Abstract
Background
Despite evidence that shows no survival advantage, many older patients receive primary androgen-deprivation therapy (PADT) shortly after the diagnosis of localized prostate cancer (PCa).
Objective
This study evaluates whether the early use of PADT affects the subsequent receipt of additional palliative cancer treatments such as chemotherapy, palliative radiation therapy, or intervention for spinal cord compression or bladder outlet obstruction.
Design, setting, and participants
This longitudinal population-based cohort study consists of Medicare patients aged ≥66 yr diagnosed with localized PCa from 1992 to 2006 in areas covered by the Surveillance Epidemiology and End Results (SEER) program. SEER-Medicare linked data through 2009 were used to identify the use of PADT and palliative cancer therapy.
Outcome measurements and statistical analysis
Instrumental variable analysis methods were used to minimize confounding effects. Confidence intervals were derived from the bootstrap estimates.
Results and limitations
This study includes 29 775 men who did not receive local therapy for T1–T2 PCa within the first year of cancer diagnosis. Among low-risk patients (Gleason score 2–7 in 1992–2002 and Gleason score 2–6 in 2003–2006) with a median age of 78 yr and a median follow-up of 10.3 yr, PADT was associated with a 25% higher use of chemotherapy (hazard ratio [HR]: 1.25; 95% confidence interval [CI], 1.08–1.44) and a borderline higher use of any palliative cancer surgery (HR: 1.07; 95% CI, 0.97– 1.19) within 10 yr of diagnosis in regions with high PADT use compared with regions with low PADT use. Because this study was limited to men >65 yr, the results may not be applicable to younger patients.
Conclusions
Early treatment of low-risk, localized PCa with PADT does not delay the receipt of subsequent palliative therapies and is associated with an increased use of chemotherapy.
Keywords: Prostatic neoplasm, Medicare, SEER program, Antineoplastic agents–hormonal
1. Introduction
Prostate cancer (PCa) is the most common nonskin cancer and the second most common cause of cancer death among American men. Because of the widespread use of prostate-specific antigen (PSA) screening, most contemporary patients are diagnosed with localized (T1–T2) PCa [1]. Standard treatment options include surgery, radiation therapy, or active surveillance (ie, deferral of treatment until evidence of progression). Although not supported by any major groups or guidelines, primary androgen-deprivation therapy (PADT) is often initiated shortly after diagnosis as primary treatment of localized PCa, especially in older men [2].
The use of PADT as an adjunct to radiation therapy for men with high-risk or locally advanced (T3) disease has been shown to improve survival [3,4]. Unfortunately, for men with low-risk disease, the early use of PADT [2,5] or Casodex [6] has been shown to worsen disease-specific and overall survival in the majority of men. Early use of PADT carries significant morbidity, including a 10–50% increase in the risks of fracture, diabetes, weight gain, hot flashes, decreased muscle tone, impotence, coronary heart disease, myocardial infarction, and sudden cardiac death [7–10]. Androgen-deprivation therapy (ADT) not only is associated with numerous treatment-related complications and more severe decline in physical well-being but also is costly [11].
The purpose of this manuscript is to address the question of whether the early use of PADT is beneficial by delaying the receipt of subsequent palliative therapies such as chemotherapy, radiation therapy, or surgical intervention.
2. Materials and methods
2.1. Data sources
Data for this study were obtained from the Surveillance Epidemiology and End Results (SEER) program and linked Medicare files. The Medicare database covers approximately 97% of US persons aged ≥65 yr, and linkage to the SEER database is complete for approximately 93% of the patients [12]. This study has been approved by the Institutional Review Board at the University of Medicine and Dentistry of New Jersey.
2.2. Study participants
The study cohort consisted of men (aged ≥66 yr) who were residents of the SEER areas existing before 2001 and were diagnosed with T1–T2 PCa in 1992–2006 (n = 189 460). We excluded men who died within 1 yr of cancer diagnosis (n = 7253); had other cancers diagnosed before their PCa (n = 18 155); or had surgery, radiation therapy, or chemotherapy within 1 yr of diagnosis (n = 104 797). To ensure that the database accurately documented a patient’s clinical course and comorbidity, patients not fully covered by Medicare 1 yr before and 1 yr after cancer diagnosis were excluded (n = 25 430). We also excluded men with unknown health service area (HSA) (n = 809), men with unknown cancer grade (n = 2411), and men who received ADT before cancer diagnosis (n = 830).
2.3. Primary androgen-deprivation therapy
Men who received ADT as primary cancer therapy (eg, no surgery or radiation therapy) within 1 yr of diagnosis were defined as receiving PADT, regardless of whether they subsequently received surgery or radiation therapy >1 yr after diagnosis. Patients who received no therapy within 1 yr of diagnosis were defined as receiving surveillance. Utilizing a previously described algorithm, we reviewed Medicare physician, inpatient, and outpatient claims to identify orchiectomy (Healthcare Common Procedure Coding System [HCPCS] codes 54520, 54521, 54522, 54530, or 54535 or International Classification of Diseases, 9th Revision, code 624) and the use of luteinizing hormone– releasing hormone agonists (HCPCS codes J0128, J1950, J3315, J9202, J9217, J9218, J9219, or J9225) [7].
2.4. Study end points and covariates
In this study, palliative therapy included palliative radiation therapy, chemotherapy, treatment of bladder outlet obstruction, and treatment of spinal cord compression that occurred >1 yr after cancer diagnosis. Palliative external-beam radiation therapy was defined as external-beam irradiation that consisted of <20 fractions within a 6-wk period without brachytherapy (pers. comm., A. Zietman, Boston, MA, USA). Chemotherapy was identified from the HCPCS codes published in the literature and by the authors (Appendix 1) [13]. Treatment of bladder outlet obstruction (transurethral resection of the prostate) and treatment of spinal cord compression are defined in Appendix 1. Charlson scores, a powerful predictor of longevity in men with localized PCa, were derived from Medicare inpatient, outpatient, and physician claims during the year prior to PCa diagnosis using a validated algorithm [14]. We used clinical extension information provided by SEER to determine cancer stage (T1, T2). For patients diagnosed in 2003– 2006, low risk included those men with Gleason score 2–6 disease. For patients diagnosed in 1992–2002, low risk included those men with Gleason score 2–7 disease, because Gleason scores 5–7 were grouped together during this period. Patients who did not have low-risk cancer were grouped in the high-risk category. We analyzed the data by year of diagnosis (1992–2002 and 2003–2006) and found the patterns of outcomes to be consistent. Accordingly, only the combined results are presented in the study.
2.5. Instrumental variable analysis
Treatment effects estimated from observational studies are often biased because of patient selection. Recently, instrumental variable analysis (IVA), a method of capturing the random component of patient treatment choice, has been applied successfully in several medical studies to mimic the results of randomized trials [15]. We selected HSA, defined as one or more counties that are relatively self-contained with respect to the provision of routine hospital care, as our instrumental variable. The instrumental variable was constructed by first calculating the proportion of patients who received PADT in each HSA. Because some HSAs had small numbers of PCa cases, each HSA with <50 cases was combined with the nearest HSA (in terms of distance between geographic centers) with ≥50 cases. The threshold of ≥50 cases was chosen because lower thresholds were associated with more imbalances in patient characteristics in high- and low-PADT utilization areas. The algorithm produced 48 utilization areas for men with low-risk disease and 30 utilization areas for men with high-risk disease. High- and low-use areas corresponded to the top and bottom tertiles of PADT utilization and were used as the (binary) instrumental variable. Patients who differ in the likelihood of receiving PADT were compared, and the treatment effect on the “marginal” population was calculated as
where the following definitions are used: IV, instrumental variable; Hi, a geographic area in the upper tertile of PADT use; Lo, a geographic area in the lower tertile of PADT use. The terms are thus: Pr(PADT|Hi/Low) indicates the probability of PADT use in high/low use region; Adjusted OutcomesHi /Lo demonstrates survival probability in high/low use region.
Previous studies have demonstrated that PADT use is highly influenced by nonmedical factors, with tumor characteristics accounting for only 9.7% of the total variance in use [16,17]. Our data confirmed that PADT use varied widely across HSAs, a key requirement of an instrumental variable. An instrumental variable must influence outcomes through its correlation with treatment status and not through any other independent effect. We verified this assumption by comparing baseline characteristics, including age at diagnosis, PSA, and Gleason score at diagnosis.
2.6. Statistical analyses
IVA methods based on the Rubin causal model were used to account for both measured confounders and unmeasured confounders (eg, PSA, family history, diet, weight) [18]. Covariates in the IVA models included age, race, comorbidity status, cancer stage, cancer grade, income status, urban residence, marital status, and year of diagnosis. All IVA results were derived from the same models. We examined all the required assumptions to ensure the validity of our IVA. Analyses were conducted using SAS v.9.1 and R v.2.14.0. (R Foundation for Statistical Computing, Vienna, Austria). We calculated PADT utilization for each cancer risk group so that it was not necessary to assume that the patterns of PADT utilization were the same for all cancer risk groups within the same area.
High- and low-use HSAs were compared using IVA, adjusting for the variables listed in Table 1. Results are presented in Table 2. Clustering because of HSAs was accommodated using a frailty term in the model using the “coxme” package in R. To compute the cumulative incidence curve of further palliative cancer therapy, we substituted the population means (for continuous covariates) into the proportional hazards model for each combination of the categorical covariates to derive adjusted survival curves. We then averaged these adjusted survival functions to obtain the population-adjusted survival function [19]. We computed the cumulative incidence probabilities of palliative cancer therapies by treating death as a competing risk. Confidence intervals for survival probabilities were obtained by computing these adjusted survival curves for each of 1000 bootstrap samples of the original data. Testing was two-sided with an α-level of 5%.
Table 1.
Characteristics of study cohort*
| Characteristics | Primary androgen deprivation therapy, n = 11 749 |
Surveillance, n = 18 026 |
|---|---|---|
| Age, yr, median (IQR) | 80 (75–84) | 77 (72–81) |
| Black race , no. (%) | 1131 (9.6) | 2163 (12.0) |
| Married at diagnosis , no. (%) | 6758 (57.5) | 10 837 (60.1) |
| Urban residence , no. (%) | 9654 (82.2) | 15 247 (84.6) |
| Income, US $ , median (IQR) | 45 361 (35 773–59 436) | 46 118 (35 733–60 306) |
| SEER regions , no. (%) | ||
| Northeast | 1701 (14.5) | 2216 (12.3) |
| North central | 3916 (33.3) | 5214 (28.9) |
| West | 5739 (48.8) | 9765 (54.2) |
| South | 393 (3.3) | 831 (4.6) |
| Cancer risk , no. (%) | ||
| Low risk | 6927 (59.0) | 15 296 (84.9) |
| High risk | 4822 (41.0) | 2703 (15.1) |
| Clinical stage at diagnosis , no. (%) | ||
| T1 | 3568 (30.4) | 8832 (49.0) |
| T2 | 8181 (69.6) | 9194 (51.0) |
| Charlson comorbidity score , no. (%) | ||
| 0–1 | 10 487 (89.3) | 16 386 (90.9) |
| ≥2 | 1262 (10.7) | 1640 (9.1) |
| Year of cancer diagnosis , no. (%) | ||
| 1992–1999 | 5638 (48.0) | 10 218 (56.7) |
| 2000–2007 | 6111 (52.0) | 7808 (43.3) |
| Survive 5 y r, no. (%) | 6355 (54.1) | 11 516 (63.9) |
| Survive 10 y r, no. (%) | 1520 (12.9) | 4047 (22.5) |
IQR = interquartile range; SEER = Surveillance Epidemiology and End Results.
Race was self-determined by the patients. Clinical extension information provided by SEER was used to determine cancer stage (T1, T2). Charlson comorbidity score was derived from Medicare claims during the year before prostate cancer diagnosis by using a validated algorithm.
Table 2.
Use of palliative treatment by cancer risk using instrumental variable analysis*
| High-PADT use | Low-PADT use | ||||
|---|---|---|---|---|---|
| Cancer risk | Events/ person-year |
Rate per 100 |
Events/ person-year |
Rate per 100 |
Adjusted HR, high PADT/low PADT (95% CI) |
| Use of any palliative treatmenta | |||||
| Low risk | 834/44 336 | 1.9 | 708/40 688 | 1.7 | 1.07 (0.97–1.19) |
| High risk | 384/10 303 | 3.7 | 373/11 325 | 3.3 | 0.99 (0.74–1.32) |
| All localized | 1388/62 609 | 2.2 | 1042/50 596 | 2.1 | 1.05 (0.97–1.14) |
| Use of chemotherapy | |||||
| Low risk | 461/52 294 | 0.9 | 336/48 648 | 0.7 | 1.25 (1.08–1.44)b |
| High risk | 180/11 956 | 1.5 | 181/12 923 | 1.4 | 1.00 (0.71–1.41) |
| All localized | 738/73 476 | 1.0 | 506/60 091 | 0.8 | 1.12 (0.91–1.39) |
| Use of palliative radiation therapy | |||||
| Low risk | 251/53 059 | 0.5 | 218/48 932 | 0.4 | 1.17 (0.88–1.55) |
| High risk | 157/12 091 | 1.3 | 149/13 166 | 1.1 | 0.98 (0.65–1.49) |
| All localized | 453/74 522 | 0.6 | 354/60 606 | 0.6 | 1.07 (0.84–1.36) |
| Use of spinal cord compression treatment | |||||
| Low risk | 99/53 371 | 0.2 | 107/49 238 | 0.2 | 0.99 (0.64–1.53) |
| High risk | 78/12 204 | 0.6 | 66/13 249 | 0.5 | 1.04 (0.71–1.51) |
| All localized | 200/75 035 | 0.3 | 185/61 016 | 0.3 | 0.88 (0.65–1.18) |
| Use of TURP/nephrostomy/cystotomy | |||||
| Low risk | 308/45 571 | 0.7 | 268/41 434 | 0.6 | 1.05 (0.86–1.29) |
| High risk | 165/10 597 | 1.6 | 140/11 649 | 1.2 | 1.22 (0.76–1.94) |
| All localized | 528/64 422 | 0.8 | 382/51 720 | 0.7 | 1.05 (0.86–1.29) |
PADT = primary androgen-deprivation therapy; HR = hazard ratio; CI = confidence interval; TURP = transurethral resection of the prostate.
Covariates included age, race, comorbidity status, cancer stage, cancer grade, income quartiles, urban residence, marital status, year of diagnosis, and state buy-in status. To calculate unbiased rates and HR for each end point, we excluded the patients with that specific event prior to or within 1 yr of prostate cancer diagnosis (ie, we excluded patients with prior TURP/nephrostomy/cystotomy or with TURP/nephrostomy/cystotomy within 1 yr of diagnosis when we calculated rates and HR for use of TURP/nephrostomy/cystotomy.
Palliative treatment includes chemotherapy, palliative radiation therapy, spinal cord compression treatment, TURP, nephrostomy tubes, and cystotomy tubes.
p < 0.05.
3. Results
The total cohort consisted of 29 775 men aged ≥66 yr with localized PCa diagnosed from 1992 to 2006. By definition, none of these men received local therapies (eg, radiation or surgery) within the first year following diagnosis. The median age of the study cohort was 78 yr, and the median follow-up was 10.3 yr. As expected, patients receiving PADT and patients managed by surveillance differed in many characteristics, suggesting that there could be differences in unmeasured characteristics (Table 1).
PADT utilization (Table 3) varied widely across HSAs within the same risk group and had similar distributions in prognostic factors such as cancer stage, PSA, and Gleason score in the high- and low-PADT areas.
Table 3.
Characteristics of men with localized prostate cancer in high- and low-use primary androgen-deprivation therapy health service areas
| Low risk | High risk | All localized cancer | ||||
|---|---|---|---|---|---|---|
| Characteristic | High use, n = 8060 |
Low use, n = 7508 |
High use, n = 2522 |
Low use, n = 2811 |
High use, n = 12 087 |
Low use, n = 10 014 |
| PADT therapy within 12 mo, no. (%) |
3174 (39.4) | 1710 (22.8) | 1818 (72.1) | 1590 (56.6) | 5687 (47.1) | 3146 (31.4) |
| Duration of PADT use, mo, mean (SD) |
44 (39) | 36 (34) | 40 (33) | 35 (30) | 43 (37) | 35 (33) |
| Age at diagnosis, yr, median (IQR) |
78 (73–82) | 77 (73–81) | 80 (76–84) | 79 (75–84) | 78 (74–82) | 78 (73–82) |
| Zip code–level income, US$ , median (IQR) |
46 199 (36 183–62 749) |
45 057 (35 792–58 482) |
42 235 (35 728–58 814) |
46 177 (33 359–55 994) |
45 361 (35 602–61 081) |
45 007 (35 792–57 636) |
| Charlson score, mean (SD) | 0.43 (0.86) | 0.35 (0.79) | 0.43 (0.85) | 0.51 (0.94) | 0.44 (0.86) | 0.37 (0.80) |
| Clinical stage T1, no. (%) | 3749 (46.5) | 3362 (44.8) | 832 (33.0) | 841 (29.9) | 5226 (43.2) | 4098 (40.9) |
| PSA, mean (SD)a | 11.2 (14.5) | 9.0 (9.5) | 22.4 (24.7) | 19.8 (22.6) | 15.0 (18.9) | 14.8 (18.8) |
| Gleason score 2–6, no. (%)a | 1071 (13.3) | 1119 (14.9) | 0 | 0 | 1186 (9.8) | 1124 (11.2) |
| Gleason score 7, no. (%)a | 0 | 0 | 486 (19.3) | 665 (23.7) | 659 (5.5) | 542 (5.4) |
| Gleason score 8–10, no. (%)a | 0 | 0 | 278 (11.0) | 380 (13.5) | 349 (2.9) | 316 (3.2) |
PADT = primary androgen-deprivation therapy; SD = standard deviation; IQR = interquartile range; PSA = prostate-specific antigen.
Limited to patients diagnosed in 2004 or thereafter.
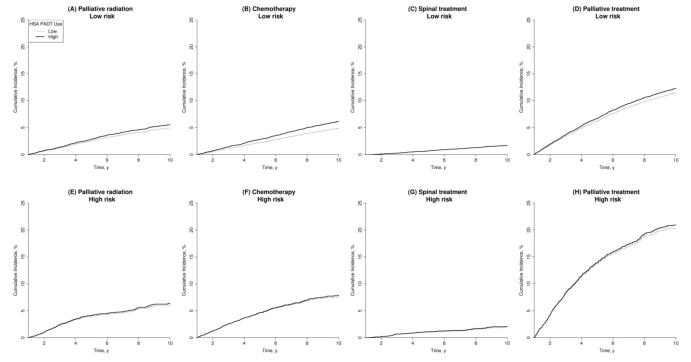
Table 2 shows that among low-risk patients, living in high-PADT areas was associated with a 25% increased use of chemotherapy (hazard ratio [HR]: 1.25; 95% confidence interval [CI], 1.08–1.44) or a 1.3% risk difference, which translates to a 7.8% (0.4– 12.6%) risk difference between patients who receive PADT compared with no PADT. In addition, the use of palliative radiation therapy and surgical procedures to relive bladder outlet obstruction also increased but did not reach statistical significance. There was a borderline increase in the use of palliative radiation therapy (HR: 1.07; 95% CI, 0.97– 1.19) Among high-risk patients, the adjusted risk of receiving palliative therapy (HR: 0.99; 95% CI, 0.74–1.32) or chemotherapy (HR: 1.00; 95% CI, 0.71–1.41) following PADT was comparable in high- and low-use areas. When the analysis was restricted to men with Gleason scores 8–10 only, the results were very similar to those reported in Table 2.
4. Discussion
Despite evidence that the early use of PADT leads to worse cause-specific and overall survival, PADT is frequently given to men with localized (T1–T2) PCa [20]. The early use of PADT in men with low-risk disease may be driven by the misconception that the therapy delays the need for palliative therapy. Utilizing IVA, we have now found that men with low-risk PCa who initiate PADT shortly after diagnosis receive subsequent palliative cancer therapy, and especially chemotherapy, more frequently than men who delay the use of PADT. For the high-risk group, there is little difference in the use of palliative therapy between high- and low-PADT areas. Our findings may be explained by several potential mechanisms. Some studies suggest that ADT may promote molecular events that yield more aggressive, castration-resistant tumors [21,22]. For example, PADT may induce amplification of the androgen receptor MYC human epidermal receptor 2 (HER-2)/neu gene expression and N-cadherin, which may contribute to cancer progression and metastasis [22–28]. A recent study showed that ADT induces epithelial– mesenchymal transition and increases stem cell–like features, which have also been implicated in cancer metastasis and drug resistance [21].
Our findings may also be explained by different practice philosophies. Physicians who initiate PADT shortly after diagnosis may also be more enthusiastic about initiating palliative therapy and chemotherapy early in the course of this disease. An alternative explanation is that the practice simply reflects practice patterns more prevalent when there were strong financial incentives to provide PADT. Most existing studies on PADT generally have not provided data specific for localized (T1–T2) disease. The European Organization for Research and Treatment of Cancer trial 30891, including patients with both localized and advanced disease (eg, T0–4N0–2), showed a modest overall survival benefit, but further analyses suggested that this benefit was limited to patients with aggressive disease (PSA >50 ng/ml or PSA doubling time <12 mo) [29]. In general, our findings are consistent with previous studies showing that men with low-risk PCa do not appear to benefit from, and may actually be harmed by, the early use of PADT. One potential advantage of this study over clinical trials is that the study includes “real-world” patients who would often be excluded from clinical trials even though they are the patients who would receive the treatment in practice. Our study has some limitations. We were able to study only men aged ≥66 yr, and therefore results may not be applicable to younger men. The SEER-Medicare database does not capture information concerning the use of antiandrogens. Previous data from the Cancer of the Prostate Strategic Urologic Research Endeavor showed that the use of antiandrogens as sole treatment of localized PCa is relatively uncommon (approximately 2%), and it is unlikely that this small subset could alter the outcomes of the men choosing surveillance [30].
The success of IVA depends on finding a suitable, partly random, varying factor (instrumental variable) that can be used to balance treatment groups. Our instrumental variable had excellent properties. However, as in randomized studies, it is possible that some unmeasured factors may have been imbalanced between groups. We conducted several sensitivity analyses using various geographic-based instruments or patients’ comorbidity status. All these analyses yielded similar results, suggesting that our analyses are robust. Because an instrumental variable may not remove confounding effects completely, further confirmatory studies will be valuable in guiding the clinical management of this disease.
5. Conclusions
In summary, this large population-based study shows that the early treatment of low-risk, localized PCa with PADT does not delay the receipt of subsequent palliative cancer therapies and is associated with an increased use of chemotherapy.
Take-home message.
Before initiating primary androgen-deprivation therapy, patients should be informed that this treatment does not delay the receipt of subsequent palliative therapies and is associated with an increased use of chemotherapy in patients with low-risk, localized prostate cancer.
Fig. 1. Adjusted cumulative incidence in high- and low-use health service areas by cancer risk. Confidence intervals (CIs) for cumulative incidence were obtained by using 1000 bootstrap samples. Palliative treatment includes palliative radiation therapy, chemotherapy, spinal cord compression treatment, and transurethral resection of the prostate, nephrostomy, or cystotomy. The difference in use of chemotherapy between high primary androgen-deprivation therapy (PADT) use and low PADT use at 10 yr for low-risk patients was significant (95% CI, 0.06–2.09; Fig. 1b). Death was treated as a competing risk.
HSA = health service area.
Acknowledgment statement
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Information Services and the Office of Strategic Planning, HCFA; Information Management Services, Inc.; and the SEER program tumor registries in the creation of the SEER-Medicare database. The authors are grateful for the input of Drs. Anthony Zietman and Sung Kim regarding the coding of palliative radiation therapy.
Funding/Support and role of the sponsor: This study was supported in part by award number DAMD17–01–1-0755 from the US Army Medical Research Acquisition Activity, Fort Detrick, MD, USA, and by the Cancer Institute of New Jersey, DOD award W81XWG-05–1-0235, and by National Cancer Institute (NCI) grant R01 CA116399 and CINJ core grant NCI CA-72720–10. NCI provided funding for this study but did not play any role in the design and conduct of the study, analysis and interpretation of the data, or preparation of the manuscript. The study received institutional review board approval from the University of Medicine and Dentistry of New Jersey as well as the Surveillance Epidemiology and End Results (SEER) program and the Center for Medicare and Medicaid Services (CMS). The performance and design of this study were reviewed and approved by both NCI and CMS. This study uses the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The content of the information does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.
Appendix 1.
Codes used to identify prostate cancer therapy
| External-beam radiation therapy |
| CPT code: 77401–77416, 77418, 77520–77525 |
| Other palliative radiation |
| CPT code: 79101 HCPCS code: A9605, A9600, C9401 |
| Chemotherapy |
| ICD-9 diagnosis code: V581, V662, V672 ICD-9 procedure code: 9925 CPT code: 96401, 96408, 96410, 96412, 96413, 96415, 96417, 96523, 96545, 96549, HCPCS code: G0921–G0932, G9021–G9032, J8530, J8560, J9035, J9045, J9060, J9062, J9070, J9080, J9090, J9093, J9094, J9170, J9181, J9182, J9264, J9293, J9360, J9390 |
| Surgery or radiation therapy for spinal cord compression or pending compression |
| Surgery: ICD-9 procedure 309 81.0x, 81.3x, 81.6x, 84.5x, with ICD-9 diagnosis 198.3, 198.5, 733.13 Radiation therapy: CPT 77401–77416 with ICD-9 diagnosis 198.3, 198.5, 733.13 |
| TURP |
| ICD-9 diagnosis code: 185 ICD-9 procedure code: 6029 |
| Nephrostomy tubes |
| CPT code: 50392, 50395 |
| Cystotomy tubes |
| CPT code: 51040, 51102 |
CPT = Current Procedure Terminology; HCPCS = Healthcare Common Procedure Coding System; ICD-9 = International Classification of Diseases, 9th Revision; TURP = transurethral resection of the prostate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Grace L. Lu-Yao had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lu-Yao, Albertsen, Yao, DiPaola.
Acquisition of data: Lu-Yao.
Analysis and interpretation of data: Lu-Yao, Li.
Drafting of the manuscript: Lu-Yao, Albertsen, Moore, Lin.
Critical revision of the manuscript for important intellectual content: Lu-Yao, Albertsen, Shih, Moore, Lin, Yao.
Statistical analysis: Li, Moore, Lin, Shih.
Obtaining funding: Lu-Yao.
Administrative, technical, or material support: None.
Supervision: Lu-Yao.
Other (specify): None.
Financial disclosures: Grace L. Lu-Yao certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Grace Lu-Yao has received clinical research funding from the New Jersey Commission on Cancer Research. Dr. Peter Albertson has received clinical research funding from Sanofi-Aventis and consultation fees from Blue Cross/Blue Shield. Dr. Weichung Shih has received clinical research funding from Myriad; Dr. Dirk Moore has received funding from Innocentive Inc., and the National Cancer Institute, and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Siu-Long Yao has been employed by Schering-Plough and Merck Research Laboratory in the area of clinical cancer research. None of these entities contributed funding, or played any role whatsoever in the design, interpretation, or drafting of our study or manuscript.
References
- [1].Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–3. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- [4].Schröder FH, Kurth KH, Fosså SD, et al. Early versus delayed endocrine treatment of pN1–3 M0 prostate cancer without local treatment of the primary tumor: results of European Organisation for the Research and Treatment of Cancer 30846—a phase III study. J Urol. 2004;172:923–7. doi: 10.1097/01.ju.0000135742.13171.d2. [DOI] [PubMed] [Google Scholar]
- [5].Wong YN, Freedland SJ, Egleston B, Vapiwala N, Uzzo R, Armstrong K. The role of primary androgen deprivation therapy in localized prostate cancer. Eur Urol. 2009;56:609–16. doi: 10.1016/j.eururo.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP, Casodex Early Prostate Cancer Trialists’ Group Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97:247–54. doi: 10.1111/j.1464-410X.2005.06051.x. [DOI] [PubMed] [Google Scholar]
- [7].Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- [8].Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- [9].Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- [10].Potosky AL, Reeve BB, Clegg LX, et al. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002;94:430–7. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
- [11].Sadetsky N, Greene K, Cooperberg MR, Hubbard A, Carroll PR, Satariano W. Impact of androgen deprivation on physical well-being in patients with prostate cancer: analysis from the CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) registry. Cancer. 2011;117:4406–13. doi: 10.1002/cncr.26064. [DOI] [PubMed] [Google Scholar]
- [12].Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(Suppl 8):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- [13].Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(Suppl 8):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- [14].Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- [15].Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–24. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- [17].Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98:839–45. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Angrist J, Imbens G, Rubin D. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91:444–55. [Google Scholar]
- [19].Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer; New York, NY: 2000. [Google Scholar]
- [20].Pagliarulo V, Bracarda S, Eisenberger MA, et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol. 2012;61:11–25. doi: 10.1016/j.eururo.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sun Y, Wang B-E, Leong KG, et al. Androgen deprivation causes epithelial– mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. doi: 10.1158/0008-5472.CAN-11-3004. In press. DOI:10.1158/0008–5472.can-11–3004. [DOI] [PubMed] [Google Scholar]
- [22].Jennbacken K, Tesan T, Wang W, Gustavsson H, Damber JE, Welen K. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr-Related Cancer. 2010;17:469–79. doi: 10.1677/ERC-10-0015. [DOI] [PubMed] [Google Scholar]
- [23].Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–9. [PubMed] [Google Scholar]
- [24].Koivisto PA, Helin HJ. Androgen receptor gene amplification increases tissue PSA protein expression in hormone-refractory prostate carcinoma. J Pathol. 1999;189:219–23. doi: 10.1002/(SICI)1096-9896(199910)189:2<219::AID-PATH423>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [25].Kaltz-Wittmer C, Klenk U, Glaessgen A, et al. FISH analysis of gene aberrations (MYC, CCND1, ERBB2, RB, and AR) in advanced prostatic carcinomas before and after androgen deprivation therapy. Lab Inves. 2000;80:1455–64. doi: 10.1038/labinvest.3780152. [DOI] [PubMed] [Google Scholar]
- [26].Shi YAN, Brands FH, Chatterjee S, et al. HER-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001;166:1514–9. doi: 10.1016/s0022-5347(05)65822-3. [DOI] [PubMed] [Google Scholar]
- [27].Ricciardelli C, Jackson MW, Choong CS, et al. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate. 2008;68:830–8. doi: 10.1002/pros.20747. [DOI] [PubMed] [Google Scholar]
- [28].Bao BY, Pao JB, Huang CN, et al. Polymorphisms inside microRNAs and microRNA target sites predict clinical outcomes in prostate cancer patients receiving androgen-deprivation therapy. Clin Cancer Res. 2011;17:928–36. doi: 10.1158/1078-0432.CCR-10-2648. [DOI] [PubMed] [Google Scholar]
- [29].Studer UE, Collette L, Whelan P, et al. Using PSA to guide timing of androgen deprivation in patients with T0–4 N0–2 M0 prostate cancer not suitable for local curative treatment (EORTC 30891) Eur Urol. 2008;53:941–9. doi: 10.1016/j.eururo.2007.12.032. [DOI] [PubMed] [Google Scholar]
- [30].Kawakami J, Cowan JE, Elkin EP, Latini DM, Duchane J, Carroll PR. Androgen-deprivation therapy as primary treatment for localized prostate cancer: data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Cancer. 2006;106:1708–14. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]