reflexes associated with pressor responses [48, 49] and ORX-containing fibers are in close apposition to RVLM neurons expressing the ORX 1 receptor [50]. Furthermore, injecting ORX into the RVLM increases local firing rates in vitro [51] and also elicits tachycardia [50, 52] and hypertension [50, 52, 53].
Both the DLPAG and VLPAG showed strong c-Fos responses to FG-7142, which were attenuated with the ORX 1 receptor antagonist. Although these regions are densely innervated by ORX neurons [3], and express ORX 1 and 2 receptors [41], ORX’s role in these regions remains largely unknown. The DLPAG and VLPAG respectively mobilize “fight-or-flight” or “freezing” responses in rats [25, 54], Pretreating rats with the ORX 1 receptor antagonist did not alter FG-7142 induced increases in c-Fos responses in the DMH/PeF, which supports the hypothesis that the ORX 1 receptor antagonist is acting at postsynaptic target sites of the hypothalamic ORX neurons, and not at ORX neurons themselves. The lack of effect on FG-7142 induced increases in c-Fos in the PVN is not unexpected since this area only expresses the ORX 2 receptor [41].
Conclusion
Overall the data presented here elucidates the neural pathways through which this centrally active ORX 1 receptor antagonist may be attenuating anxiety-associated behaviors and cardioexcitation (see hypothetical illustration in Fig. 6) and suggest that ORX 1 receptor antagonists could constitute a potential novel treatment strategy for panic and related acute anxiety states.
Figure 6.
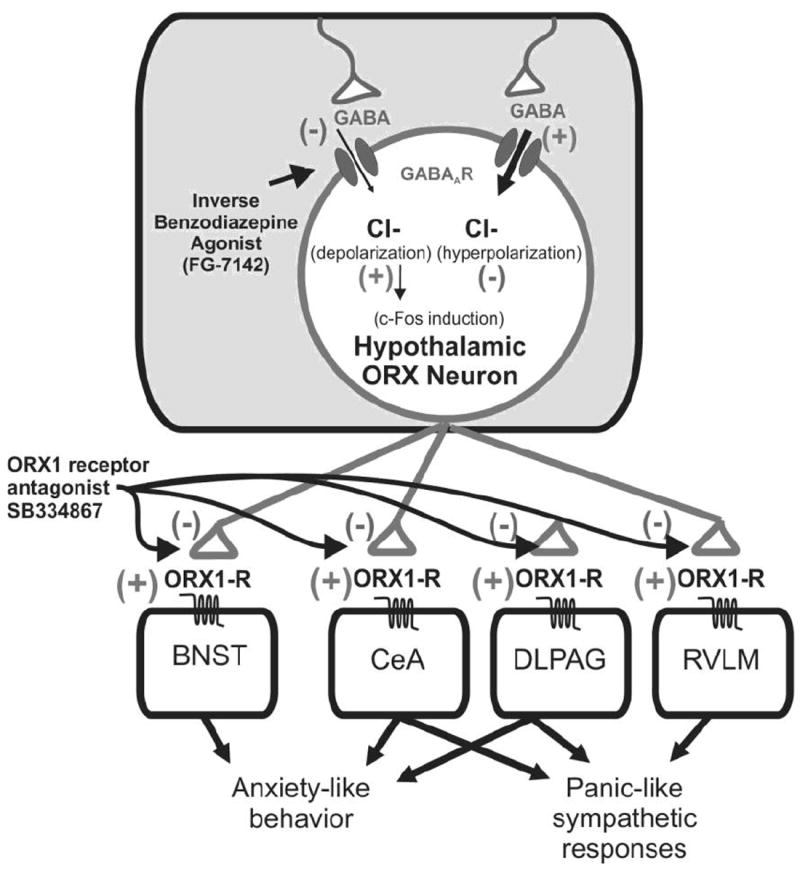
Hypothetical model depicting mechanisms through which FG-7142, a beta-carboline, negatively modulates GABA-GABAA receptor-mediated chloride influx [55] on orexin neurons that leads to depolarization and subsequent release of orexin at postsynaptic targets in the brain and spinal cord that express orexin 1 receptors to increase anxiety-associated behavior and cardioexcitation. Abbreviations: BNST, bed nucleus of the stria terminalis; CeA, central amygdala, DLPAG, dorsolateral periaqueductal gray; ORX, orexin; ORX1-R, orexin 1 receptor; RVLM, rostroventrolateral medulla.
Figure 5.
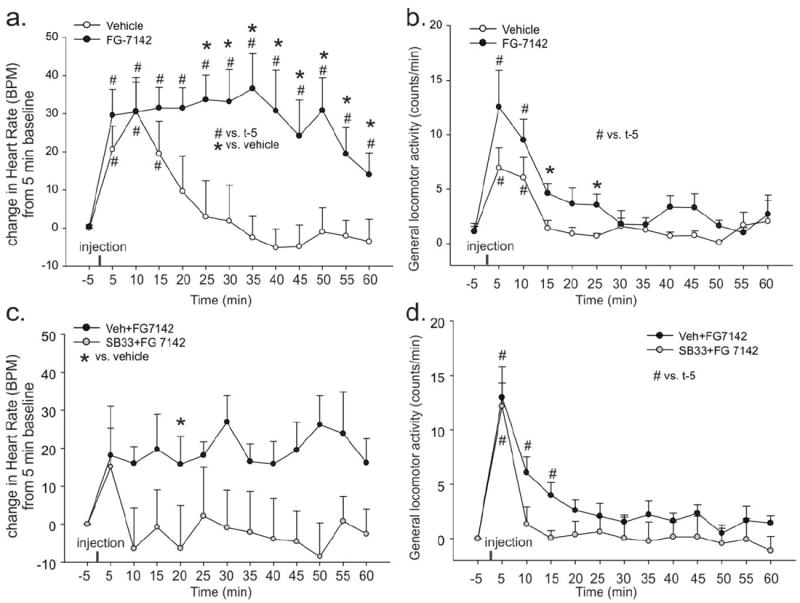
Effects of FG-7142 and the ORX 1 receptor antagonist SB334867 (SB33) on heart rate (HR) and locomotor activity. a, b) Line graphs illustrate changes in a) heart rate [HR in beats/min (BPM)] comparing 5 min baseline values (pre-injection) to values measured post-injection, and b) general activity counts between rats systemically injected with vehicle or FG-7142. c, d) Line graphs illustrate changes in c) heart rate [HR in beats/min (BPM)] comparing 5 min baseline values (pre-injection) to values measured post-injection, and d) general activity counts between rats systemically pre-injected with vehicle or SB334867 30 min prior to a systemic FG-7142 injection. *, p < 0.05, between-subjects effects (2-tailed paired t-test). #, p < 0.05, within-subjects effects over time (Dunnett’s test). There were no significant baseline HR or activity differences between groups (see results section). Values of lines represent means, whereas error bars represent S.E.M.s.
Highlights.
-
A centrally active orexin 1 receptor antagonist attenuates panicogenic drug induced:
anxiety and cardioexcitation and;
brain responses in the amygdala, periaqueductal gray and rostroventrolateral medulla.
Acknowledgments
We gratefully acknowledge Jolane K. Abrams for sectioning the brain tissue and Amy Dietrich for immunohistochemical assistance from Experiment 1. This work was supported by Indiana CTSI Project Development Team Pilot Grant (RR025761to PLJ) undergraduate research fellowships (to MCE and NHO from the Indiana CTSI (UL1 RR025761 to AS), NIH Student LRP and NARSAD Young Investigator Award to PLJ; R01 MH52619 to AS and R01 MH065702 to AS and CAL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 2005;1044:116–21. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- 6.Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept. 2002;104:91–5. doi: 10.1016/s0167-0115(01)00352-4. [DOI] [PubMed] [Google Scholar]
- 7.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–R5. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PJ, Samuels BC, Fitz SD, Barton S, Lightman SL, Lowry CA, et al. Activation of the orexin 1 receptor is a critical component of CO2 mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.38. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nature medicine. 2010;16:111–5. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamec RE. Evidence that long-lasting potentiation in limbic circuits mediating defensive behaviour in the right hemisphere underlies pharmacological stressor (FG-7142) induced lasting increases in anxiety-like behaviour: role of benzodiazepine receptors. J Psychopharmacol. 2000;14:307–22. doi: 10.1177/026988110001400401. [DOI] [PubMed] [Google Scholar]
- 11.Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2:98–9. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- 12.Lin AS, Uhde TW, Slate SO, McCann UD. Effects of intravenous caffeine administered to healthy males during sleep. Depress Anxiety. 1997;5:21–8. [PubMed] [Google Scholar]
- 13.Minor TR, Rowe MK, Soames Job RF, Ferguson EC. Escape deficits induced by inescapable shock and metabolic stress are reversed by adenosine receptor antagonists. Behav Brain Res. 2001;120:203–12. doi: 10.1016/s0166-4328(00)00376-4. [DOI] [PubMed] [Google Scholar]
- 14.Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–83. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- 15.Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–70. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 17.Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav. 1996;55:249–56. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- 18.Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, et al. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res. 2005;157:331–41. doi: 10.1016/j.bbr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 20.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–80. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 21.DiMicco JA, Abshire VM, Hankins KD, Sample RH, Wible JH., Jr Microinjection of GABA antagonists into posterior hypothalamus elevates heart rate in anesthetized rats. Neuropharmacology. 1986;25:1063–6. doi: 10.1016/0028-3908(86)90203-0. [DOI] [PubMed] [Google Scholar]
- 22.Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R8. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- 23.Shekhar A. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. I. Behavioral measures. Brain Res. 1993;627:9–16. doi: 10.1016/0006-8993(93)90742-6. [DOI] [PubMed] [Google Scholar]
- 24.Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav Brain Res. 2008;188:1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann N Y Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 27.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–47. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 28.Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 29.Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1675–87. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 31.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–41. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 33.McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Ann N Y Acad Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- 34.Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L. The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience. 1998;84:967–96. doi: 10.1016/s0306-4522(97)00560-5. [DOI] [PubMed] [Google Scholar]
- 35.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 36.Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–98. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- 37.Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: Possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–76. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- 38.Ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Research Bulletin. 1986;16:231–48. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- 39.Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–8. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 42.Truitt W, J PJ, Dietrich A, Kelley PE, F SD, S A. Anxiety-like responses induced by orexin A in the BNST are attenuated by NMDA antagonism. Society for Neuroscience. 2009 [Google Scholar]
- 43.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–80. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 44.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–15. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, et al. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R93. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- 47.Furlong TM, Carrive P. Hypocretin/orexin neurons and the perifornical hypothalamus play a more important role in contextual fear than in restraint stress. Society for Neuroscience. 2005 [Google Scholar]
- 48.Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, et al. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci. 1984;4:474–94. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada KA, McAllen RM, Loewy AD. GABA antagonists applied to the ventral surface of the medulla oblongata block the baroreceptor reflex. Brain Res. 1984;297:175–80. doi: 10.1016/0006-8993(84)90556-0. [DOI] [PubMed] [Google Scholar]
- 50.Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1801–R7. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- 52.Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R692–R7. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- 53.Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept. 2002;104:75–81. doi: 10.1016/s0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- 54.Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791–9. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- 55.Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7,142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


