Abstract
A theme in sensory perception it that exposure to a stimulus causes perception of subsequent stimuli to be shifted in the opposite direction. Such phenomenon are known as aftereffects and have been extensively described in the visual system as well as recently described for the vestibular system during translation. It is known from aviation studies that after a maneuver in roll pilots can experience a false perception of roll in the opposite direction. The magnitude and duration of this effect as well as the potential influence of the gravity vector have not previously been defined. In the current paper this roll aftereffect (RAE) is examined in response to whole body roll about an earth-horizontal axis in eight healthy human subjects. The peak velocity of a 0.5 s duration roll was varied based on previous responses to find the point where subjects perceived no motion. Without an preceding stimulus the starting position (upright, 9° left, or 9° right) did not influence roll perception. The RAE was measured in a completely dark room using an adapting (firstinterval) stimulus consisting of 9° of roll over 1.5 s (peak velocity 12°/s), delivered 0.5, 3, or 6s prior to test (second-interval) stimulus. A significant RAE was seen in all subjects. Half a second after the adapting stimulus a test stimulus had to be on average 1.5 ± 0.4°/s in the opposite direction to be perceived as stationary. When the subject remained upright after the adapting stimulus the RAE diminished with time, although it remained significantly larger at 3s and 6s when the subject remained tilted after the adapting stimulus. These data demonstrate that roll perception can be influenced by small preceding stimuli and tilt causes a persistence of the RAE.
Keywords: Gillingham illusion, Post-roll illusion, aftereffects
Introduction
One hallmark of sensory systems is that exposure to a stimulus makes it seem more neutral over time. As a result of this, after adapting to a stimulus, a subsequent neutral stimulus may actually be perceived as opposite that of the adapter. Such aftereffects have been extensively studied in vision such as the motion aftereffect (MAE) in which after viewing a moving image, a static pattern appears to move in the opposite direction. This was classically described as the “waterfall illusion” more than 175 years ago (Addams 1834) but has also been implicated in other areas of visual perception including color, line curvature, and facial identification (Thompson and Burr 2009). Analogous effects have been described for perception of sound intensity (Reinhardt-Rutland 1998), voice perception (Bestelmeyer et al. 2010), and proprioception (Seizova-Cajic et al. 2007).
Given that aftereffects are ubiquitous in sensory physiology it is not surprising that they should also apply to vestibular perception. Recently a vestibular aftereffect has been described in response to whole-body translation (Crane 2012). However, the semicircular canals are not involved in sensation of translation, and there is some evidence that the canals may behave differently. Vestibular evoked eye movements such as the vestibuloocular reflex (VOR) can persist in the absence of vestibular stimulation such as occurs with velocity storage during prolonged yaw rotation (Raphan et al. 1979; Hess and Angelaki 1997). Velocity storage has been observed to have perceptual consequences (Bertolini et al. 2011), which is interesting because this leads to persistence of the initial perception – the opposite of other sensory aftereffects. However, unlike yaw rotation, roll is not influenced by velocity storage (Tweed et al. 1994; Bertolini et al. 2008) which allows an opportunity for studying aftereffects in the semicircular canals without the potentially confounding influence of velocity storage.
Spatial disorientation is an important cause of aviation accidents (Gillingham 1992; Lyons et al. 1994; Heinle and Ercoline 2002). A common type of spatial disorientation is the post-roll illusion also known as the “Gillingham” illusion after Kent Gillingham who studied the effect which was named for him posthumously when he died in an aviation accident in 1993 (Lyons et al. 1994). After making an abrupt roll, pilots can erroneously perceive a roll in the opposite direction which can cause them to make an inappropriate “correction” by continuing the roll in the initial direction (Ercoline et al. 2000). Thus, the post-roll illusion is essentially a sensory aftereffect although it has not classically been considered in those terms. The phenomenon has been hypothesized to be the cause of several aviation accidents (Lyons et al. 1994), and has been demonstrated in aircraft during controlled conditions (Ercoline et al. 2000) as well as in simulated flight conditions which demonstrated the effect even after rolls as short as 2 s (Nooij and Groen 2011). Various terms have been used for this phenomenon: Gillingham illusion, post-roll illusion, and roll aftereffect. In this paper it will be referred to as the roll aftereffect (RAE) because it describes the phenomenon, the term “aftereffect” is most commonly used to describe these types of phenomena in the sensory literature, and the current paper does not examine the phenomenon in an aviation specific context.
Although the previous literature demonstrates a RAE, questions remain. The RAE has previously been studied only in an aviation context so that the main outcome is the amount of force applied to a control stick (Nooij and Groen 2011) or the bank angle the subject oriented a plane after a turn (Ercoline et al. 2000). In these prior studies many (Nooij and Groen 2011) or all (Ercoline et al. 2000) of the subjects also had pilot training which almost certainly influenced their responses given the context of the experiments.
The potential influence of the gravity vector in the RAE has also not previously been explored. During a coordinated turn in an aircraft the change with respect to the gravity vector caused by the bank of the turn is cancelled by centrifugal force such that the net acceleration vector experienced by pilots and passengers continued to be straight down. Thus, sensation of tilt is absent and in the absence of visual feedback and instruments the semicircular canals provide the only cue to roll. When the RAE was previously studied in a flight simulator it was done with the subject on their back so that roll could be delivered without changes with respect to the gravity vector (Nooij and Groen 2011). Furthermore, even when the gravity vector is tilted, the orientation of a surrounding visual environment, such as the inside of an aircraft cabin, leads to a strong perception that one’s orientation is upright (Howard and Hu 2001; Groen et al. 2002).
The goals of the current paper are to describes the RAE using standard psychophysics methods and determine the potential influence of tilt on the RAE. A robust RAE was observed ever after a modest 1.5 s, 12°/s peak velocity adapting stimulus. It will be demonstrated that although perception of roll is independent of tilt for an isolated stimulus, tilt can lead to persistence of the RAE.
Materials and Methods
Equipment
Motion stimuli were delivered using a 6-degree-of-freedom motion platform (Moog, East Aurora, NY, model 6DOF2000E) similar to that used in other laboratories for human and monkey motion perception studies (Grabherr et al. 2008; Fetsch et al. 2009; MacNeilage et al. 2010) and previously used for translation aftereffect studies in the current laboratory (Crane 2012). Subjects were seated upright in a padded racing seat (Corbeau, Sandy UT, model FX-1) mounted on the platform which included high lumbar and seat bolsters. The head was fixed using a appropriately sized football style helmet which was rigidly fixed to the motion platform with an inflatable liner to prevent decoupling of the head as previously described (Crane 2012).
Masking noise was delivered as previously described (Roditi and Crane 2012a). The rotation axis was adjusted for each subject so it was always located between the ears to minimize lateral acceleration of the labyrinth due to translation.
A hand-held control box with three buttons was used to collect responses. The center button was pressed by the subject to initiate each stimulus. The two buttons at either end were used to identify the perceived direction of roll as left or right.
Stimuli
Both the adapter (the initial stimulus of constant amplitude) and test stimuli (second stimulus which is adjusted based on subject’s responses) consisted of a sine wave in acceleration, which lasted 1.5s for the adapter or 0.5s for the test stimulus. The equations which describe these stimuli have been previously published (Crane 2012) and are similar to those used in other motion perception studies (Benson et al. 1986; Grabherr et al. 2008).
A small amount of mechanical oscillation limited to the forward-backward direction was added to every test stimulus presentation to create a small amount of noise and vibration to minimize non-vestibular cues (Crane 2012).
The experiment was arranged in blocks of trials grouped based on common test parameters such as starting position and inter-stimulus interval (ISI). In the first three blocks of trials only the test stimulus and no adapting stimulus was given. In one block the starting position was tilted 9° right, in one it was upright (0°), and in the other it was tilted 9° left. After each test stimulus the subject returned to the starting position. The order in which these three blocks were completed was randomized for each subject. The purpose of these blocks was to measure the baseline bias (the stimulus velocity at which no motion is perceived) in vestibular perception as well as any bias that may be introduced by an initial tilt. The maximum test stimulus amplitude was 3° over 0.5 s (2.0 Hz, peak velocity 12°/s, peak acceleration 75°/s/s). A staircase was used to determine which stimuli to present next based on previous responses. The staircases were designed to start with stimuli which were likely be unambiguously perceived, and work toward smaller stimuli. Interleaved independent staircases were used, one staircase started with rightward rotation and the other with leftward rotation. This was done to eliminate a potential artifacts based on the initial test stimulus, and minimize the ability of subjects to identify patterns in the stimulus presentation. Each staircase contained 25 stimulus presentations. For each response in the direction of the staircase the stimulus velocity was moved in the opposite direction. The test stimulus velocity was varied on a continuum such that each staircase could step through zero. Thus, the staircases tended to deliver most stimuli in the range where subjects where equally likely to perceive a movement in either direction, and there were not necessarily equal numbers of test stimuli on either side of zero. With each reversal in response direction the step size decreased by half down to a minimum of 0.4 °/s. The level was changed in a 1-up, 1-down manner – i.e. a leftward response causes the next stimulus to be delivered in a more rightward direction and vice versa. If the subject did not respond with a perceived direction within 2 seconds no response was recorded and the stimulus was re-presented when that staircase was active again. These types of lapses were rare and occurred in <1% of stimulus presentations.
For the remainder of trial blocks, a two-interval procedure was used to measure potential aftereffects. The adapting (first interval) stimulus was always 9° of roll over 1.5 s (0.66 Hz, peak velocity 12°/s, peak acceleration 25°/s/s) although the direction could be right or left. Right and left adapting stimuli were randomly interleaved such that within a trial block 50% of stimuli had an adapter in each direction. After an inter-stimulus interval (ISI) in which no motion occurred, a test stimulus (second interval) was delivered similar to that described above for the single interval condition. For each adapter there were two staircases: one that started with 3° rightward roll and the other that started with 3° leftward roll. Thus each of these trial blocks included 4 randomly interleaved staircases each of which included 25 stimulus presentations. Thus these aftereffect blocks included 100 stimulus presentations.
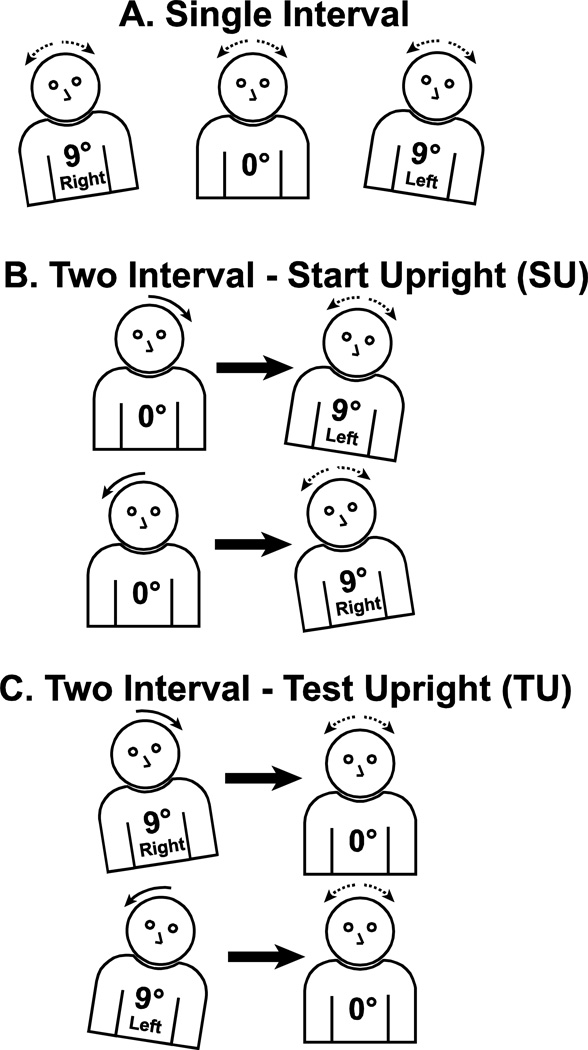
The three single interval blocks were always run first, although the order of these blocks was randomly varied between subjects (Fig. 1A). Each two-interval block of trials was limited to a single ISI, 0.5, 3.0, or 6 s. For each ISI there was one trial block in which the subject started upright (SU) prior to the adapting stimulus, thus the test stimulus occurred when the subject was tilted at ±9° (Fig. 1B). There were separate trial blocks in which the subject started at ±9° such that the test stimulus began with the subject upright – test upright (TU, Fig. 1C). The order in which these two-interval trial blocks were completed was varied between subjects.
Figure 1.
Diagram demonstrating the three types of trial blocks performed in the experiment. Panel A: Single interval experiments in which starting position was fixed within the trial block, but could be upright or tilted 9° in either direction. Panel B: Two interval start upright (SU) trials. The subject began the first interval in the upright position so that after the first (adapting) stimulus they were tilted right or left. After an inter-stimulus interval (ISI) of 0.5, 3, or 6 s the test stimulus was delivered. Thus the subject was tilted at the start of the test stimulus. Each trial block included a single ISI with right and leftward rotations randomly interleaved. Panel C: Two interval test upright (TU) condition. The subject began each stimulus shifted right or left 9° so that the adapting stimulus left them upright for the start of the test stimulus. Trials with rightward and leftward rotation were randomly interleaved.
Subjects
A total of 8 human subjects participated in the experiment. Mean age was 42 ± 18 (mean ± SD, range 20 – 65). Subjects were equally divided between men and women. To prevent fatigue, trial blocks were completed over 2 or 3 testing sessions which took place on separate days. Subjects took breaks between trial blocks on the same day.
Subjects understood that the test stimulus would be roll towards either the right or left. Informed written consent was obtained from all participants. The protocol was approved by the University of Rochester Research Science Review Board.
Subjects were screened prior to participation. The screening included caloric testing, an audiogram, visual acuity testing, and screening questions to rule out any known history of vestibular disease or cognitive deficit. Based on these results the subjects had normal peripheral vestibular function and hearing.
Analysis
The percentage of rightward responses for each stimulus level was plotted as a function of the test stimulus delivered (Fig. 2). A cumulative Gaussian function with confidence intervals was determined from those data points using a Monte Carlo maximum-likelihood criteria as previously described (Wichmann and Hill 2001a; Wichmann and Hill 2001b) and used by others(Fetsch et al. 2009; MacNeilage et al. 2010) as well as in the current laboratory (Crane 2012; Roditi and Crane 2012b). Data from each subject was resampled and fit 2,000 times so that multiple estimates of the mean could be generated and 95% confidence intervals determined (Fig. 2C). The level of significance in the difference in two distributions as determined as previously described (Crane 2012).
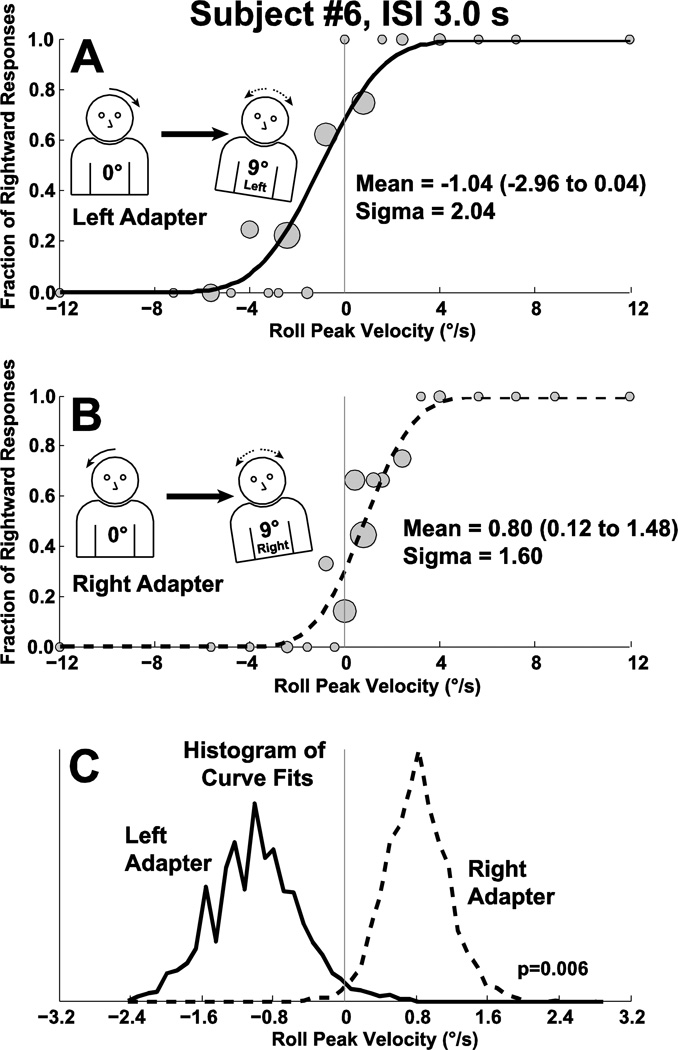
Figure 2.
A block of trials demonstrating the roll aftereffect in sample subject (#6) with an ISI of 3 s. Gray circles in panels A&B are sized proportionally to the number of responses they represent with the largest representing 9 and the smallest 1 stimulus presentation. Panel A: Best fit of a cumulative distribution function (CDF) to trials in which the adapting stimulus was to the left. The CDF is shifted to the left indicating that a neutral stimulus is more likely to be perceived as right. Panel B: The CDF fit to trials in which the adapting stimulus was roll to the right. A neutral stimulus is now more likely to be perceived as left. Panel C: The CDF was fit to the data in panels A&B after being randomly resampled 2,000×. The histograms of these fits are shown which demonstrates a significant difference between the two curves. The y-axis is of arbitrary units and is not labeled.
The repeated measures analysis of variance (ANOVA) was used to compare the bias between subjects and test conditions for two interval experiments. Factors included adapter direction, and ISI. The T-test was used to measure significance of means between two groups of samples. Pearson’s correlation coefficient was used to test the significance of correlations between groups. Statistical significance was defined as p < 0.05.
Results
All the subjects completed all the test conditions. All subjects were able to correctly and reliably identify direction of the test stimulus at the extreme range (i.e beginning of the staircase).
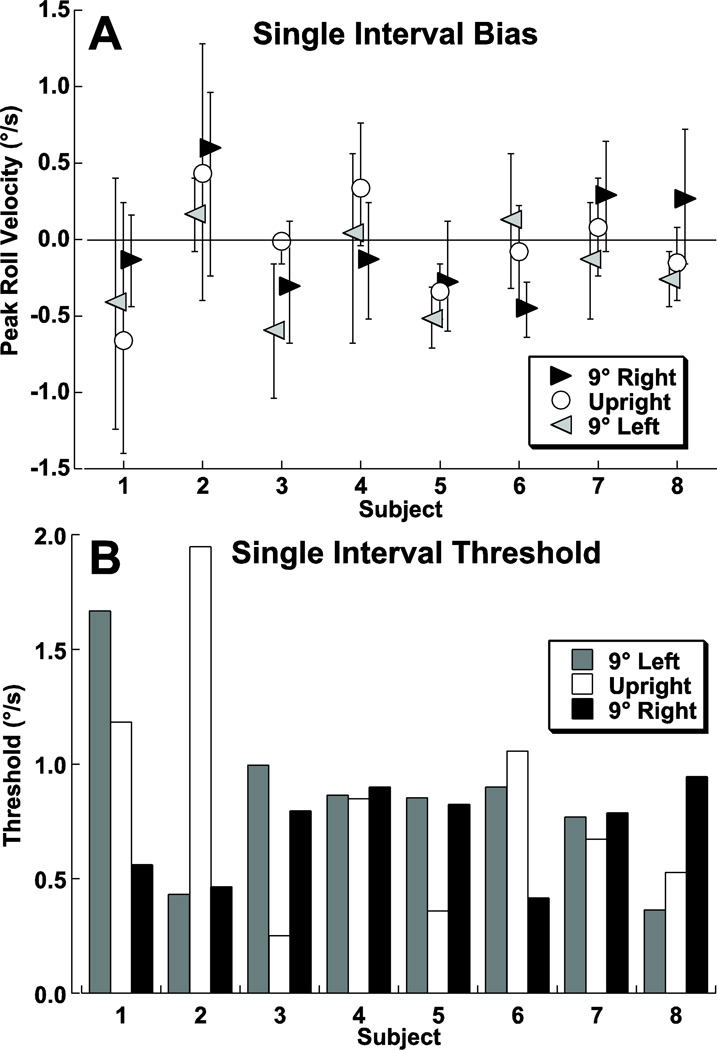
The baseline bias was determined using a set of single interval trials using the mean of the psychometric function that best fit the responses (Fig. 1A). The baseline bias (the displacement of point of subjective equality) was near zero in every subject and in all cases < 0.8°/s. The mean bias had a peak velocity of 0.3 ± 0.2 °/s (mean of absolute values ± SD, Fig. 3A). For these single interval experiments the starting position (centered, 9° right, or 9° left) had no significant influence on the bias (ANOVA, p = 0.49, F=0.73). The threshold was determined from the sigma of the psychometric function (Fig. 3B) and was 0.78 ± 0.41°/s (mean ± SD) which also was not dependent on the starting position (ANOVA, p > 0.5, F=0.23). Thus the starting position alone (upright vs. tilted) did not bias the perception or change the threshold of detecting a subsequent roll rotation.
Figure 3.
Direction determination for single interval roll rotation for each subject. Panel A: Bias of the psychometric function with 95% confidence intervals. Panel B: Threshold (sigma, or width of the CDF fit to the response) for each condition.
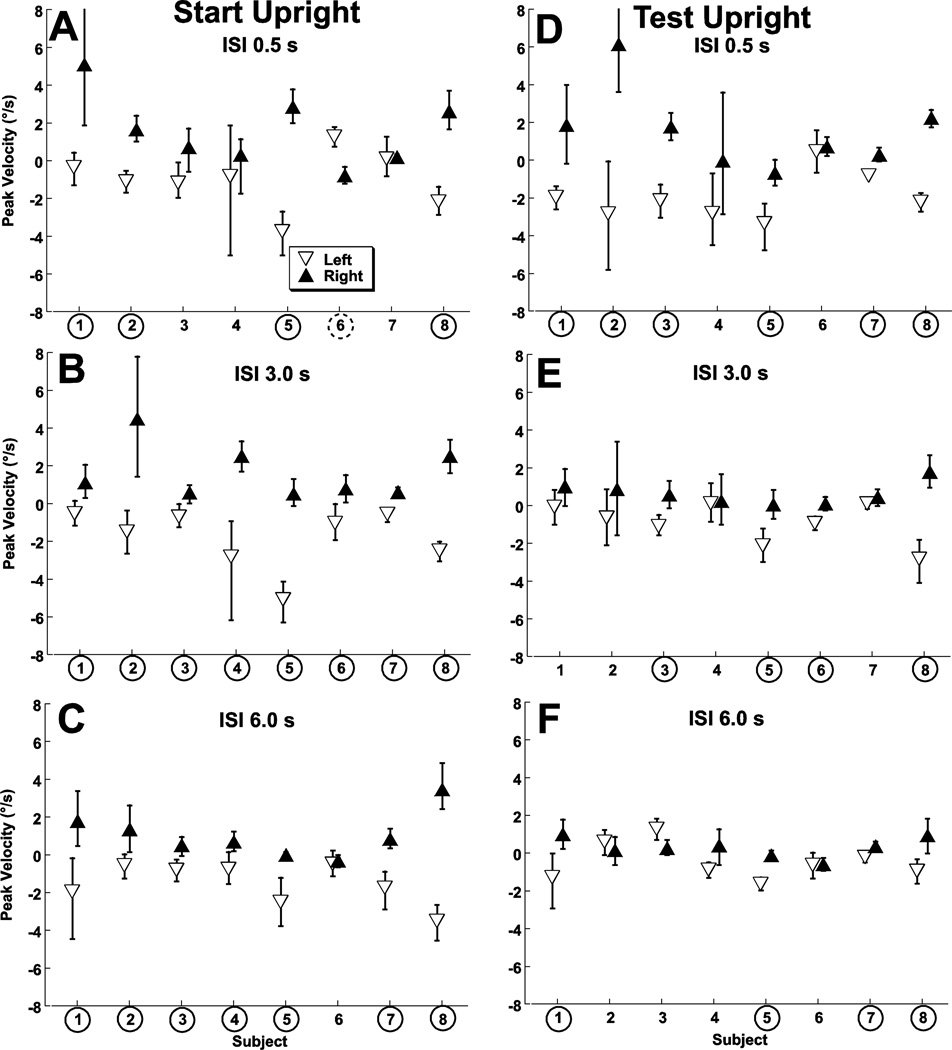
The addition of a preceding adapting stimulus often influenced perception of the test stimulus such that perception was biased in the direction opposite the adapter, or an aftereffect (Fig. 4). Significant effects of the direction of the adapter were determined for each subject using the fraction of overlapping fits technique described in the Methods with the significance defined as p < 0.01. In a single subject (#6) and single test condition (SU with an ISI of 0.5 s, Fig. 4A) there was a significant priming effect: Perception of the test stimulus was biased in the same direction as the adapting stimulus. In all the other subjects and test conditions any significant differences in biases related to the direction of the adapting stimulus were aftereffects.
Figure 4.
The influence of an adapting stimulus on bias (i.e. the test stimulus velocity that is perceived as no motion) by subject. Subject numbers are circled if there was a significant difference in the bias based the direction of the adapting stimulus at the p < 0.01 level. This significant effect was almost always in the direction opposite the adapting stimulus but in one subject (dashed circle, panel A) was in the opposite direction. Error bars represent 95% confidence intervals.
With the SU stimulus subjects were tilted to the right or left by 9° at the start of the test stimulus depending on the direction of the previous adapting stimulus. Significant aftereffects were seen in 4 of 8 subjects at an ISI of 0.5s (Fig. 4A), all 8 subjects at an ISI of 3 s (Fig. 4B), and 7 of 8 subjects at an ISI of 6 s (Fig. 4C). In two subjects data was carried out to an ISI of 12 s (subjects #7 and #8, data not shown) which continued to demonstrate a significant aftereffect, however this trial block was too long to be practically performed in all subjects. With the TU stimulus the orientation was the same (upright) at the start of the test stimulus regardless of the direction of the previous adapting stimulus which began from a tilted position. Significant aftereffects occurred in 6/8 subjects at an ISI of 0.5 (Fig. 4D), 4/8 subjects at an ISI of 3 s (Fig. 4E), and 4/8 subjects at an ISI of 6 s (Fig. 4F). Thus significant aftereffects were less common with the TU stimulus with an ISI of 3 and 6 s.
The size of the RAE (bias in perception of the test stimulus caused by the adapting stimulus) was similar between the TU and SU conditions. At an ISI of 0.5 the difference in bias between a leftward and rightward adapter was 3.0 ± 2.7 °/s (TU and SU similar at p = 0.4, T-test). However the RAE was significantly smaller in the TU condition relative to the SU condition at ISIs of 3s (p = 0.04) and 6s (p = 0.01, Fig. 5A). This is consistent with aftereffect persisting longer when the subject remains titled after delivery of the adapting stimulus.
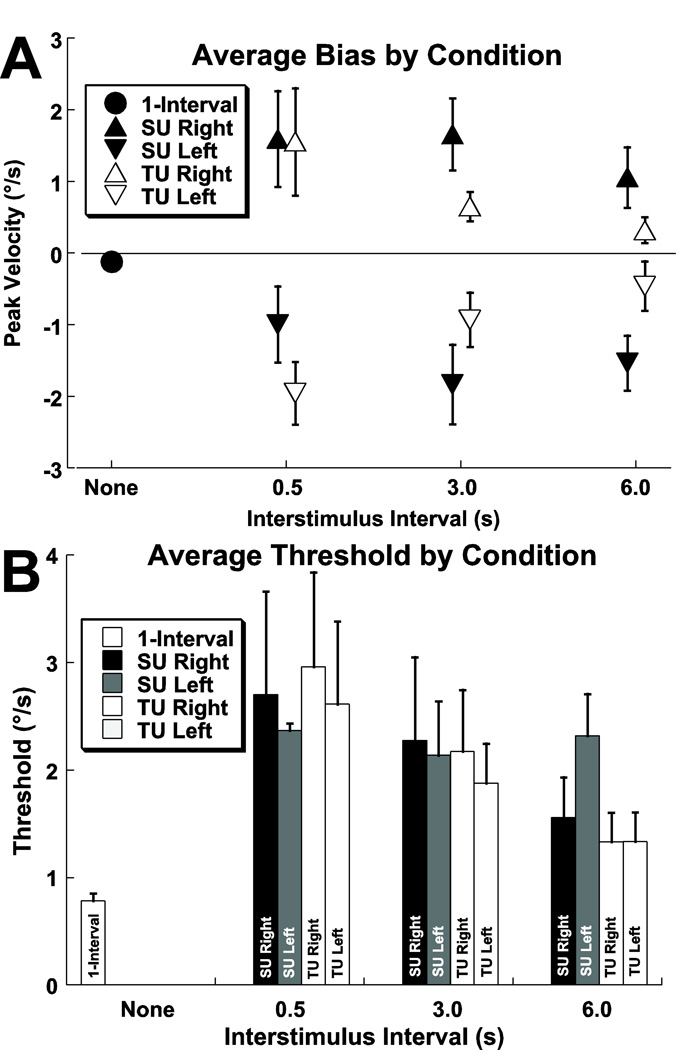
Figure 5.
Mean biases and thresholds for the study population by ISI. Error bars represent ± 1 SEM. Panel A: Bias. Panel B: Thresholds.
The threshold for determination of roll direction increased in conditions that caused a bias. The threshold was significantly lower (p < 0.001, T-test) during the single interval test at 0.78 ± 0.41°/s (mean ± SD) when compared with adapter conditions 2.1 ± 1.7°/s (Fig. 5B). The SU and TU conditions had similar thresholds with ISI s of 0.5 and 3.0 (T-test, p > 0.1 for both). However at the ISI of 6 s, the SU threshold was significantly higher (T-test, p = 0.02) at 2.0 ± 1.3°/s for SU vs. 1.3 ± 0.7°/s for TU. Overall the size of the aftereffect was significantly correlated with the threshold for two interval trials (R=0.54, p << 0.001, Pearson’s Correlation Coefficient).
Discussion
In this study the threshold for roll rotation was measured to be 0.8 ± 0.4°/s or 5 ± 3°/s/s which with the 2 Hz stimulus used corresponded to a tilt of 0.2°. In the current study roll was always associated with a change with respect to the gravity vector since the rotation vector was earth horizontal. The threshold for roll in this study is nearly the same as that previously reported in two recent studies of perception of yaw rotation at 1 Hz (0.7°/s) (Grabherr et al. 2008) and in the current laboratory where it was measured a 0.9°/s at 1 Hz (Roditi and Crane 2012a). The threshold for perception of static tilt (postural vertical) has been previously measured as 4.4° (Mann and Dauterive 1949; Mann 1950) which is similar to the threshold measured using a centrifuge to change the vertical acceleration vector (Graybiel and Patterson 1955). A more recent study described the threshold of human tilt perception over a group of individuals as 1.9° – 5.6° (Janssen et al. 2011). Thus in the current study the thresholds were almost certainly determined by the semicircular canal response to roll rather than by otolith and proprioceptive influences to tilt because at the threshold of roll perception the tilt (0.2°) was an order of magnitude smaller than the previously described thresholds of tilt perception (1.9 to 5.6°).
A RAE has previously been described in pilots flying aircraft (Ercoline et al. 2000) as well as in flight simulators (Nooij and Groen 2011). However, these previous studies were focused on developing techniques for pilot training rather than characterizing the RAE itself. This is an important difference because in prior studies the task was to fly a real or simulated aircraft after a maneuver that involved roll. Thus the response potentially depended not only on the RAE but also on the perception of the initial maneuver, orientation in space, prior pilot training, and motor influences.
The current study focused on the RAE itself. Standard psychophysics techniques allowed the latency of as well as the magnitude of the RAE to be independently determined. In the current experiments the direction of gravity provides a potential cue to roll direction. In prior studies the subject was oriented nose up (Nooij and Groen 2011) or the roll was due to a coordinated turn (Ercoline et al. 2000) so that the orientation of the gravity vector did not change relative to the subject. Since in our everyday experiences we are upright and head roll is associated with changes in the gravity vector it is important to understand the influence of gravity. Although, it was thought that the availability of a changing gravity vector might provide a powerful cue which would eliminate the RAE (Nooij and Groen 2011), this was not the case.
The RAE seems not to be an immediate effect, but occurs at finite period after rotation. In aircraft simulators the latency of the effect was estimated to be 0.82s in darkness and 0.44s when the interior of the cockpit was visible based on the average time between when the stimulus ended and the subject made a correction which suggested a RAE (Nooij and Groen 2011). The current data confirm that the aftereffect was more reliably present at 3s than at 0.5s using the SU protocol. Previous data for translation also revealed a similar effect which was more reliable at a ISI of 1 s than at 0.5 s (Crane 2012). This suggests that aftereffects of the vestibular system occur at a much longer latency than those of the visual system which are on the order of 300 ms (Kanai and Verstraten 2005).
The RAE is a robust phenomenon. In the current study all of the participants demonstrated a significant RAE during at least one condition (SU, with a ISI of 3 s). This is in contrast to the translation aftereffect which was only significant in about half the subjects tested (Crane 2012). In Nooij and Groen study the magnitude of the RAE was found to increase with increasing duration of the adapting stimulus and increasing velocity of the adapting stimulus (Nooij and Groen 2011). The lowest velocity stimulus used in that study was 10°/s which lasted 12 s and the shortest duration stimulus was 2 s but used a faster rotation at 30°/s. In these conditions the total displacement of the adapter was 120 or 60°, significantly larger than the 9° used here. Both of these conditions yielded a similar RAE estimated of about 5°/s, and from this the authors concluded that the RAE can occur with short high velocity adapters or long low velocity adapters. In the current study the adapting stimulus was even shorter at 1.5 s, and used a peak velocity of 12°/s, thus even short, low velocity, and low amplitude stimuli can produce an aftereffect which in this study had an average magnitude of about 1.5°/s (Fig. 5A).
Tilt perception did not inhibit the RAE, but rather had a significant influence on the persistence of the RAE. Tilt alone without a preceding semicircular canal rotation did not introduce a bias or change the threshold of single-interval roll perception (Fig. 3A). Thus, it does not seem that tilt influenced subjects to report their postural orientation relative to gravity rather than the direction of roll. Tilt at the onset of the test stimulus had no significant influence on the initial RAE at 0.5 s (Fig. 5). However, there was a significantly larger RAE at ISI of 3 and 6 s when the test stimulus was delivered while the subject was tilted (SU vs. TU conditions). This finding indicates that tilt at the end of a roll adapter causes the RAE to persist. The 9° tilt at the end of the adapting stimulus, although small, is well above the tilt perception threshold (Janssen et al. 2011), and prolonged the RAE perhaps by facilitating persistence of the effect of the adapting roll.
The current data demonstrate that the RAE can last several times the duration of the short adapting stimulus. If there is a persistent perception of roll then subjects must at some point face sensory conflict as their perceived roll will no longer be consistent with the tilt perceived by the otoliths. This conflict could be resolved by elimination of the RAE which might explain the observed RAE duration. The previous literature demonstrates the threshold of otolith perception of tilt is 2–5° (Mann 1950; Janssen et al. 2011). A typical aftereffect 0.5 s after the end of the stimulus was 1.5°/s (Fig 5A). Thus it might take 2 or 3 s of experiencing an RAE for 50% (a typical definition of a threshold) of subjects to realize their otolith perception of tilt and their perception of roll were inconsistent. The observed RAE decreased by 50% between the ISIs of 0.5 and 3 s in the TU condition (Fig. 5A), which supports this hypothesis. In the SU condition the RAE persisted longer perhaps due to the persistent body tilt prolonging the time required to recognize the perceived roll is no longer consistent with the tilt experienced by the otoliths.
A previous attempt to explain the RAE has focused on the time constant of the semicircular canal cupula (Nooij and Groen 2011) which has been estimated as 4.2s (Dai et al. 1999). A control systems model was presented which uses this time constant to predict the observed magnitude of the RAE (Nooij and Groen 2011). However, this explanation has serious shortcomings: Most notably the RAE causes perception to shift in the direction opposite the initial stimulus rather than a persistence of the initial rotation that would be predicted by the time required for the cupula to return to a neutral position after rotation ends. An origin of the RAE at the cupula also cannot explain the significant latency of the RAE previously described or the influence of visual feedback (Nooij and Groen 2011). Furthermore the aftereffect is not seen in eye movements with the roll vestibulo-ocular reflex (Bertolini et al. 2008) as would be expected if it were an end organ phenomenon. The current data demonstrate tilt after roll can modify the time course of the RAE which would also be difficult to explain with semicircular canal physiology. Thus it is more likely that the origin of the RAE is in the brainstem or cortical neuronal circuits, although further speculation is beyond the scope of the current data.
It is well known that tilt and linear acceleration are ambiguous in the absence of semicircular canal input (Clark and Graybiel 1968; Seidman et al. 1998; Carriot et al. 2006) as linear acceleration can represent either tilt or translation (Guedry 1974). It has been shown that high frequency acceleration is usually interpreted as translation while lower frequency acceleration is interpreted as tilt (Paige and Tomko 1991) even when semicircular canal stimulation is absent (Graybiel et al. 1979; Seidman et al. 1998). In the current experiments the adapting stimulus always included semicircular canal stimulation and a static tilt alone did not yield a RAE, but does not address the issue of whether it is the perception of change in tilt or the semicircular canal stimulation itself that leads to the RAE. A prior study demonstrated that the RAE can occur with no change in otolith stimulation due to tilt (Nooij and Groen 2011), so a perceived change in tilt is not needed for the RAE. However, the possibility that the RAE could occur with no head rotation remains open and might be investigated by delivering a stimulus to the otoliths consistent with tilt without a corresponding roll. This might be done by accelerating a subject along a linear track. To reproduce even the shortest ISI stimulus demonstrated here would require about 10 m of range for linear acceleration plus additional range for safe deceleration, and twice this range if the directions were to be randomized. Much more space would be required to test the ISI of 3 and 6 s. These experiments are well outside the capabilities available in the current laboratory but might address the question of if the RAE is directly related to semicircular canal activity or if it is a function of high level perception of a change in tilt. A second way to disambiguate tilt from roll would be to rotated a subject about an earth vertical axis for more than a minute until there was no longer any semicircular canal activation. The subject could then be moved away from the axis of rotation such that they could experience a centripetal acceleration without any change in roll. Such an experiment has previously been performed shown to be perceived as tilt (Seidman et al. 1998). Although this could theoretically be performed in a smaller space that the large linear sled required to deliver a purely linear acceleration it would also be technically difficult. One issue would be that the acceleration sensed by the two otoliths and possibly other areas of the body would significantly different unless the axis of rotation were large. A second issue would be finding a way to tilt the subject to measure an aftereffect while in a centrifuge.
One theory of sensory aftereffects is that they occur due to a dynamic recalibration of a sensory system. This could serve as a method of error correction so that offsets can be nulled over time using the assumption that a time averaged response represents no motion. Such a purpose has been implicated for aftereffects in the visual system (Ullman and Schechtman 1982; Anstis et al. 1998), and the vestibular system also adapts to chronic stimulation (Miles and Eighmy 1980) or sensory conflict with vision (Cohen et al. 1992; Crane and Demer 2000). With training, this adaptive effect can be as short as a few minutes (Merfeld et al. 2006), but it is unclear if these mechanisms persist down to stimuli as short as the modest rotations of 1.5 s duration such as the adapting stimulus used here.
An alternative theory of sensory aftereffects which may be more consistent with short term adaptation is that they may modify neuronal coding so that a larger dynamic range can be covered (Barlow 1990). This model predicts that after an initial movement, thresholds for detection of subsequent motion would be higher which was observed. The increase in threshold after the adapting stimulus was such that the average threshold was more than three-fold higher 0.5 s after an adapting stimulus than it was for an isolated test stimulus (Fig. 5B). When after effects to translation were previously examined the increase in threshold after an adapting stimulus was a much more modest 1.5× (Crane 2012). This difference may be related to the stronger and more consistent aftereffect seen here with roll relative to what was seen with translation.
The current study conclusively demonstrates the RAE as a significant perceptual bias that can occur after modest low amplitude roll and persist for several seconds. The RAE also has a multisensory component that causes it to persist when subject remains tilled after rolling. The RAE has practical relevance as a potential cause for aviation accidents that can occur due to disorientation after roll (Lyons et al. 1994), and the current data demonstrate it may be a common illusion that occurs even after relatively small stimuli.
Acknowledgements
This work was funded by a grant from the National Institute on Deafness and Other Communication Disorders K23 DC011298. Additional support was provided by a clinician-scientist grant from the Triological Society. Technical support was provided by Shawn Olmstead-Leahey. Thanks to my former mentor, Dr. Joseph L. Demer, for reviewing a prepublication version of this manuscript.
Bibliography
- Addams R. An account of a peculiar optical phaenomenon seen after having looked at a moving body etc. Magaz. J. Sci. 3rd series. 1834;5:373–374. [Google Scholar]
- Anstis S, Verstraten FA, Mather G. The motion aftereffect. Trends in cognitive sciences. 1998;2:111–117. doi: 10.1016/s1364-6613(98)01142-5. [DOI] [PubMed] [Google Scholar]
- Barlow HB. A theory about the functional role and synaptic mechanism of visual aftereffects. In: Blakemore C, editor. Vision: Coding and Efficiency. Cambridge University Press; 1990. pp. 363–375. [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986;57:1088–1096. [PubMed] [Google Scholar]
- Bertolini G, Bockisch CJ, Straumann D, Zee DS, Ramat S. Do humans show velocity-storage in the vertical rVOR? Progress in brain research. 2008;171:207–210. doi: 10.1016/S0079-6123(08)00628-6. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol. 2011;105:209–223. doi: 10.1152/jn.00154.2010. [DOI] [PubMed] [Google Scholar]
- Bestelmeyer PE, Rouger J, DeBruine LM, Belin P. Auditory adaptation in vocal affect perception. Cognition. 2010;117:217–223. doi: 10.1016/j.cognition.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Carriot J, Barraud PA, Nougier V, Cian C. Difference in the perception of the horizon during true and simulated tilt in the absence of semicircular canal cues. Exp Brain Res. 2006;174:158–166. doi: 10.1007/s00221-006-0434-6. [DOI] [PubMed] [Google Scholar]
- Clark B, Graybiel A. Influence of contact cues on the perception of the oculogravic illusion. Acta Otolaryngol. 1968;65:373–380. doi: 10.3109/00016486809120978. [DOI] [PubMed] [Google Scholar]
- Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res. 1992;90:526–538. doi: 10.1007/BF00230935. [DOI] [PubMed] [Google Scholar]
- Crane BT. Fore-aft translation aftereffects. Exp Brain Res. 2012;219:477–487. doi: 10.1007/s00221-012-3105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane BT, Demer JL. Effect of adaptation to telescopic spectacles on the initial human horizontal vestibuloocular reflex. J Neurophysiol. 2000;83:38–49. doi: 10.1152/jn.2000.83.1.38. [DOI] [PubMed] [Google Scholar]
- Dai M, Klein A, Cohen B, Raphan T. Model-based study of the human cupular time constant. J Vestib Res. 1999;9:293–301. [PubMed] [Google Scholar]
- Ercoline WR, Devilbiss CA, Yauch DW, Brown DL. Post-roll effects on attitude perception: "the Gillingham Illusion". Aviation, space, and environmental medicine. 2000;71:489–495. [PubMed] [Google Scholar]
- Fetsch CR, Turner AH, Deangelis GC, Angelaki DE. Dynamic re-weighting of visual and vestibular cues during self-motion perception. J Neurosci. 2009;29:15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham KK. The spatial disorientation problem in the United States Air Force. J Vestib Res. 1992;2:297–306. [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Graybiel A, Johnson WH, Money KE, Malcolm RE, Jennings GL. Oculogravic illusion in response to straight-ahead acceleration of CF-104 aircraft. Aviat Space Environ Med. 1979;50:382–386. [PubMed] [Google Scholar]
- Graybiel A, Patterson JL., Jr Thresholds of stimulation of the otolith organs as indicated by the oculogravic illusion. J Appl Physiol. 1955;7:666–670. doi: 10.1152/jappl.1955.7.6.666. [DOI] [PubMed] [Google Scholar]
- Groen EL, Jenkin HL, Howard IP. Perception of self-tilt in a true and illusory vertical plane. Perception. 2002;31:1477–1490. doi: 10.1068/p3330. [DOI] [PubMed] [Google Scholar]
- Guedry FE., Jr . Psychophysics of vestibular sensation. In: Kornhuber HH, editor. Handbook of Sensory Physiology. New York: Springer-Verlag; 1974. pp. 3–154. [Google Scholar]
- Heinle TE, Ercoline WR. In: Spatial disorientation: Causes, consequences, and countermeasures for the USAF. USAF, editor. 2002. [Google Scholar]
- Hess BJ, Angelaki DE. Inertial vestibular coding of motion: concepts and evidence. Curr Opin Neurobiol. 1997;7:860–866. doi: 10.1016/s0959-4388(97)80147-x. [DOI] [PubMed] [Google Scholar]
- Howard IP, Hu G. Visually induced reorientation illusions. Perception. 2001;30:583–600. doi: 10.1068/p3106. [DOI] [PubMed] [Google Scholar]
- Janssen M, Lauvenberg M, van der Ven W, Bloebaum T, Kingma H. Perception threshold for tilt. Otol Neurotol. 2011;32:818–825. doi: 10.1097/MAO.0b013e31821c6c7b. [DOI] [PubMed] [Google Scholar]
- Kanai R, Verstraten FA. Perceptual manifestations of fast neural plasticity: motion priming, rapid motion aftereffect and perceptual sensitization. Vision Res. 2005;45:3109–3116. doi: 10.1016/j.visres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Ercoline WR, Freeman JE, Gillingham KK. Classification problems of U.S. Air Force spatial disorientation accidents 1989–91. Aviat Space Environ Med. 1994;65:147–152. [PubMed] [Google Scholar]
- MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE. Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci. 2010;30:9084–9094. doi: 10.1523/JNEUROSCI.1304-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CW. Studies in space perception. Pensacola, FL: Naval School of Aviation Medicine; 1950. [Google Scholar]
- Mann CW, Dauterive HJ., Jr The perception of the vertical. I. The modification of non-labyrinthine cues. J Exp Psychol. 1949;39:700–707. doi: 10.1037/h0063533. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng. 2006;53:2362–2372. doi: 10.1109/TBME.2006.883645. [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB. Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol. 1980;43:1406–1425. doi: 10.1152/jn.1980.43.5.1406. [DOI] [PubMed] [Google Scholar]
- Nooij SA, Groen EL. Rolling into spatial disorientation: simulator demonstration of the post-roll (Gillingham) illusion. Aviation, space, and environmental medicine. 2011;82:505–512. doi: 10.3357/asem.2946.2011. [DOI] [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol. 1991;65:1170–1182. doi: 10.1152/jn.1991.65.5.1170. [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1979;35:229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Reinhardt-Rutland AH. Increasing-loudness aftereffect following decreasing-intensity adaptation: spectral dependence in interotic and monotic testing. Perception. 1998;27:473–482. doi: 10.1068/p270473. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol. 2012a;13:381–401. doi: 10.1007/s10162-012-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Suprathreshold asymmetries in human motion perception. Exp Brain Res. 2012b;219:369–379. doi: 10.1007/s00221-012-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman SH, Telford L, Paige GD. Tilt perception during dynamic linear acceleration. Exp Brain Res. 1998;119:307–314. doi: 10.1007/s002210050346. [DOI] [PubMed] [Google Scholar]
- Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC. Proprioceptive movement illusions due to prolonged stimulation: reversals and aftereffects. PloS one. 2007;2:e1037. doi: 10.1371/journal.pone.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Burr D. Visual aftereffects. Current biology : CB 19. 2009:R11–R14. doi: 10.1016/j.cub.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Tweed D, Fetter M, Sievering D, Misslisch H, Koenig E. Rotational kinematics of the human vestibuloocular reflex. II. Velocity steps. J Neurophysiol. 1994;72:2480–2489. doi: 10.1152/jn.1994.72.5.2480. [DOI] [PubMed] [Google Scholar]
- Ullman S, Schechtman G. Adaptation and gain normalization. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. Royal Society. 1982;216:299–313. doi: 10.1098/rspb.1982.0076. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001a;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001b;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]