Abstract
Forkhead box P3 (Foxp3) is well known for its highly restricted expression in T regulatory cells (Tregs). A recent study suggested the existence of a Foxp3 positive macrophage subpopulation in mouse bone marrow, spleen, liver, lymph nodes, and thymus that exhibited immune regulatory effect similar to Tregs. Before this report was retracted, we attempted to study the function of this macrophage subpopulation in a mouse model of hyperlipidemia. Bone marrow and spleen cells isolated from C57BL/6 apo E−/− mice were stained with anti-CD11b, anti-F4/80 and anti-Foxp3 and analyzed by flow cytometry. Our results showed that 3.06–8.08% of CD11b+F4/80+ macrophages from bone marrow cells and 0.24–2.21% from splenic were Foxp3-positive. Unexpectedly, unstained or isotype stained controls also showed strong autofluorescence and similar percentages of these cells fell within the same FL1 channel that counted the anti-Foxp3 stained population. Back gating of the autofluorescent population onto a SSC/FSC plot showed that this population of cells had a higher side scatter. The peritoneal macrophages (PMø) exhibited similar autofluorescence. We used qPCR to further evaluate the expression of Foxp3 mRNA in PMø that were treated with M-CSF, M-CSF+IL-4, M-CSF+TGFβ1 or in BMDM treated with TGFβ1 in the presence of anti-CD3 and CD28 antibody co-stimulators. No expression of Foxp3 mRNA was detected in either cell culture systems, whereas robust Foxp3 gene expression was induced in naïve CD4+ cells stimulated with TGFβ1. Consistent with these findings, fluorescence microscopy showed no Foxp3 protein expression in PMø, however Foxp3 expression was easily detected in induced Tregs. We conclude that the reported expression of Foxp3 in macrophages is likely an artifact and that a stringent multimodality approach is critical to demonstrate candidate gene expression in any cell type.
1. Introduction
Naturally occurring regulatory T cells (Treg) are important for the induction of self-tolerance and the control of autoimmunity. Forkhead box P3 (Foxp3) has been well recognized as a specific transcription factor for Treg cells, acting as a lineage-specific factor or master regulator (Miyao T, 2012). However, some reports show that Foxp3 is expressed in other non-Treg cells, including epithelial cells (Chen GY, 2008) and breast cancer cells (Zuo T, 2007 ). Recently, a distinct Foxp3 expressing macrophage subpopulation was described in mouse bone marrow, spleen, liver, lymph nodes, and thymus; it was characterized as a CD11b+F4/80+Foxp3+ or CD11b+CD68+Foxp3+ macrophage subpopulation which suppressed immune effector cell capability and promoted tumor growth (Manrique SZ, 2011a). This report of macrophage expression of Foxp3 raised interest in the scientific community (Giri PK, 2011; O, 2011; Tsun A, 2011), however this report was subsequently retracted (Manrique SZ, 2011b). After this paper was originally published, we initiated studies to investigate the role of Foxp3+ macrophages in the pathogenesis of atherosclerosis using our experimental mouse model. Specifically, we examined the changes in the Foxp3-expressing macrophage population during hyperlipidemia by comparing apo E−/− mice fed a high fat diet to control mice fed a normal mouse chow. We adopted similar strategies as those described in the original article to identify the Foxp3+ macrophage subpopulation in the bone marrow, spleen, and liver of mice cohorts [4]. We were unable to replicate the presence of CD11b+F4/80+ macrophage population that express Foxp3 and noted that the Foxp3 positive staining is most likely an artifact, attributable to autofluorescence.
2. Material and Methods
2.1. Animals and cells
C57BL/6 apo E−/− mice (16 – 26 weeks old) were housed under specific pathogen-free conditions at the animal facility at the Cedars-Sinai Medical Center. The experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center.
Mouse bone marrow cells were isolated from femoral bones using standard methods. Splenocytes were obtained by mechanical homogenization and passing the homogenate through a 40μm cell strainer (BD). Mouse splenic naïve CD4+ cells were isolated by using CD4+CD62L+ T Cell Isolation Kit II (Miltenyi Biotec, Auburn, USA) following manufacturer’s instructions.
Raw264.7 cells (Mouse leukemic monocyte macrophage cell line) were obtained from the American Type Culture Collection (ATCC) (Manassas, Virginia, USA). Thioglycollate-elicited peritoneal macrophages were harvested from mice that had received an intra-peritoneal injection of 2 ml of thioglycollate solution (4%), three days prior to harvest. Raw264.7 cells or mouse peritoneal macrophages were cultured in RPMI1640 media containing 10% FCS, 1% penicillin/streptomycin and L-glutamine. To further enrich the peritoneal macrophage population, unattached cells were washed off the culture plates with medium on second day after seeding. Bone marrow derived macrophages (BMDM) were prepared as previously described (Sharifi et al, 2006)(Sharifi et al., 2006).
2.2. Cell staining and flow cytometry analysis
The staining of macrophages from bone marrow, splenocytes, peritoneal macrophages and Tregs induced from naïve CD4+ cells was performed in two steps. First, cells were surface-stained with APC-anti-CD11b antibody and PE-anti-F4/80 antibody (Biolegend). Following this, the cells were fixed and permeabilized with FOXP3 Fix/Perm Buffer Set (cat# 421403, BioLegend), and then stained with Alexa Fluor® 488 anti-mouse Foxp3. In some of the mouse peritoneal sample staining experiments, different fluorophores were used; labeled anti-F4/80 antibody (Alexa Fluor® 488) and anti-mouse Foxp3 (PE labeled) antibody (Biolegend). Beckman-Coulter (Dako) CyAn was used for data acquisition at the Flow Cytometry Core of Cedars-Sinai Medical Center, and the data were analyzed with Summit software. After flow cytometry, the remaining cells were DAPI-stained and representative images were taken (AxioImager, Zeiss).
2.3. In vitro induction of regulatory T cells from naïve CD4+ cells
Tregs were induced from mouse naïve CD4+ cells as previously described (Fantini MC, 2007). Briefly, 1×106 isolated naïve CD4+ cells per well were seeded onto 12 well plates, and they were stimulated with plate bound anti-CD3 (5 μg/ml) and soluble anti-CD28 (2.5 μg/ml, Biolengend) in the absence or presence of 5 ng/ml transforming growth factor β1 (TGFs1, R&D systems) for 72 hrs. The culture medium used was X-VIVO 20 (Lonza, Walkersville, MD) containing 20 ng/ml IL-2 (Peprotech, Rocky Hill, NJ). Stimulated cells were used as a positive Foxp3 intracellular staining control.
2.4. RNA extraction and real time -PCR
Total RNA was extracted from cells using TRIzol Reagent (Invitrogen) following manufacturer’s instructions. cDNA was prepared with iScript cDNA synthesis kit (Bio-Rad). Relative Foxp3 expression level was determined by qPCR with EvaGreen Supermix (Bio-Rad). The mouse Foxp3 primer sequences used were forward 5′-GGTACACCCAGGAAAGACAG-3′, reverse 5′-ATCCAGGAGATGATCTGCTTG-3′. GAPDH used as internal control, primer sequences are forward 5′-TCAAGCTCATTTCCTGGTATGAC-3′, and reverse 5′-TTACTCCTTGGAGGCCATGT-3′. The PCR was performed with an initial incubation step at 94 °C for 3 min, 40 cycles of 94 °C for 10 s, 60 °C for 30 s, and followed by a melting curve analysis step.
3. Results
3.1. False-positive Foxp3 intracellular staining of macrophages is due to an autofluorescent macrophage population
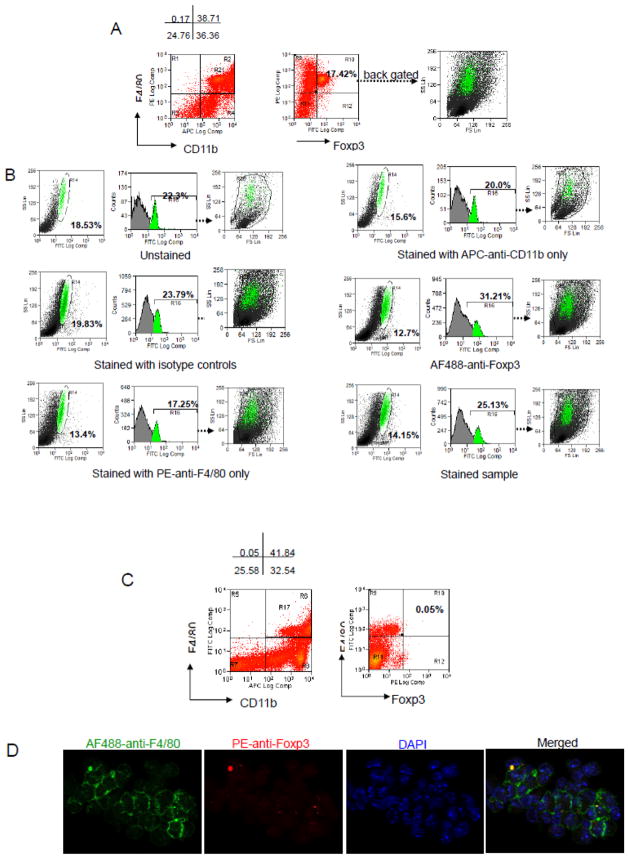
We were able to reproduce the results previously reported [4]. Bone marrow cells and splenocytes contained a population of 3.06–8.08% and 0.24–2.21% CD11b+F4/80+ “Foxp3 expressing” macrophages, respectively. A representative FACS data of bone marrow cell and splenocyte staining is shown (Fig. 1 and Fig. 2, respectively); the “CD11b+F4/80+Foxp3+” cells that were counted in bone marrow (7.95%) (Fig. 1A) and spleen (1.15%) (Fig. 2A) are also shown. However, the unstained, isotype, or single color stained controls contained a strongly autofluorescent population in mouse bone marrow cells in the FL1 channel (Fig. 1B). It corresponded to 7.43% of the unstained cells in the plot of SS vs FL1 (FITC), and 8.85% in the histogram of FL1 (Fig. 1B, unstained). Isotype control and single color stained samples had similar populations. When we compared isotype with stained sample only, there was a slight increased staining (0.75% in SSC/FSC, 0.23% in FL1 histogram). Back gating of this autofluorescent population to the SSC/FSC dot plot showed a higher side scatter (represented by green dots in the SSC/FSC plot). In stained samples, the “CD11b+F4/80+Foxp3+” subpopulation distribution in the SSC/FSC dot plot (Fig. 1A) showed the same pattern that was seen in all the control staining (Fig. 1B). Back gating of the autofluorescent population (gate R31; Fig. 1B) to the F4/80/Foxp3 plot in (Fig. 1A), confirmed the “CD11b+F4/80+Foxp3+” population was the autofluorescent population (figure not shown). In splenocytes, a much lower percentage (1.15%) of “CD11b+F4/80+Foxp3+” subpopulation was observed (Fig. 2A). However, this macrophage population exhibited similar characteristics as we had seen in bone marrow cells, which was an autofluorescent population and had a higher SSC in the SSC/FSC dot plot..
Figure 1. FACS analysis of bone marrow cells with macrophage- and Foxp3-specific antibodies.
(A) CD11b+F4/80+ macrophage population was gated (gate R22), and “Foxp3 positive population” was determined (7.95%). The population was back gated in the SSC/FSC plot and distribution was showed in green dots. (B) Unstained, isotype, each individual antibody and antibody cocktail stained sample were analyzed. Autofluorescent population percentages were shown in the SSC/FL1(FITC) plots and FL1(FITC) histogram. Distribution of the population was shown in the SSC/FSC plots.
Figure 2. FACS analysis of splenocytes with macrophage- and Foxp3-specific antibodies.
(A) CD11b+F4/80+ macrophage population were gated (gate R22), and “Foxp3 positive population” was determined(1.15%). The population was back gated in the SSC/FSC plot and distribution was showed in pink dots. (B) Unstained, isotype, each individual antibody and antibody cocktail stained sample were analyzed. Autofluroscent population percentages were shown in the plots of SSC/FL1(FITC)(gate R31). Distribution of the population was shown in the SSC/FSC plots. All data presented were from an individual C57 apoE(−/−) mouse on normal diet; similar staining was seen in the other mice within the same group and those fed a high fat diet.
Mouse peritoneal macrophages were also stained to further investigate the putative ‘Foxp3-positive cell’ subpopulation. Macrophages were harvested from the peritoneal cavities of thioglycollate-stimulated mice and stained with anti-CD11b, anti-F4/80 and anti-Foxp3, following the previously used methodology. 38.71–41.84% of the cells isolated from peritoneal cavity were CD11b+F4/80+ (Fig. 3A and 3C) and 17.42% of these macrophages were “Foxp3+ve” (Fig. 3A). However, similar to our observation in bone marrow cells, unstained, isotype, and single stained controls contained a strong autofluorescent subpopulation. This accounted for 18.53% and 19.83% of the ‘positive cells’ in unstained and isotype control, respectively, in the SS vs FL1 plot. In stained sample controls, the percentage was 14.15%. Back gating of this subpopulation to SSC/FSC plot, showed that this population had similar properties to the autofluorescence population in bone marrow and spleen; the “CD11b+F4/80+Foxp3+” cells were again an autofluorescent population, although the median fluorescence intensity had increased in the histogram of FL1. When we changed AF488 labeled anti-Foxp3 antibody to PE labeled anti-Foxp3, and AF488-anti-F4/80 to PE-anti-F4/80, we eliminated the false CD11b+F4/80+Foxp3+ population as shown (Fig. 3C), suggesting that a green fluorophore is prone to misinterpretation. In keeping with the FACS data, no Foxp3 expressing macrophages were found when the stained samples were observed using a U.V. microscope (Fig. 3D).
Figure 3. FACS analysis and fluorescent microscopy of “Foxp3 expression” in mouse peritoneal macrophages (PMφ).
(A) Cells were stained with APC-anti- CD11b, PE-anti-F4/80, and AF488-anti-Foxp3. CD11b+F4/80+ macrophage population was gated (R21 in Fig. 3A or R17 in Fig. 3C ). A discrete “Foxp3 falsepositive population” accounted for 17.42% of total PMφ cells were detected. (B) Unstained, Isotype, and single color stained controls of PMφ. Autofluorescencent population was detected in a subpopulation of PMφ in the SSC/FL1(AF488) plots, histogram plots of FL1(AF488), and the population was back gated in the FSC/SSC dot plot showing it had a higher side scatter(green dots). (C) Cells were stained with APC-anti-CD11b, AF488-anti-F4/80, and PE-anti-Foxp3, less autofluorescence in FL2(PE) channel was seen when using PE labeled anti- Foxp3 antibody. The remaining cells were DAPI-stained and representative images were taken; F4/80 (green), Foxp3 (red), and DAPI (blue). Data presented are from an individual C57 apoE(−/−) mouse with normal diet. Similar staining was observed in the other experimental group.
3.2. TGFβ1 signal fails to induce macrophages to express Foxp3
According to Manrique et al (Manrique SZ, 2011a), CD11b+F4/80+Foxp3− macrophages could be converted into CD11b+F4/80+Foxp3+ macrophages by TGFβ1. we re-examined this using either peritoneal macrophages or BMDM. M-CSF was used to generate more homogenous and mature macrophages, M-CSF plus IL-4 to skew macrophages to alternatively activated (M2) phenotype, and M-CSF plus TGFβ1 to induce Foxp3 expression during three day cultures of peritoneal macrophages. BMDM stimulation conditions were exactly the same as in vitro induction of Foxp3 expression with naïve CD4+ cells, except one group without addition of anti-CD3 and anti-CD28. Naïve CD4+ cells isolated from mouse spleen were used as a positive control.
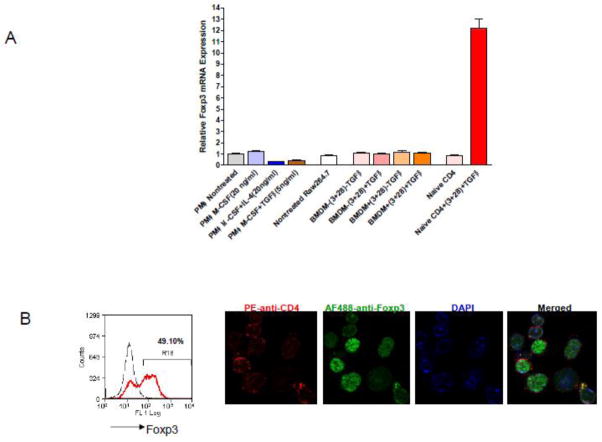
Real-time PCR showed that Foxp3 expression was not up-regulated by M-CSF alone, M-CSF plus IL-4, or M-CSF plus TGFβ1 treatments in peritoneal macrophages. Foxp3 expression in BMDM was also unchanged. The relative expression levels were as low and similar to those seen in Raw 264.7 cells, in all the treated and untreated cells. In contrast, TGFβ1 actively converted naïve CD4+ cells into Foxp3 expressing Tregs (Fig. 4B). Positive Foxp3 nuclear staining in these cells was readily detected under microscope (Fig. 4B). These results suggest that the Foxp3 transcript in primary mouse macrophages has extremely low copy number and Foxp3 expression is not inducible by TGFβ1.
Figure 4. Analysis of Foxp3 mRNA expression in macrophages and naïve CD4+ cells.
(A) qPCR of Foxp3 mRNA expression in stimulated and unstimulated cells. PMφ were stimulated with MCSF( 5ng/ml), M-CSF+IL-4 (20ng/ml), and M-CSF+TGFβ1(5ng/ml) for 72 hrs; BMDM or naïve CD4+ cells were stimulated with TGFβ (5ng/ml) in the presence of plate bound anti-CD3(2.5μg/ml) and anti-CD28(5μg/ml), IL-2 (20ng/ml) in the medium for 72 hrs; unstimulated Raw264.7 cells were the negative control. (B) Unstimulated (dashed line) and stimulated (red) naïve CD4 cells were stained with PE-anti-CD4 and AF488-anti-Foxp3 for flow cytometry analysis. The remaining cells were DAPI-stained and representative images were taken.
4. Discussion
Mammalian cells contain cellular components, such as NADPH and avins, which are intrinsically fluorescent when exposed to certain wavelengths (specifically 488 nm in flow cytometry) without the addition of external fluorophores, resulting in auto fluorescence. Depending on the biological and physiological conditions of cells and the types of cells, the levels of autofluorescence can vary (M., 2005; Hulspas R, 2009). Our observation was that small lymphocytes in bone marrow or spleen have less autofluorescence than larger macrophages; the autofluorescent CD11b+F4/80+ macrophage population which was shown as SSChigh on flow cytometry suggests that a high content of granularity is associated with the autofluorescence. Daigneault et al. (Marc Daigneault, 2010) made a similar observation confirming that the enhanced granularity, associated with the increased number of cellular organelles in the cytoplasm, of differentiated macrophages resulted in autofluorescence.
Thus, autofluorescence can be a significant problem in flow cytometry analysis, particularly when working with macrophages which are well known for being sticky. In our hands, mouse bone marrow cells, splenocytes, or peritoneal macrophages were all shown to contain a subpopulation of autofluorescent cells that was detected in the FL1 channel. We used a green fluorophore-labeled antibody (Alexa488 labeled anti-Foxp3), therefore, both the unspecific stained population and/or the autofluorescent subpopulation could give false-positive Foxp3 staining. Bone marrow and spleen contain a mixture of cell types with widely different physical properties but only a small proportion of granular macrophages are responsible for this effect. Moreover, if the cells do not express Foxp3, or the Foxp3 intracellular staining is not bright enough, autofluorescence may completely hinder the detection of these cells. We feel that this false positivity is particularly significant when intracellular epitopes are examined as compared to cell surface antigens. However, in circumstances involving weak fluorescence intensity, such as labeling cell surface antigens with low affinity antibody or nonspecific binding of Fc molecule to cell surface receptors, the autofluorescence may interfere with data interpretation and more rigorous methods need to be employed to indentify the antigen.
When this report was being prepared, two papers describing “Foxp3 expressing macrophages” were published. Put et al. (Put S, 2012) showed that a Foxp3-positive macrophage population was due to high background fluorescence and macrophages have no lineage history of Foxp3. Similarly, Mayer et al. (Mayer CT, 2012) demonstrated that when CD11b−Foxp3+ Tregs from the spleens of Foxp3DTR-eGFP(DEREG) mice were depleted following DT administration, whereas “CD11b+Foxp3+” cells remained, the “CD11b+Foxp3+” macrophage population did not express Foxp3 and the observation was attributable to auto fluorescence. These studies, along with our study, provide further evidence that macrophages do not express Foxp3.
Flow cytometry has been widely used as a powerful tool to quantify any cell population by size, granularity and the presence of specific markers. However, its accuracy to distinguish true-positive from false-positive cell populations relies on an optimized labeling protocol with specific antibodies and appropriate control samples. As a quantitative measurement, flow cytometry should be combined with other qualitative measurement, such as Western blotting, qPCR, to confirm target antigen expression to avoid false-positive data.
Highlights.
Foxp3 has been reported to be expressed in macrophages
We determined the macrophage phenotype in bone marrow and spleen of apo E−/− mice
Flow cytometry analysis showed that the Foxp3-positive macrophages are an artifact
qPCR analysis confirmed flow cytometry data
Foxp3 expression in macrophages is an artifact of autofluorescence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen GY, CC, Wang L, Chang X, Zheng P, Liu Y. Cutting edge: Broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol. 2008;180:5163–6. doi: 10.4049/jimmunol.180.8.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini MC, DS, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–94. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- Giri PK, SA, Estes DM. New players in immune regulation. Immunotherapy. 2011;3:1290–1. [PubMed] [Google Scholar]
- Hulspas R, OGM, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin Cytom. 2009;76:355–64. doi: 10.1002/cyto.b.20485. [DOI] [PubMed] [Google Scholar]
- MM Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev 2005. 2005;11:227–56. 11, 227–56. doi: 10.1016/S1387-2656(05)11007-2. [DOI] [PubMed] [Google Scholar]
- Manrique SZ, CM, Hoelzinger DB, Dominguez AL, Mirza N, Lin HH, Stein-Streilein J, Gordon S, Lustgarten J. Foxp3-positive macrophages display immunosuppressive properties and promote tumor growth. J Exp Med. 2011a;208:1485–99. doi: 10.1084/jem.20100730. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Manrique SZ, CM, Hoelzinger DB, Dominguez AL, Mirza N, Lin HH, Stein-Streilein J, Gordon S, Lustgarten J. Retraction. Foxp3-positive macrophages display immunosuppressive properties and promote tumor growth. J Exp Med. 2011b;208:2561. doi: 10.1084/jem.2010073020812r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigneault Marc, JAP, Marriott Helen M, Whyte Moira KB, Dockrell David H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CT, KA, Loddenkemper C, Sparwasser T. Lack of Foxp3+ macrophages in both untreated and B16 melanoma-bearing mice. Blood. 2012;119:1314–5. doi: 10.1182/blood-2011-11-392266. [DOI] [PubMed] [Google Scholar]
- Miyao T, FS, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–75. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- OL Immune regulation: macrophages join the FOXP3 suppressor gang. Nat Rev Immunol. 2011;11:438. doi: 10.1038/nri3015. [DOI] [PubMed] [Google Scholar]
- Put S, AA, Humblet-Baron S, Schurgers E, Liston A, Matthys P. Macrophages have no lineage history of Foxp3 expression. Blood. 2012;119:1316–8. doi: 10.1182/blood-2011-11-391755. [DOI] [PubMed] [Google Scholar]
- Sharifi BG, Zeng Z, Wang L, Song L, Chen H, Qin M, Sierra-Honigmann MR, Wachsmann-Hogiu S, Shah PK. Pleiotrophin Induces Transdifferentiation of Monocytes Into Functional Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1273–1280. doi: 10.1161/01.ATV.0000222017.05085.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsun A, LY, Li B. You, me and Foxp3: immune regulation for two. Immunotherapy. 2011;3:1139–42. doi: 10.2217/imt.11.118. [DOI] [PubMed] [Google Scholar]
- Zuo T, WL, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, Liu JQ, Love R, Liu CG, Godfrey V, Shen R, Huang TH, Yang T, Park BK, Wang CY, Zheng P, Liu Y. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–86. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]