Abstract
The Bcl-2 interacting death suppressor (Bis) protein is known to be involved in a variety of pathophysiological conditions. We recently generated bis-deficient mice, which exhibited early lethality with typical nutritional deprivation status. To further investigate the molecular basis for the malnutrition phenotype of bis deficient mice, we explored Bis expression in the digestive system of normal mice. Western blot analysis and quantitative real time reverse transcription polymerase chain reaction analysis indicated that Bis expression is highest in the esophagus, followed by the stomach, colon, jejunum and ileum. Immunohistochemical data indicated that Bis expression is restricted to the stratified squamous epitheliums in the esophagus and forestomach, and was not notable in the columnar epitheliums in the stomach, small intestine and colon. In addition, strong Bis immunoreactivity was detected in the striated muscles surrounding the esophagus and smooth muscles at a lesser intensity throughout the gastrointestinal (GI) tract. Ganglionated plexuses, located in submucous layers, as well as intermuscular layers, were specifically immunoreactive for Bis. Immunofluorescence studies revealed that Bis is co-localized in glial fibrillary acidic protein-expressing enteric glial cells. Immunostaining with neuron specific esterase antibodies indicate that Bis is also present in the cell bodies of ganglions in the enteric nervous system (ENS). Our findings indicate that Bis plays a role in regulating GI functions, such as motility and absorption, through modulating signal transmission between the ENS and smooth muscles or the intestinal epitheliums.
Keywords: Bag3, Enteric nervous system, Enteric glial cell, Mouse, Immunohistochemistry
Introduction
Bis, also referred as Bag3, was originally identified as a Bcl-2 binding protein that enhances the antiapoptotic activity of Bcl-2 in vitro [1, 2]. The prosurvival function of Bis has been supported by more recent reports showing that Bis is overexpressed in a variety of tumors, including leukemia, pancreas cancers, thyoid cancers, prostate cancers, as well as glioblastomas [3-9]. In addition, the suppression of Bis expression sensitizes cell death upon various stimuli [9-11]. Bis is also known to have an anti-stress function, since its expression is up-regulated upon various stresses in vitro and in vivo, as well [4, 12-16]. Another recently reported function of Bis is that it participates in protein quality control via stimulating macroautophagy. The target proteins are aggregation-prone proteins, as evidenced by poly Q, or proteins found in aging brains, where oxidative stress is increased [17, 18]. It has been suggested that the molecular basis for its role in the selective autophagy of misfolded proteins is that it interacts with microtubule-motor dynein and subsequently directs heat shock protein 70 (Hsp70) substrates to aggresomes [19, 20]. Therefore, Bis is thought to play an important role in determining the fates of cells in various pathophysiological conditions.
We, and separately by another group, have recently reported the development of bis-deficient mice, using a cre-loxP system targeting exon 4 and retroviral insertion, respectively. In both cases, the mice exhibit early lethality before weaning as a common phenotype [21, 22]. Muscle weakness and subsequent respiratory problems were proposed as the cause of the early lethality by another group, based on the presence of massive apoptotic figures in skeletal muscles. However, even though z-disk disruption was also observed in our model, massive apoptotic findings were not found. Instead, the bis-deficient mice developed by us revealed significant hypoglycemia, fatty livers, loss of peripheral fat reserves and increased glucocorticoid levels, all of which are typical adaptive response to malnutrition [22, 23]. Thus, a serious nutritional problem might be more closely connected with the early lethality of bis-/- mice, but the precise molecular basis for this are not understood.
Bis has been demonstrated to express ubiquitously and at high levels by skeletal muscles and the heart [1]. We previously investigated the expression profile of Bis in the developing brain, as well as the adult rat brain. The findings showed that Bis expression was observed transiently in a specific population of neurons in the cortex and hippocampus and midline glial cells in the brain stem and spinal cord, while Bis expression in the glial cells in the rostral migratory system are maintained in adults [24-26]. These results suggest that the expression of Bis in the glial cells is involved in the differentiation and migration of a specific population of neurons, which is supported by in vitro experiments, showing that the knockdown of Bis expression led to an impairment in glial differentiation, as well as disorganization of spreading of mature neurons and migrating neurons [27]. It is therefore possible that Bis is involved in the development or functional maturation of enteric nervous system (ENS), leading to the regulation of the physiology of the gastrointestinal (GI) system, but the localization of Bis in ENS has not yet been investigated. The malnutrition phenotype observed in bis-deficient mice could be attributed a problem associated with the intake or absorption of milk, as well as in the motility of the intestine, which is mainly under the control of ENS. In this report, to investigate the possible role of Bis in the physiological function of the GI tract, we examined the expression of Bis in the GI tracts of mice, covering the esophagus, stomach, small intestine and colon. Particular attention was paid to the presence of Bis in the nerve plexus in the muscle layer and submucosal layer, which could be associated with the dysfunction of the GI tract of bis-deleted mice.
Materials and Methods
Animals
Bis expression in GI tract was examined in wild C57BL/6 mice at 14-16 days after birth since bis-deficient mice exhibit serious catabolic phenotypes at those ages [22]. All research procedures involving animals were performed in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals and the Guidelines and Policies for Rodent Experiment provided by the Institutional Animal Care and Use Committee (IACUC) at College of Medicine, the Catholic University of Korea.
Western blot analysis
Tissues were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris·HCl, pH 7.6, 0.1% sodium dodecyl sulfate, 1% NP-40, 0.5% sodium deoxycholate) and sonicated three times on ice. An equal amount of proteins were separated by sodium dodecyl sulfate-polyacyrlamide gel electrophoresis under reducing conditions and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The blots were incubated with anti-Bis serum raised in rabbit (1 : 10,000) or Hsp70 (1 : 1,000, BD Science, San Jose, CA, USA) [1], and subsequently with peroxidase-conjugated anti-rabbit IgG (1 : 2,000, Millipore). The visualization of Bis immunoreactive bands were performed with an enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA, USA). Quantification for the intensities of each band was carried out on Multi Gauge 2.2 software (Fuji Photo Film, Tokyo, Japan). The ratios of the density of the Bis band to that of heat shock protein Hsp70 band were compared and the relative levels of Bis were determined between fractions from mouse GI tracts.
Quantitative real-time reverse transcription polymerase chain reaction
To verify bis mRNA expression in GI tract, total RNA was extracted with RNA-zol Bee (Tel-Test, Friendswood, TX, USA), and cDNA was synthesized using reverse transcriptase (RevertAid, Thermo Fisher Scientific). The relative expression of bis mRNA was determined by quantitative real-time PCR (ABI 7300, Life Technologies, Carlsbad, CA, USA) with SYBR Premix Ex Taq (Takara Bio, Shiga, Japan) and specific primers for bis and β-actin: bis, 5'-ACTCTAAGCCTGTTTCCCAGAAGT-3' and 5'-AGACTTGTACTTGACCTGGGACAT-3' (NM_013863.5); β-actin, 5'-TTCGTT GCCGGTCCACA-3' and 5'-ACCAGCGCAGCGATATCG-3' (X03672.1). After normalized to β-actin mRNA levels, the relative expression of bis mRNA from each part of the GI tracts was represented as a relative value compared from that of the ileum, which was designated as 1.0.
Immunohistochemistry
The tissues were fixed in 10% neutral buffered-formalin and embedded in paraffin. Paraffin sections were cut in 4 µm, deparaffinized and dehydrated. Endogenous peroxidase activity was blocked for 30 minutes by treatment with hydrogen peroxide block (Thermo Fisher Scientific). To reduce nonspecific staining, sections were blocked with 10% normal goat serum (GBI, Mukilteo, WA, USA) and then incubated with primary antibody against Bis (1 : 2,000) or against neuron specific esterase (NSE; 1 : 100, Chemicon, Temecula, CA, USA) at 4℃ overnight. After washing with 0.01 M phosphate-buffered saline (PBS; pH 7.4), the sections were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1 : 400, Novus Biologicals, Littleton, CO, USA) and then rinsed in 0.01 M PBS. Sections were placed in 3,3'-diaminobenzidine tetrahydrochloride (DAB plus substrate System, Thermo Fisher Scientific). After 1-2 minutes, the reaction was stopped by several washes with distilled water.
Immunofluorescence
The tissues were embedded in Optimal Cutting Temperature compound (OCT; Tissue-Tek, Torrance, CA, USA). The sections were cut in 6 µm. The sections were dried for 30 minutes at room temperature, fixed in acetone for 10 minutes at -20℃, and blocked by treatment with 10% normal goat serum (GBI) for 30 minutes. For double-fluorescence staining, these sections were incubated with primary antibody against Bis (1 : 4,000) and antibody against glial fibrillary acidic protein (GFAP; 1:700, Millipore) overnight at 4℃. After washing in PBS, sections were incubated with Cy3-conjugated goat anti-rabbit IgG (1 : 2,000, Jackson, West Grove, PA, USA) and Alexa Fluor 488-conjugated goat anti-mouse IgG (1 : 300, Invitrogen, Carlsbad, CA, USA) for 40 minutes at room temperature. Counter-staining was performed with 4',6-diamidino-2-phenylindole (DAPI; 1 : 2,000, Roche Diagnostics GmbH, Mannheim, Germany) for 5 minutes at room temperature. Slides were viewed using a confocal microscope (LSM 510 Meta, Carl Zeiss Microimaging GmbH, Jena, Germany). Images were converted to TIFF format, and contrast levels were adjusted by Adobe Photoshop ver. 7.0 (Adobe Systems, San Jose, CA, USA).
Statistical analysis
Data were expressed as the means±standard errors (SE). Differences between the two groups were examined for statistical significance, using a two-tailed Student's t-test. A P-value of less than 0.05 was considered significant.
Results
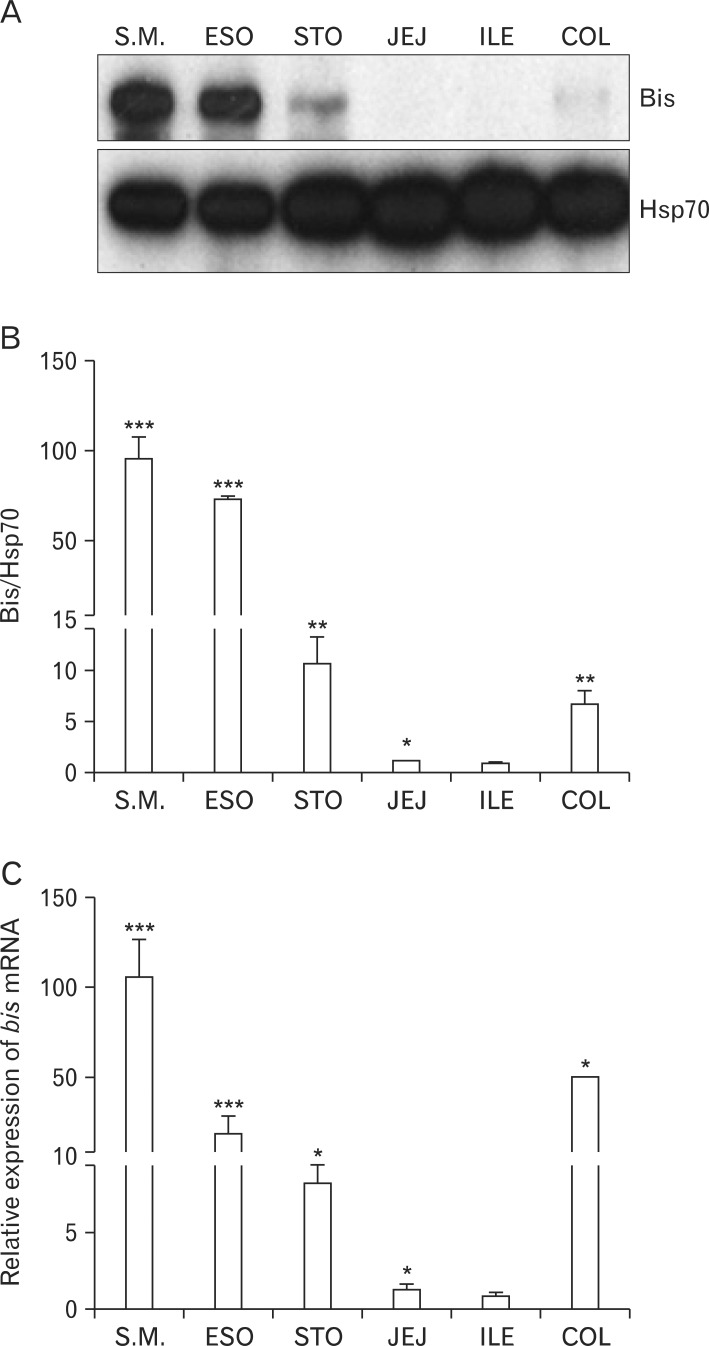
Tissue fractions were isolated from each part of the GI tract of mice at postnatal 14-16 days, and prepared for western analysis, quantitative real time analysis and immunohistochemistry. Western analysis, using specific Bis antibody, demonstrated that the degree for Bis expression varied throughout the GI tract, although the expression levels were lower than those of the skeletal muscles (Fig. 1A). Among the GI tract, the highest expression of Bis was by the esophagus, while only traces were found for the jejunum and ileum. The quantitative determination of band intensities indicated that Bis expression in the esophagus was 77 fold higher than that for the ileum (Fig. 1B). The relative bis mRNA levels were the highest in the esophagus, 38 fold higher than that for the jejunum or ileum, which is correlated with the Bis protein expression pattern in a Western assay (Fig. 1C).
Fig. 1.
Expression of Bis in the mouse gastrointestinal (GI) tract. (A) Western blot analysis for Bis in the each part of GI tracts from 14-day-old mice. A representative result was provided with heat shock protein 70 (Hsp70) as a loading control. (B) A quantitative determination of Bis expression using densitometric analysis after normalizing to Hsp70 (mean±SE, n=3). The relative density of the Bis bands to that of Hsp70 from ileum was designated as 1.0. (C) Determination of the relative expression of bis mRNA in the mouse GI tract by quantitative real time polymerase chain reaction analysis. Data are normalized relative to β-actin mRNA in the same samples, and the value for the ileum was arbitrarily set as 1.0 (mean±SE, n=3). S.M., skeletal muscle; ESO, esophagus; STO, stomach; JEJ, jejunum; ILE, ileum; COL, colon. *P<0.05, **P<0.01 and ***P<0.005 compared with the value from ileum.
To identify the Bis expressing cells in the GI tract, we performed immunohistochemistry with tissue sections from each part of the mouse GI tract. An intense immunostaining for Bis was observed in the stratified squamous epithelium lining in the esophagus (Fig. 2A). In the stomach, Bis immunoreactivity was also prominently observed in the squamous epithelium of the forestomach, but this abruptly disappeared at the junction of the forestomach and glandular stomach, where the stratified squamous epithelium of the esophagus changes abruptly to the simple columnar epithelium of the stomach (Fig. 2B, C). Bis expression was also very low in the columnar epitheliums of the jejunum, ilum and colon (Fig. 3 and data not shown). Thus, Bis expression appears to be limited to the squamous epithelium and does not occur in the columnar epithelium in the GI tract. Bis has been reported to be strongly expressed in the skeletal and cardiac muscles [1]. In the GI tract, the striated muscles surrounding the esophagus also exhibit intense immunostaining for Bis (Fig. 2A). Although the intensity is weaker than in the striated muscles, Bis immunoreactivity was detected in the smooth muscles throughout the entire GI tract, from the esophagus to the colon, including the inner circular and outer longitudinal layers (Fig. 2B, D, 3). Therefore, the highest expression of Bis in the esophagus found in the western assay is likely due to a significant level of Bis expression in the squamous epitheliums and in the striated muscles.
Fig. 2.
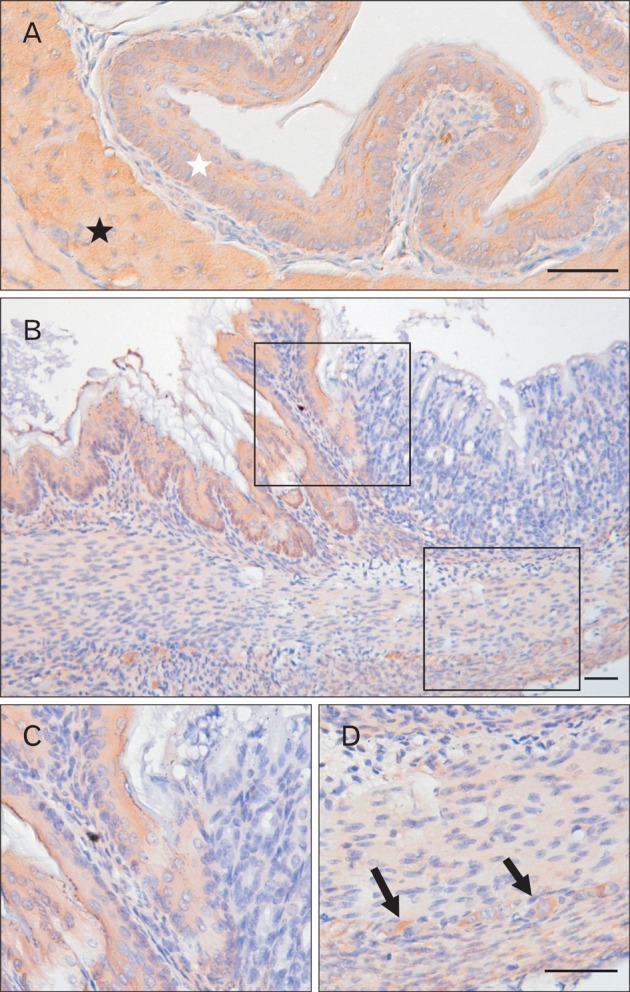
Immunohistochemical analysis of Bis in the esophagus (A) and stomach (B-D). Intense Bis immunoreactivity was observed in the stratified squamous epithelium and striated muscles (marked as white or black asterisk, respectively) in the esophagus (A). In epithelia of the grandular stomach, only trace levels of Bis expression were detected (B, C) and weak immunostaining for Bis was detected in the smooth muscles of the stomach, while specific localization of Bis was detected in myenteric plexus (arrows) (B, D). Higher magnifications of the boxed areas in B are shown in C and D. Scale bars=50 µm (A-D).
Fig. 3.
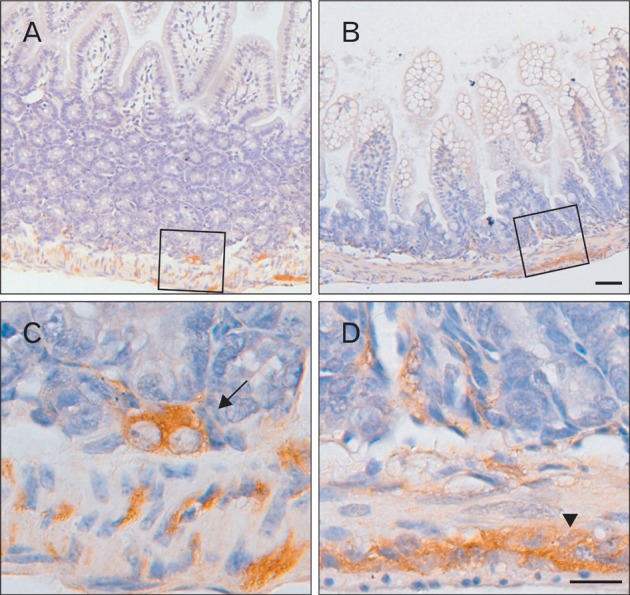
Bis immunoreactivity in the small intestine. Bis immunoreactivity was clearly localized in the ganglionated networks of the submucous plexus (arrow) and myenteric plexus (arrowhead) in jejunum (A, C) and ileum (B, D). Higher magnifications of the boxed areas in A and B are shown in C and D, respectively. Scale bars=50 µm (A, B), 20 µm (C, D).
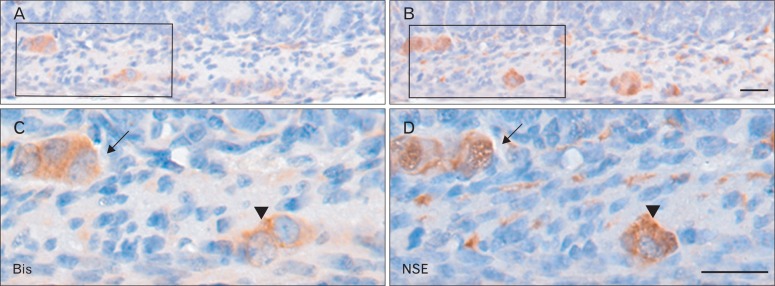
In addition to the squamous epitheliums and smooth muscles, Bis immunoreactivity was specifically detected in the myenteric nerve plexus, also called Auerbach's plexus [28], which is located between inner and outer smooth muscles that cover the esophagus, stomach and intestines (Figs. 2D, 3B, 5A). Another enteric nerve plexus, which is mainly present in the submucosa of the small intestine, called Meissner's plexus, also showed Bis immunoreactivy, the level of which were comparable to that in Auerbach's plexus (Figs. 3A, 5A). The enteric nerve plexus is composed by several types of cell populations, including neurons, glia, and interstitial cells of Cajal, and mesenchymal fibroblasts [28, 29]. An examination at higher magnification indicates that Bis immunoreactivity is localized in two populations in the enteric nerve plexus. Bis expresses in the cytoplasm of cell bodies in a population with a large nucleus and obvious nucleoli representing neurons (Fig. 3A, C). Bis expression was also detected in the cytoplasm and the extending process of the cells, which have a smaller nucleus than smooth muscle cells, indicating enteric glial cells (Fig. 3B, D). To identify the Bis-immunoreactive cells in the enteric nerve plexus, double immunofluorescence labeling was conducted, using a Bis antibody and gial GFAP antibody as a marker of enteric glial cells. Fig. 4 shows that nearly all of the GFAP expressing enteric glial cells are also immunoreactive for Bis, corresponding to a fraction of the Bis-expressing cells. We also performed immunohistochemistry with an antibody for NSE, using serial sections next to the Bis-stained sections, to compare with the distribution and density of those immunoreactive cells. As shown in Fig. 5B and D, NSE immunoreactive cells in the submucosal layer, as well as in myenteric plexuses have large immunostained cell bodies with granular cytoplasmic stained patterns and Bis immunoreactivity was also observed in the cell bodies in the same cells in subsequent sections (Fig. 5A, C). Therefore, Bis is expressed in the cell bodies of neurons in the ENS. The pattern for the expression profile of Bis in the colon was similar to that for the small intestine, showing the immunostaining in the ENS and smooth muscles (data not shown). The relatively thicker smooth muscle layers in the colon appear to be the source of the higher Bis expression in the colon, rather than in the jejunum or ileum, as found in a Western assay (Fig. 1A).
Fig. 5.
Comparison of immunoreactivity for Bis (A, C) and neuron specific esterase (NSE) (B, D) in adjacent sections of the jejunum. The large cell bodies, which were immunostained with Bis antibodies (A, C), were also positive for NSE (B, D) in submucosal plexus (arrows), as well as in myetneric plexus (arrowheads). Higher magnifications of the boxes in A and B were shown in C and D, respectively. Scale bars=20 µm (A-D).
Fig. 4.
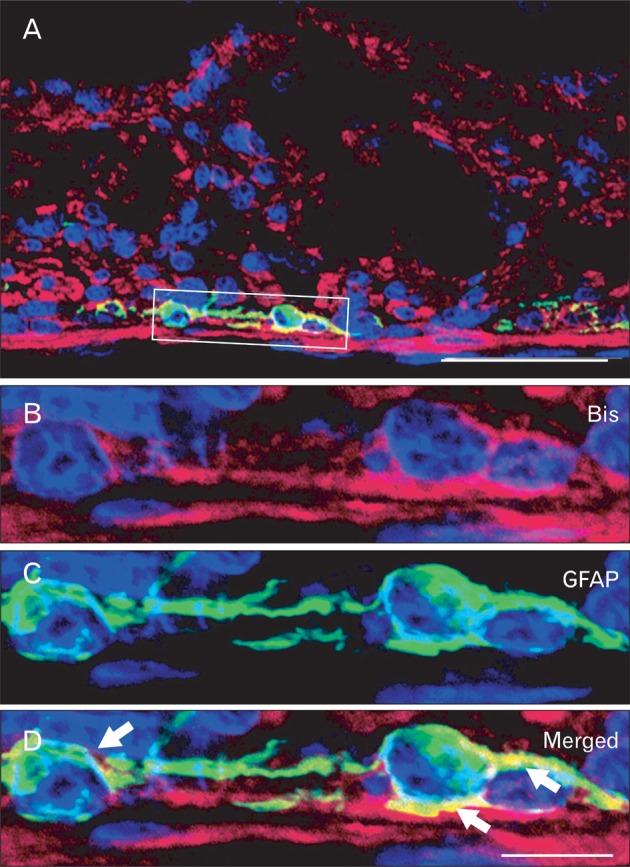
Immunofluorescence staining of Bis and glial fibrillary acidic protein (GFAP) in Jejunum. Confocal laser microscopic imaging of immunofluorescence for Bis (B), GFAP (C) and overlay (A, D) in the myenteric plexus of jejunum. Note that Bis immunoreactvity was observed in GFAP-expressing the enteric glial cells (marked as arrows).The higher magnification views of the boxed area in A are shown in B-D. Scale bars=50 µm (A), 10 µm (B-D).
Discussion
In the present study, we examined the expression profile of Bis in the mouse GI tract, using Western assay, quantitative determination of bis mRNA, and immunohistochemistry. Our results showed that Bis is expressed in the striated smooth muscles of the esophagus, as well as the smooth muscles through the entire intestinal wall. In addition, Bis expression is specifically detected in the stratified squamous epithelium, rarely in the columnar epithelium. Finally, Bis is strongly detected in the enteric ganglion cells and enteric glial cells. Therefore, the expression profile of Bis in GI tract, showing the specific localization in the cells of less self-renewing capacity, suggest the possible role for Bis in the maintenance of cell survival probably by suppressing cell death. The death suppressor function of Bis has been demonstrated by previous experiments performed in vitro, as well as in vivo data, showing higher expression of Bis in the various kinds of cancers [1, 2, 9-11]. Thus, our results presenting the expression of Bis in the normal cells lacking proliferation potential, but with long life span, support the pro-survival role of Bis in the physiological condition, in addition to a pathological condition.
ENS is organized in a series of several plexus that modulates a variety of physiological functions of the GI system, including motility, secretion, microcirculation, and inflammation [28]. Two ganglionated plexuses, the submucosal and myenteric plexus, are representative plexuses in the ENS, and are composed of neurons and enteric glial cells in a ratio of 1 : 4 [29]. Myenteric ganglia, located in the septum between the circular and longitudinal smooth muscle layers, are mainly involved in regulating the motility of the GI tract, such as mixing movement and peristalsis [30, 31], and the submucosal plexus, also referred to as Meissner's plexus, is known to regulate blood flow and electrolyte transport through the intestinal epithelium [32, 33]. The interstitial cells of Cajal are also known to coordinate information between two plexus through interconnecting fibers. Our results show that Bis expression was specifically detected in two major enteric nerve plexus areas, the submucosal and myenteric plexus, from the esophagus to the colon in the mouse GI system (Fig. 2, 3). As evidenced by colocalization with GFAP immunoreactivities, Bis was shown to be present in GFAP-positive enteric glial cells (Fig. 4). Furthermore, immunostaining of serial sections with NSE and Bis antibodies reveal that Bis is also expressed in the cytoplasm of NSE-expressing ganglion cells in small intestines (Fig. 5). The specific expressions of the Bis protein in the ENS, therefore, suggest that Bis might play an important role in the neural activity of the ENS to modulate absorption or motility processes of GI tract.
On the other hand, another prominent finding of our studies is that Bis immunoreactivity is strictly confined to the squamous epitheliums of the esophagus and forestomach, while immunoreactivity of Bis was rarely detected in the columnar epitheliums in the stomach, jejunum, ileum and colon, which mediate the transport of nutrients or electrolytes (Figs. 2, 3). Thus, it is not likely that Bis is directly involved in the digestion, secretion or absorption processes. Moreover, our findings showing that Bis is expressed by smooth muscles throughout the intestinal wall, as well as the striated muscles in the esophagus indicate that Bis is related to motility, rather than digestion or absorption. In a previous study, Bis was shown to be strongly expressed in skeletal and cardiac muscles and bis deleted mice exhibited z-disc disruption [1, 22]. Several cases of patients with myofirillar myopathy (MFM) showed a heterozygous p.Pro209Leu (c.626C>T) mutation in exon 3 of the bis gene, without a mutation in any other genes causing MFM. These patients suffer from axial muscle weakness, cardiomyopathy and respiratory insufficiency [34, 35]. In addition to typical microscopic findings of myofibrillar myopathy in a muscle biopsy, recent studies reported that bis gene-mutation caused myofibrillar myopathy patients to present features of significant axonal neuropathy with the presence of giant axons, which is associated with the accumulation of neurofillaments, in a nerve biopsy [36-38]. These results suggest that Bis might be involved in the maintenance of the integrity of the cytoskeleton in neurons, as well as in muscle fibers. Likewise, the presence of Bis in ENS and smooth muscles in the GI tract imply a regulatory role for Bis in the transfer of intrinsic regulatory signals from the ENS and the motility of the GI tract.
The targeted deletion of the bis gene results in the deterioration of nutritional status and subsequent early lethality before weaning [22, 23]. Considering that the intrinsic motor function of the GI tract is peristaltic movement or mixing for solid food, an impairment in GI motility is likely not the only cause of the malnutrition phenotype of bis-deficient mice provided by milk from the mother. The nerve terminals in the ENS could sense luminal contents, including nutrients via afferent fibers from mucosal cells, which contain specific chemoreceptors and initiate appropriate reactions, such as absorption, secretion and peristalsis [39, 40]. Moreover, the presence of receptors for small molecules, such as cholecystokinin, vasoactive intestinal peptide or nitric oxide, and receptors for peptide neurotransmitters, such as adregergic, cholinergic or dopaminergic receptors, have been demonstrated in the enteric epitheliums [41-45]. Finally, luminal perfusion studies with agonists and antagonists for adrengergic receptors indicate that the transport of glucose, amino acids or oligopeptide to enteric epithelial cells is, at least in part, under the neural control of intrinsic ENS [46-48]. Therefore, the possibility for the involvement of Bis in the absorption of nutrients in the GI tract should not be excluded by a lack of expression of Bis in the columnar epithelium in the GI tract.
With more recent information, the role of enteric glial cells in the GI system has changed from the simple mechanical support for enteric neurons to the active modulators for GI functions, such as enteric neurotransmission, inflammatory events, as well as GI motor function [49, 50]. In mouse models, the loss of enteric glia results in neuronal degeneration [51]. Genetically modulated mice, in which the glial cell line-derived neurotropic factor is deleted, exhibited a complete failure to develop ENS [52]. In addition, enteric glial ablation was found to cause a marked change in the neurochemical coding of enteric neurons: a reduction in the vasoactive intestinal peptide, substance P, and nitric oxide synthase immnoreactive neurons and an increase choline acetyl transferase expressing neurons in ENS [53]. In rats, the selective ablation of the enteric glial cells via the administration of a gliotoxin caused a decrease in small intestinal motility and transit [54]. Evidence for the involvement of enteric glial cells in abnormal GI motility in humans is evidenced by several pathological conditions, showing a significant decrease in the enteric glial cells and interstitial cells of Cajal in patients with colonic diverticular diseases or idiopathic severe slow-transit constipation [55-57]. Overall, these findings suggest that the enteric glial cells are closely linked to the development or functional maturation of the enteric neurons. We previously demonstrated that Bis expression is increased time dependently during the in vitro differentiation of the P19 embryonic carcinoma cells and the knockdown of Bis expression led to impairment in glial differentiation, as well as the disorganization of spreading of mature neurons and migrating neurons [27]. Therefore, it is possible that Bis may play an important role in the relationship between glial cells and neurons in the ENS, as well the central nervous system, affecting the structural and functional maturation of neurons. This possibility should be examined in further studies using mice carrying the glia-specific ablation of the bis gene.
Acknowledgements
We wish to thank W. Y. Kim for photographic assistances. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2007589).
References
- 1.Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999;18:6183–6190. doi: 10.1038/sj.onc.1203043. [DOI] [PubMed] [Google Scholar]
- 2.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 3.Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Büchler MW. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001;503:151–157. doi: 10.1016/s0014-5793(01)02728-4. [DOI] [PubMed] [Google Scholar]
- 4.Romano MF, Festa M, Pagliuca G, Lerose R, Bisogni R, Chiurazzi F, Storti G, Volpe S, Venuta S, Turco MC, Leone A. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ. 2003;10:383–385. doi: 10.1038/sj.cdd.4401167. [DOI] [PubMed] [Google Scholar]
- 5.Chiappetta G, Ammirante M, Basile A, Rosati A, Festa M, Monaco M, Vuttariello E, Pasquinelli R, Arra C, Zerilli M, Todaro M, Stassi G, Pezzullo L, Gentilella A, Tosco A, Pascale M, Marzullo L, Belisario MA, Turco MC, Leone A. The antiapoptotic protein BAG3 is expressed in thyroid carcinomas and modulates apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J Clin Endocrinol Metab. 2007;92:1159–1163. doi: 10.1210/jc.2006-1712. [DOI] [PubMed] [Google Scholar]
- 6.Staibano S, Mascolo M, Di Benedetto M, Vecchione ML, Ilardi G, Di Lorenzo G, Autorino R, Salerno V, Morena A, Rocco A, Turco MC, Morelli E. BAG3 protein delocalisation in prostate carcinoma. Tumour Biol. 2010;31:461–469. doi: 10.1007/s13277-010-0055-3. [DOI] [PubMed] [Google Scholar]
- 7.Ammirante M, De Laurenzi V, Graziano V, Turco MC, Rosati A. BAG3 is required for IKKalpha nuclear translocation and emergence of castration resistant prostate cancer. Cell Death Dis. 2011;2:e139. doi: 10.1038/cddis.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Festa M, Del Valle L, Khalili K, Franco R, Scognamiglio G, Graziano V, De Laurenzi V, Turco MC, Rosati A. BAG3 protein is overexpressed in human glioblastoma and is a potential target for therapy. Am J Pathol. 2011;178:2504–2512. doi: 10.1016/j.ajpath.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco R, Scognamiglio G, Salerno V, Sebastiani A, Cennamo G, Ascierto PA, Botti G, Turco MC, Rosati A. Expression of the anti-apoptotic protein BAG3 in human melanomas. J Invest Dermatol. 2012;132:252–254. doi: 10.1038/jid.2011.257. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Xu B, Li J, Lu H. BAG3 gene silencing sensitizes leukemic cells to Bortezomib-induced apoptosis. FEBS Lett. 2009;583:401–406. doi: 10.1016/j.febslet.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Wang HQ, Liu BQ, Gao YY, Meng X, Guan Y, Zhang HY, Du ZX. Inhibition of the JNK signalling pathway enhances proteasome inhibitor-induced apoptosis of kidney cancer cells by suppression of BAG3 expression. Br J Pharmacol. 2009;158:1405–1412. doi: 10.1111/j.1476-5381.2009.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MY, Kim SY, Shin SL, Choi YS, Lee JH, Tsujimoto Y, Lee JH. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol. 2002;175:338–346. doi: 10.1006/exnr.2002.7903. [DOI] [PubMed] [Google Scholar]
- 13.Lee MY, Kim SY, Choi JS, Choi YS, Jeon MH, Lee JH, Kim IK, Lee JH. Induction of Bis, a Bcl-2-binding protein, in reactive astrocytes of the rat hippocampus following kainic acid-induced seizure. Exp Mol Med. 2002;34:167–171. doi: 10.1038/emm.2002.24. [DOI] [PubMed] [Google Scholar]
- 14.Pagliuca MG, Lerose R, Cigliano S, Leone A. Regulation by heavy metals and temperature of the human BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett. 2003;541:11–15. doi: 10.1016/s0014-5793(03)00274-6. [DOI] [PubMed] [Google Scholar]
- 15.Rosati A, Leone A, Del Valle L, Amini S, Khalili K, Turco MC. Evidence for BAG3 modulation of HIV-1 gene transcription. J Cell Physiol. 2007;210:676–683. doi: 10.1002/jcp.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung SE, Kim YK, Youn DY, Lim MH, Ko JH, Ahn YS, Lee JH. Down-modulation of Bis sensitizes cell death in C6 glioma cells induced by oxygen-glucose deprivation. Brain Res. 2010;1349:1–10. doi: 10.1016/j.brainres.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 2008;283:1437–1444. doi: 10.1074/jbc.M706304200. [DOI] [PubMed] [Google Scholar]
- 18.Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28:889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 2011;12:149–156. doi: 10.1038/embor.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Qian SB. Chaperone-mediated hierarchical control in targeting misfolded proteins to aggresomes. Mol Biol Cell. 2011;22:3277–3288. doi: 10.1091/mbc.E11-05-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. 2006;169:761–773. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youn DY, Lee DH, Lim MH, Yoon JS, Lim JH, Jung SE, Yeum CE, Park CW, Youn HJ, Lee JS, Lee SB, Ikawa M, Okabe M, Tsujimoto Y, Lee JH. Bis deficiency results in early lethality with metabolic deterioration and involution of spleen and thymus. Am J Physiol Endocrinol Metab. 2008;295:E1349–E1357. doi: 10.1152/ajpendo.90704.2008. [DOI] [PubMed] [Google Scholar]
- 23.Youn DY, Yoon JS, Kim YK, Yeum CE, Lee SB, Youn HJ, Tsujimoto Y, Lee JH. Deletion of the bis gene results in a marked increase in the production of corticosterone that is associated with thymic atrophy in mice. Am J Physiol Endocrinol Metab. 2011;301:E223–E231. doi: 10.1152/ajpendo.00604.2010. [DOI] [PubMed] [Google Scholar]
- 24.Park HJ, Choi JS, Chun MH, Chung JW, Jeon MH, Lee JH, Lee MY. Immunohistochemical localization of Bis protein in the rat central nervous system. Cell Tissue Res. 2003;314:207–214. doi: 10.1007/s00441-003-0786-1. [DOI] [PubMed] [Google Scholar]
- 25.Choi JS, Lee JH, Kim HY, Chun MH, Chung JW, Lee MY. Developmental expression of Bis protein in the cerebral cortex and hippocampus of rats. Brain Res. 2006;1092:69–78. doi: 10.1016/j.brainres.2006.02.137. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Lee JH, Shin YJ, Lee JY, Yun H, Chun MH, Lee MY. Transient expression of Bis protein in midline radial glia in developing rat brainstem and spinal cord. Cell Tissue Res. 2009;337:27–36. doi: 10.1007/s00441-009-0794-x. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JS, Lee MY, Lee JS, Park CS, Youn HJ, Lee JH. Bis is involved in glial differentiation of p19 cells induced by retinoic Acid. Korean J Physiol Pharmacol. 2009;13:251–256. doi: 10.4196/kjpp.2009.13.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 29.Jessen KR. Glial cells. Int J Biochem Cell Biol. 2004;36:1861–1867. doi: 10.1016/j.biocel.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3rd ed. Vol. 1. New York: Rven Press; 1994. pp. 423–482. [Google Scholar]
- 31.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi H, Suzuki T, Yamamoto T, Suzuki Y. Cholinergic inhibition of electrogenic sodium absorption in the guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2003;284:G617–G628. doi: 10.1152/ajpgi.00201.2002. [DOI] [PubMed] [Google Scholar]
- 33.Cooke HJ, Reddix RA. Neural regulation of intestinal electrolyte transport. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 2083–2132. [Google Scholar]
- 34.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, Engel AG. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009;65:83–89. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HC, Cherk SW, Chan SK, Wong S, Tong TW, Ho WS, Chan AY, Lee KC, Mak CM. BAG3-related myofibrillar myopathy in a Chinese family. Clin Genet. 2012;81:394–398. doi: 10.1111/j.1399-0004.2011.01659.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Allen E, Ding J, Wang W. Giant axonal neuropathy. Cell Mol Life Sci. 2007;64:601–609. doi: 10.1007/s00018-007-6396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, Malerba G, Bertolasi L, Rizzuto N. Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain. 2007;130(Pt 2):394–403. doi: 10.1093/brain/awl284. [DOI] [PubMed] [Google Scholar]
- 38.Jaffer F, Murphy SM, Scoto M, Healy E, Rossor AM, Brandner S, Phadke R, Selcen D, Jungbluth H, Muntoni F, Reilly MM. BAG3 mutations: another cause of giant axonal neuropathy. J Peripher Nerv Syst. 2012;17:210–216. doi: 10.1111/j.1529-8027.2012.00409.x. [DOI] [PubMed] [Google Scholar]
- 39.Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277(5 Pt 1):G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- 40.Raybould HE, Glatzle J, Freeman SL, Whited K, Darcel N, Liou A, Bohan D. Detection of macronutrients in the intestinal wall. Auton Neurosci. 2006;125:28–33. doi: 10.1016/j.autneu.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Baglole CJ, Davison JS, Meddings JB. Epithelial distribution of neural receptors in the guinea pig small intestine. Can J Physiol Pharmacol. 2005;83:389–395. doi: 10.1139/y05-024. [DOI] [PubMed] [Google Scholar]
- 42.Berlioz F, Maoret JJ, Paris H, Laburthe M, Farinotti R, Rozé C. Alpha(2)-adrenergic receptors stimulate oligopeptide transport in a human intestinal cell line. J Pharmacol Exp Ther. 2000;294:466–472. [PubMed] [Google Scholar]
- 43.Cooke HJ. Neurobiology of the intestinal mucosa. Gastroenterology. 1986;90:1057–1081. doi: 10.1016/0016-5085(86)90889-9. [DOI] [PubMed] [Google Scholar]
- 44.Kimm MH, Hardin JA, Gall DG. The role of nitric oxide in the regulation of macromolecular transport in rat jejunum. J Physiol. 1996;490(Pt 1):243–248. doi: 10.1113/jphysiol.1996.sp021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong TP, Debnam ES, Leung PS. Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol. 2007;584(Pt 2):613–623. doi: 10.1113/jphysiol.2007.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barry MK, Aloisi JD, Yeo CJ. Luminal adrenergic agonists modulate ileal transport by a local mechanism. J Surg Res. 1993;54:603–609. doi: 10.1006/jsre.1993.1092. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa Y, Eguchi T, Ishida H. Mechanism of beta-adrenergic agonist-induced transmural transport of glucose in rat small intestine. Regulation of phosphorylation of SGLT1 controls the function. Biochim Biophys Acta. 1997;1357:306–318. doi: 10.1016/s0167-4889(97)00043-8. [DOI] [PubMed] [Google Scholar]
- 48.Kreydiyyeh SI. Alpha and beta adrenoceptors mediate the inhibitory effect of epinephrine on the mucosal uptake of phenylalanine in the rat jejunum. Can J Physiol Pharmacol. 1997;75:1312–1315. [PubMed] [Google Scholar]
- 49.Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuro-gliopathies. World J Gastroenterol. 2007;13:4035–4041. doi: 10.3748/wjg.v13.i30.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rühl A, Nasser Y, Sharkey KA. Enteric glia. Neurogastroenterol Motil. 2004;16(Suppl 1):44–49. doi: 10.1111/j.1743-3150.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- 51.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 52.Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 53.Aubé AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55:630–637. doi: 10.1136/gut.2005.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Rühl A, Sharkey KA. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol. 2006;291:G912–G927. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- 55.Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006;18:53–61. doi: 10.1111/j.1365-2982.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 56.Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41–46. doi: 10.1136/gut.2005.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bassotti G, Villanacci V, Cathomas G, Maurer CA, Fisogni S, Cadei M, Baron L, Morelli A, Valloncini E, Salerni B. Enteric neuropathology of the terminal ileum in patients with intractable slow-transit constipation. Hum Pathol. 2006;37:1252–1258. doi: 10.1016/j.humpath.2006.04.027. [DOI] [PubMed] [Google Scholar]