Abstract
Cognitive control is necessary to navigating through an uncertain world. With the stop signal task (SST), we measure how cognitive control functions in a controlled environment. There has been conflicting evidence on whether trait impulsivity might reflect differences in cognitive control during the SST. While some studies find that trait impulsivity relates to measures of response inhibition, such as the stop signal reaction time (SSRT), other studies do not. Here, in 92 young adult participants (58 females; age 25 ± 4 years), we examined whether trait impulsivity, measured by the Barratt Impulsivity Scale (BIS-11), is associated with differences in performance and regional brain activations for the component processes of cognitive control during the SST. Across participants, trait impulsivity showed a trend-level correlation with SSRT (F(1,90)=3.18, p<.07; Pearson regression). In simple regressions, activation of the right anterior dorsal insula and middle frontal cortex (MFC) during stop as compared to go trials negatively correlated with motor and non-planning impulsivity score. Using the generalized form of psychophysiological interaction (gPPI), we showed that functional connectivity of the right insula and MFC with the left dorsolateral prefrontal cortex and bilateral visual areas were also negatively correlated with impulsivity. None of the other component processes of cognitive control, including response inhibition, error processing, post-error slowing, were significantly related to Barratt impulsivity. These results suggest that trait impulsivity as measured by BIS-11 may have distinct effects on saliency processing in adult individuals.
Keywords: fMRI, impulsivity, cognitive control, insula
Introduction
Impulsivity can be defined by the following elements: “1) decreased sensitivity to negative consequences of behavior; 2) rapid, unplanned reactions to stimuli before complete processing of information; and 3) lack of regard for long-term consequences” (Moeller et al., 2001). Impulsivity affects and sometimes defines a number of psychiatric disorders, including attention deficit hyperactivity, substance abuse, gambling, schizophrenia, borderline personality and bipolar disorders, many of which are associated with altered inhibitory control (Aron et al., 2003; Dagher & Robbins, 2009; Delisle & Braun, 2011; Goudriaan et al., 2008; Hinvest et al., 2011; Kaladjian et al., 2011; Li et al., 2007; Michalczuk et al., 2011; Odlaug et al., 2011; Schuermann et al., 2011; Winstanley et al., 2006). Impulsivity is also a psychological construct that varies as a spectrum across individuals (Dimoska & Johnstone, 2006; Hinvest et al., 2011; Lijffijt et al., 2004; Manuck et al., 1998; Spinella, 2007). Thus, individuals who are otherwise healthy may engage in behavior that disposes them toward negative consequences. It would therefore be useful to understand the role that impulsivity alone plays in cognitive control outside psychiatric disorders and to understand the psychological processes and neural bases of impulsivity.
Cognitive control is widely investigated with the stop signal task (SST; Li et al., 2006; 2008a, b; Logan & Cowan, 1984). The SST involves frequent go stimuli, which require the subject to quickly respond, interspersed with an infrequent stop stimulus that follows the go stimulus and requires subjects to stop responding. Our earlier work suggests that a number of different psychological processes are involved during performance of the SST. Using a staircase procedure in which the difficulty of the stop trials was adjusted according to participants’ performance, we separated the processes of response inhibition, error processing, and post-error behavioral adjustment, which are key component processes of cognitive control (Li et al., 2006; 2008a, b). Response inhibition along with attentional monitoring can be used to describe successful stop trials when contrasted with unsuccessful stop trials, or stop errors (Li et al., 2006). Error processing describes the opposite phenomena where the subjects fail to inhibit their response during a stop trial (Li et al., 2008a). Post-error behavioral adjustment is characterized by the tendency of subjects to slow down after they make an error (Li et al., 2008b).
The stop signal reaction time (SSRT), estimated from each subjects’ performance, is a measure of inhibitory control, where shorter SSRTs indicate greater capacity of inhibitory control (Logan, 1994), and vice versa. SSRT has been found to positively correlate with impulsivity in healthy adults (Avila & Parcet, 2001; Logan et al., 1997; Marsh et al., 2002). On the other hand, other studies with a similar sample size have found that impulsivity has no effect on SSRT or inhibitory control (Asahi et al., 2004; Lijffijt et al., 2004; Rodríguez-Fornells et al., 2002).
In order to better address these differences in findings and explore the relationship between trait impulsivity and other component processes of cognitive control, we sought to examine how differences in trait impulsivity might influence SST performance in a larger sample of participants. In particular, while much previous work was limited to behavioral performance, here, we investigated the neural correlates of impulsivity with functional magnetic resonance imaging (fMRI) during the SST to understand how brain activity may vary with a participant’s impulsivity. To this end, we assessed 92 healthy adults with the Barratt Impulsiveness Scale, version 11 (BIS-11). We examined how behavioral performance and regional brain activations as well as functional connectivity varied with trait impulsivity during the SST.
Methods
Subjects and behavioral task
A total of 92 healthy participants (58 females; age 25 ± 4 years) participated in this study. Impulsivity was derived from scores on the Barratt Impulsiveness Scale, version 11 (BIS-11; Barratt & Patton, 1983; Patton et al., 1995). Three subscores of the BIS-11 were also computed for “attentional” or an inability to focus, “motor” or acting without prior thought, and “nonplanning” or not thinking and planning carefully, impulsivities (Patton, Stanford & Barratt, 1995).
We employed a simple reaction time task in this stop-signal paradigm (Figure 1; Logan, Cowan & Davis, 1984; Hu and Li, 2011; Hu et al., 2012; Ide and Li, 2011a; Li et al., 2006; 2009). There were two trial types: “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention at the beginning of a go trial. After a randomized time interval (fore-period) between 1 and 5 s, the dot turned into a circle (the “go” signal), which served as an imperative stimulus, prompting the subject to quickly press a button. The circle vanished at a button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. The remaining one quarter were stop trials. In a stop trial, an additional “X,” the “stop” signal, appeared after and replaced the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. The stop signal delay (SSD) – the time interval between the go and stop signal – started at 200 ms and varied from one stop trial to the next according to a staircase procedure, increasing and decreasing by 67 ms each after a successful or failed stop trial (Levitt, 1970; De Jong et al., 1990). There was an inter-trial-interval of 2s. Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. In the scanner each subject completed four 10-minute runs of the task with the SSD updated manually across runs. Depending on the actual stimulus timing (trials varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. With the staircase procedure, we anticipated that the subjects would succeed in withholding their response in approximately half of the stop trials. The stop signal reaction time was computed by subtracting the critical stop signal delay, or the estimated SSD required for a subject to get half of stop trials correct, from the median go reaction time (Li et al., 2008a).
Figure 1.
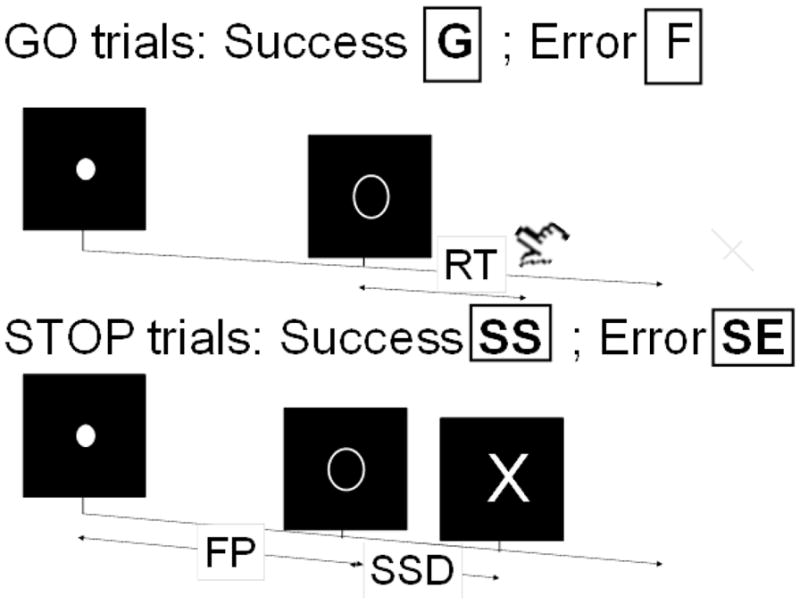
Stop signal paradigm. In “go” trials (~75%) observers responded to the go signal (a circle) and in “stop” trials (~25%) they had to withhold the response when they saw the stop signal (an X). In both trials the go signal appeared after a randomized time interval between 1 to 5 s (the fore-period or FP) following the appearance of the fixation point. The stop signal followed the go signal by a time delay – the stop signal delay (SSD). The SSD was updated according to a staircase procedure, whereby it increased and decreased by 64 ms following a stop success (SS) and stop error (SE) trial, respectively.
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4mm and no gap. Functional, blood oxygenation level dependent (BOLD) signals were then acquired with a single-shot gradient echo echo-planar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2,000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4mm and no gap. Three hundred images were acquired in each run for a total of four runs.
Data analysis and statistics
Data were analyzed with Statistical Parametric Mapping version 8 (SPM8, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each run were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion-corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner & Friston, 1999; Friston et al., 1995a). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
In the first general linear model (GLM), four main types of trial outcome were distinguished: go success (G), go error (F), stop success (SS), and stop error (SE) trial. The SS and SE trials were identical in stimulus condition, with SS and SE trials each involving inhibition success and failure, respectively. The contrast SS > SE thus engaged processes related to attentional monitoring and response inhibition (Duann et al., 2009; Li et al., 2006). The opposite contrast SE>SS highlights processes related to error detection (Hendrick et al., 2010; Ide and Li, 2011b). An SS or SE, together stop (S), trial involves incongruent goals between the prepotency to respond and the motor intention to withhold the response. S trials are also infrequent compared to go trials, making them highly salient. Thus, we interpreted the contrast of S>G as reflecting saliency processing (Hendrick et al., 2011).
In a second GLM, G, F, SS, and SE trials were first distinguished. G trials were divided into those that followed a G (pG), SS (pSS), and SE (pSE) trial. Furthermore, pSE trials were divided into those that increased in RT (pSEi) and those that did not increase in RT (pSEni), to allow the isolation of neural processes involved in post-error behavioral adjustment (Li et al., 2008a). To determine whether a pSE trial increased or did not increase in RT, it was compared with the pG trials that preceded it in time during each session. The pG trials that followed the pSE trial were not included for comparison because the neural/cognitive processes associated with these pG trials occurred subsequent to and thus could not have a causal effect on the pSE trial (Li et al., 2008a). We constructed for each individual subject 2 contrasts: SS > SE, to compare with the first GLM and verify the model; and pSEi versus pSEni, to identify activations associated with post-error slowing (PES).
A statistical analytical design was constructed for each individual subject, using the general linear model (GLM) with the onsets of go signal in each of these trial types convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (Friston et al., 1995b). Realignment parameters in all 6 dimensions were also entered in the model. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts. Serial autocorrelation of the time series violated the GLM assumption of the independence of the error term and was corrected by a first-degree autoregressive or AR(1) model (Friston et al., 2000). The GLM estimated the component of variance that could be explained by each of the regressors.
The con or contrast (difference in β) images of the first-level analysis were used for the second-level group statistics (random effects analysis; Penny and Holmes, 2004). These images were correlated with the total BIS score in a simple regression across subjects and the results were reported at a threshold of p<.001, uncorrected for multiple comparisons. To explore the association with the subcomponent of Barratt impulsivity, these images were also correlated with the three subscores of BIS-11 and the results were reported at a threshold of p<.05, family wise error (FWE) corrected for the whole brain. Brain regions were identified using an atlas (Duvernoy, 1999). All templates are in Montreal Neurological Institute (MNI) space and voxel activations are presented in MNI coordinates.
Connectivity analysis: psychophysiological interaction (PPI)
PPI describes how functional connectivity between brain regions is altered as a result of psychological context or variables (Friston et al., 1997; Gitelman et al., 2003). To accommodate three task conditions (G, SS, SE), we used a generalized form of context-dependent psychophysiological interaction (gPPI, http://brainmap.wisc.edu/PPI, McLaren et al., 2008). Briefly, in gPPI, the condition onset times for G, SS, and SE are separately convolved with the canonical hemodynamic response function (HRF) for each condition, forming the psychological regressors. The time series of the first eigenvariate of the BOLD signal were temporally filtered, mean corrected, and deconvolved to generate the time series of the neuronal signal for the selected ROIs (from the results of the BIS-11 regression) for each individual subject to compose the physiological variable. We performed this separately for the insula and middle frontal cortical ROIs (see Results below). The interaction term – PPI – was computed by multiplying the time series from the psychological regressors with the physiological variable. This interaction term and the resultant images for each subject were then used in a regression with BIS-11 scores to examine how PPI varied with impulsivity.
We focused on saliency processing (S>G) in PPI analyses, combining the SS and SE as compared to G, because this was the only contrast that demonstrated regional activities that were significant at a corrected threshold in linear regressions against BIS scores (see below).
Results
Impulsivity and behavioral performance
The total BIS-11 scores of the 92 subjects ranged from 41 to 82 (mean ± SD = 60 ± 9) out of a possible 120, where a higher score indicates greater impulsivity. The average go trial response rate was 96.0 ± 1.6%, while the stop success rate was 53.4 ± 3.8%. Average go trial reaction time (GoRT) and SSRT was 653 ± 117 ms and 216 ± 44 ms, respectively. In a simple regression across subjects, trait impulsivity showed a weak, trend-level correlation with SSRT (R=.19, F(1,90)=3.49, p<.064; Pearson regression). Motor impulsivity (range: 15–31) showed a slightly stronger albeit trend-level correlation with SSRT (R=.20, F(1,90)=3.64, p<.059), while nonplanning (range: 15–35) and attentional (range: 8–22) impulsivity did not correlate with SSRT (R=.16, F(1,90)=2.50, p<.11; R=.10, F(1,90)=.91, p<.34, respectively). Impulsivity also did not show a significant correlation with post-error slowing (total score: R= .14, F(1,90)=1.73, p<.19; motor subscore: R= .14, F(1,90)=1.88, p<.17; nonplanning subscore: R= .14, F(1,90)=1.70, p<.19; attentional subscore: R= .08, F(1,90)=.57, p<.45).
Impulsivity and response inhibition, error processing, and post-error slowing
Regressions of the BIS-11 impulsiveness ratings with contrasts for attentional monitoring and response inhibition (SS>SE), error processing (SE>SS), and post-error slowing did not reveal any significant regional brain activations, even at a threshold of p<.005, uncorrected.
Impulsivity and saliency processing
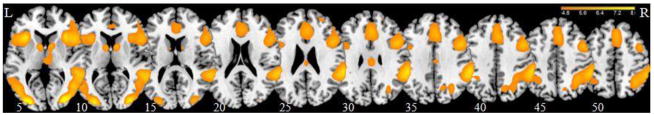
In a one sample t-test across all subjects for the stop as compared to go contrast, we observed the typical activations of anterior cingulate cortex and supplementary motor area, insula and middle frontal cortex, inferior parietal cortex, and some subcortical structures including thalamus and posterior part of the caudate head, at p<.05, FWE corrected (Figure 2).
Figure 2.
Regional activations (warm colors) during stop as compared to go trials for all subjects in a one sample t-test at p<.05, FWE corrected. BOLD contrasts are superimposed on a T1 structural image in axial sections from z=5 to z=50. The adjacent sections are 5mm apart. Color scale indicates voxel T-value.
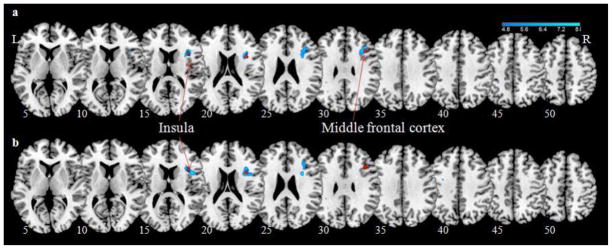
In a simple regression against BIS scores, with age and gender as covariates, activations of the right anterior dorsal insula (MNI coordinates= 36, 8, 19; Z(peak)= 3.87; Extent= 24 voxels) and right middle frontal cortex (MFC; MNI coordinates= 36, 20, 28; Z(peak)= 3.57; Extent= 18 voxels) during stop as compared to go trials were negatively correlated with BIS score, at p<.001, uncorrected. The subscore of motor impulsivity negatively correlated to activation of both the right insula (MNI coordinates= 30, 8, 19; Z(peak)= 4.24; Extent= 117 voxels) and MFC (MNI coordinates= 35, 23, 28; Z(peak)= 4.05; Extent= 117 voxels) during stop as compared to go trials (p<.046, FWE corrected for the whole brain; Figure 3a). The nonplanning impulsivity subscore inversely correlated with an activation in the right insula (MNI coordinates= 30, 8, 22; Z(peak)= 4.17; Extent= 112 voxels; p<.051, FWE corrected for the whole brain; Figure 3b). Attentional impulsivity did not show any correlated brain activations.
Figure 3.
Regional activations (blue) during stop as compared to go trials that are negatively correlated with (a) motor impulsivity and (b) non-planning impulsivity subscales of the BIS-11, at cluster level p<.05, FWE corrected. BOLD contrasts are superimposed on a T1 structural image in axial sections from z=5 to z=50. The adjacent sections are 5mm apart. Color scale indicates voxel T-value. The red cluster shows the results from the same regression against the total BIS-11 score, at p<.001, uncorrected.
Impulsivity and functional connectivity during saliency processing
Using each of the ROIs generated from the regression analysis, we then performed psychophysiological interaction analysis (gPPI) using stop as compared to go trials as the psychological regressor and each ROI separately as a physiological variable.
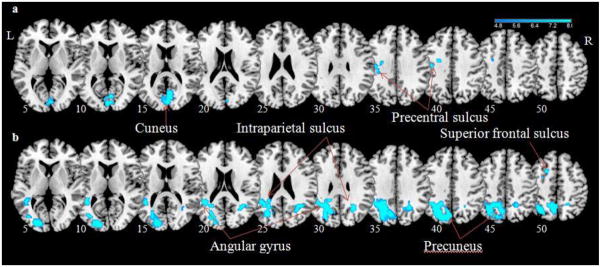
In a regression of the PPI term against the total BIS score, impulsivity was negatively correlated with PPI of the right insula and the left inferior precentral sulcus/precentral gyrus (MNI coordinates= −27, −7, 37; Z(peak)= 4.02; Extent= 51 voxels) and bilateral cuneus (MNI coordinates= 3,−79, 1; Z(peak)= 3.54; Extent= 125 voxels); and with PPI of the right MFC and the left DLPFC/superior frontal sulcus (MNI coordinates= −24, 14, 55; Z(peak)= 4.29; Extent= 54 voxels), precuneus (MNI coordinates= −12, −58, 46; Z(peak)= 4.80; Extent= 1127 voxels), and intraparietal sulcus/angular gyrus (MNI coordinates= −30, −70, 46; −21, −88, 7; 27, −55, 31; Z(peak)= 4.80; 4.58; Extent= 1127; 125 voxels), all at p<.05, FWE corrected (Figures 4a and 4b).
Figure 4.
Regions with psychophysiological interaction with (a) right dorsal anterior insula and (b) right middle frontal cortex negatively correlated with Barratt impulsivity during stop as compared to go trials (cluster p<.05, FWE corrected). BOLD contrasts are superimposed on a T1 structural image in axial sections from z=5 to z=50. The adjacent sections are 5mm apart. Color scale indicates voxel T-value.
Discussion
Barratt impulsivity and saliency processing
Barratt impulsivity is associated with decreased activation of the right anterior insula and middle frontal cortex during saliency processing. The right anterior insula and middle frontal cortex respond to both stop success and error trials in the stop signal and go-nogo tasks (Garavan et al., 1999; Hampshire et al., 2010; Kawashima et al., 1996; Rubia et al., 2003; Swainson et al., 2003). These activations have been hypothesized to signal saliency processing, a response to the infrequent stop, as compared to the frequent go signal during the stop signal task. Compared to the go signal, the stop signal is salient also because it instructs a change in act: to inhibit the prepotent go response. For this reason, some investigators have argued that a contrast between stop and go trials may implicate response inhibition, in addition to saliency processing (Dimoska et al., 2003; Pliszka et al., 2000; Rubia et al., 2001). On the other hand, studies have reported greater insular activation during stop error as compared to stop success trials (Garavan et al., 1999; 2002; Hester et al., 2004). These latter results suggest that insular activation most likely reflects saliency processing in association with the infrequency (or “oddball”) effects of stop/nogo trials or a signal of surprise associated with errors, since inhibition is in place to a greater extent in stop success than error trials. In further support of a role of the insula in saliency processing, Ramautar et al. (2006) found greater bilateral insular activation during stop error trials in a block with less frequent stop trials than one with more frequent stop trials.
Salient stimuli are abundant in the environment. Thus, the anterior insula responds to social exclusion of friends versus strangers (Meyer et al., in press), novel and ambiguous versus unambiguous visual inputs (Jepma et al., 2012), exteroceptive versus interoceptive attention (Farb et al., in press), and unexpected versus expected taste stimuli (Veldhuizen et al., 2011). In other studies, insula has been found to respond to salience over valuation during decision making (Litt et al., 2011), stimulus valence and cognitive demand (Gu et al., in press), moment to moment adjustment in task set maintenance (Wilk et al., 2012), and attentional processing in a cingulo-opercular attentional network that controls goal-directed behaviors (Dosenbach et al., 2007). Additionally, the dorsal anterior insula showed greater responses to risky as compared to non-risky choices, with the responses scaling in proportion to an anxiety rating (Tang et al., 2011). Other studies showed that activations of anterior insula to threatening/emotional as compared to safe/neutral stimuli increased during an anxiety state (Ball et al. 2012; Choi et al., 2012), consistent with a bulk of earlier work on the relationship between insular functions and anxiety trait or clinical anxiety disorders (see Etkin, 2010; Holzschneider and Mulert, 2011; Miskovic and Schmidt, 2012 for review). These findings mirror our results of decreased insular activation in impulsive individuals because anxiety and impulsivity are anti-correlated personality traits (Li and Chen, 2006).
Similarly, the middle frontal cortical (MFC) cluster, as part of the broadly defined lateral prefrontal cortex, is widely implicated in attentional processing (Bunge et al., 2005; Crone et al., 2006; Dosenbach et al., 2006; Fan et al., 2005). This MFC region increased activation during “orienting” trials where subjects had to shift their attention to a different location in space (Fan et al., 2005). This area was shown to maintain but not manipulate item information during a working memory task, suggesting that it helps sustain attention (Crone et al., 2006). The current results are also consistent with an earlier report that activation of a similar right lateral prefrontal area during nogo as compared to go trials inversely correlated with motor impulsivity during the go-nogo task (Asahi et al., 2004).
Nonetheless, we acknowledge that the contrast of stop as compared to go trials may also involve some level of response inhibition in addition to saliency processing. For instance, Chikazoe et al. (2009) reported that the insula and middle frontal regions showed greater responses to stop and to uncertain go trials as compared to certain go trials, suggesting that these regions may be involved in both preparation to stop and may mediate response inhibition and not simply saliency processing. These regions have also been linked to the preparation to inhibit, such as during the fixation period before a no-go or incongruent trial, which does not involve explicit processing of visual stimuli (Hester et al., 2004; Fassbender et al., 2006).
An additional issue is that saliency has been broadly defined across a wide array of behavioral tasks. For instance, fearful faces, painful stimulation, and negative reward evoked activations in the amygdala and midbrain in a saliency network (Berns et al., 2008; Garrido et al., 2012; Vuilleumier et al., 2003). In addition, visual search and target detection – highly salient events – are strongly linked to the frontoparietal attention network and the visual cortices (Beck et al., 2001; Corbetta & Shulman, 2002; Shulman et al., 2001). Similarly, ACC/SMA, insula, and DLPFC are known to be involved in spatial reorienting to salient stimuli, for instance after a change in feature or color, across a variety of tasks (Downar et al., 2000; 2002; Corbetta & Shulman, 2002; Peelen et al., 2004). Evidence from lesion studies shows that middle frontal cortex is particularly important to detecting and orienting attention to infrequent, or salient, events (Daffner et al., 2000; Knight & Scabini, 1998). Thus, the current findings characterize saliency processing in a broad sense, in accord with these earlier studies, but need to be contrasted with saliency related areas in behavioral tasks implicating affective and/or reward processing.
Impulsivity and functional connectivity during saliency processing
Connectivity analyses on PPI demonstrated an inverse correlation with impulsivity in functional connectivity between insula/MFC and the left superior frontal sulcus, bilateral cuneus/dorsal precuneus, and the intraparietal sulcus/angular gyrus during stop as compared to go trials. These frontal parietal cortical areas and dorsal precuneus are parts of a well defined visuospatial attention network (Corbetta & Shulman, 2002; Dosenbach et al., 2007; Nobre et al., 1997; Kastner et al., 1999; Zhang & Li, 2012b). Both the angular gyrus and the intraparietal sulcus are part of the inferior parietal lobe, which is known to mediate visuospatial attention (Chambers et al., 2004; Corbetta & Shulman, 2002; Egner et al., 2008). The dorsal precuneus is involved in attentional orienting across a variety of behavioral tasks (Cavanna & Trimble, 2006; Culham et al., 1998; Le et al., 1998; Nagahama et al., 1999). In particular, functional connectivity mapping showed that the dorsal precuneus along with an array of cortical and subcortical structures is involved in cognitive challenges that require visuospatial and motor attention. Similarly, cuneus increased activation to salient stimuli (Carretie et al., 2004) and modulation in value and salience during a decision-making task (Litt et al., 2011). Several imaging studies have shown that attention modulates visual cortical activation and that allocation of attention to either the peripheral or central visual field changes visual cortical activation accordingly (Brefczynski & DeYoe, 1999; Slotnick et al., 2003; Smith et al., 2000). Altogether, these results support the finding that individuals who are less impulsive have more attention-related functional connectivity between insula and MFC with visual processing areas and prefrontal cortex during saliency processing than those who are more impulsive.
The superior frontal cortex has also been implicated in a variety of cognitive control tasks (Chao et al., 2009; Greening et al., 2011; Jamadar et al., 2010; Kadota et al., 2010; Li et al., 2006; Mansouri et al., 2007; Zhang & Li, 2012a). For instance, this dorsal prefrontal region responded to nogo trials during go/nogo tasks (Nakata et al., 2008; Rubia et al., 2001) and to inhibition of stereotyped responses in a modified rock paper scissors task (Kadota et al., 2010). Lesions of the dorsal prefrontal cortex led to deficits in cognitive control in a modified Wisconsin Card Sorting Task in non-human primates (Mansouri et al., 2007). Thus, impulsivity may impart decreased connectivity to this prefrontal structure for cognitive control during saliency processing.
Barratt impulsivity and other attentional functions
Impulsivity has been seen to negatively affect attention in other behavioral tasks. For instance, during a visual oddball task, Russo et al. (2008) found that subjects with higher Barratt impulsivity showed lower P300 wave amplitude, a measure of stimulus evaluation and classification time, likely due to their inability to inhibit task-irrelevant behavior and maintain sustained attention. In a rapid serial visual presentation (RSVP) task, participants are required to identify targets among a series of distracters. Typically, the ability to identify a target decreases right after the detection of a target and this “attentional blink” quantifies one’s attentional capacity. Attentional blink has been found to be more severe in individuals with higher impulsivity (Li et al., 2005). Importantly, attentional processing during the RSVP task involves the middle frontal cortex, likely as part of the attentional network (Marois et al., 2000; Marcantoni et al., 2003). Altogether, this supports an association between Barratt impulsivity and impaired attentional processing in a cognitive task.
Barratt impulsivity and inhibitory control
We observed that SSRT only correlates to impulsivity at a trend-level of statistical significance in our cohort of 92 subjects. Along with earlier studies that showed mixed results in the association of trait impulsivity and inhibitory control (Avila & Parcet, 2001; Lijffijt et al., 2004; Logan et al., 1997; Marsh et al., 2002; Rodríguez-Fornells et al., 2002), this finding suggests that trait impulsivity could at best explain a small fraction (R2=.04) of the variance in inhibitory control, as measured by the SSRT. We also did not observe a correlation of Barratt impulsivity total or subscore with post-error slowing (PES) or regional activations during PES. These findings suggest that Barratt impulsivity accounts for little inter-subject variability in inhibitory control and performance monitoring, as assessed by the SST.
It is important to note that there are different instruments to assess impulsive personality. For instance, the commonly used version of Eysenck’s scales, the I-7, measures two broad constructs of impulsivity: “impulsiveness” and “venturesomeness” (Eysenck et al., 1985). BIS-11 and I-7 both have good test-retest reliability and often relate to behavioral measures of impulsivity such as by the Matching Familiar Figures Test (MFFT; Asahi et al., 2004; Carrillo-de-la-Pena et al., 1993; Gerbing et al., 1987; Kagan et al., 1964; Kagan, 1966; Luengo et al., 1991). Eysenck’s and BIS scores obtained on the same subjects correlated to each other, with the BIS motor impulsivity subscale showing the highest correlation to total impulsiveness scores on the Eysenck’s scale (Carillo-de-la-Pena et al., 1993; Luengo et al., 1991). On the other hand, neither scale appeared to fully explain behavioral measures nor teacher ratings of impulsivity (Carillo-de-la-Pena et al., 1993; Luengo et al., 1991), suggesting that there are still aspects of impulsivity that are not described by these self-report assessments.
Another popular instrument is the UPPS (urgency, premeditation, perseverance, and sensation seeking) Impulsive Behavior Scale, which measures four subscales: urgency, (lack of) premeditation, (lack of) perseverance, and sensation seeking (Whiteside & Lynam, 2001). Whiteside and Lynam (2001) found that the impulsiveness subscale of the Eysenck’s loaded onto their premeditation factor while venturesomeness loaded onto the sensation seeking factor. Patients with borderline personality disorder, which has impulsivity as one of the nine diagnostic criteria in the DSM-IV, scored higher than healthy controls on all three of the BIS-11 subscales, but only the impulsiveness subscale of the Eysenck’s and only the perseverance and urgency subscales of the UPPS (Jacob et al., 2010). These results provide further support for the multidimensionality of impulsivity. While all impulsivity scales measure impulsiveness, they vary in the dimensions of the impulsivity that they are able to capture. Future studies are required to examine whether impulsivity measured by instruments other than BIS-11 may be related to response inhibition and performance monitoring in the stop signal task.
Potential clinical implications
As described earlier, impulsivity is an important characteristic of many clinical conditions. Decreased insular activation during risk-taking is associated with hazardous drinking (Claus and Hutchison, in press). Individuals with generalized anxiety disorders show greater anterior insular activation to fearful as compared to happy faces (Klumpp et al., 2012). Anterior insula increased and supplementary motor area decreased in activation during motor preparation in conversion disorder patients, suggesting that heightened arousal and limbic activity may disrupt top-down motor control (Voon et al., 2011). During reward tasks, increased impulsivity is related to lower ventrial striatal activations in alcoholics (Beck et al., 2009). Poorer performance on the Iowa Gambling Task was also associated with increased impulsivity in MDMA users (Hanson et al., 2008). Thus, the current results may add to this literature by providing insights to cognitive deficits in individuals of impulse control disorders or other mental conditions where impulsivity represents a clinical concern.
Conclusions
Barratt impulsivity is associated with hypoactivation of frontal cortex and anterior insula and decreased functional connectivity with an attention network of brain regions during saliency processing. On the other hand, the current results do not appear to support a commonly assumed link between Barratt impulsivity and response inhibition or post-error slowing during cognitive control.
Acknowledgments
This study was supported by NIH grants R01DA023248, R21AA018004, K02DA026990, and P20DA027844, a NARSAD Young Investigator Award, and the Tourette Syndrome Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Drug Abuse, National Center for Research Resources or the National Institutes of Health. We thank Sarah Bednarski and Emily Erdman in subject recruitment and assessment as well as running of some of the imaging studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54(12):1465–8. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: an fMRI study. Eur Arch Psychiatry Clin Neurosci. 2004;254:245–51. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila C, Parcet MA. Personality and inhibitory deficits in the stop-signal task: The mediating role of Gray’s anxiety and impulsivity. Personality and Individual Differences. 2001;31:975–986. [Google Scholar]
- Ball TM, Sullivan S, Flagan T, Hitchcock CA, Simmons A, Paulus MP, Stein MB. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. Neuroimage. 2012;59(2):1879–87. doi: 10.1016/j.neuroimage.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Patton JH. Impulsivity: cognitive behavioral, and psychophysiological correlates. In: Zuckerman M, editor. Biological Bases of Impulsiveness and Sensation Seeking. Erlbaum; Hillsdale, NJ: 1983. [Google Scholar]
- Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness. Nature Neurosci. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66:734–42. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berns GS, Capra CM, Moore S, Noussair C. Three studies on the neuroeconomics of decision-making when payoffs are real and negative. Adv Health Econ Health Serv Res. 2008;20:1–9. doi: 10.1016/s0731-2199(08)20001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the “spotlight” of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15:239–49. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Carillo-de-la-Pena MT, Otero JM, Romero E. Comparison among various methods of assessment of impulsiveness. Perceptual and Motor Skills. 1993;77:567–575. doi: 10.2466/pms.1993.77.2.567. [DOI] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Martín-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Hum Brain Mapp. 2004;22:290–9. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nat Neurosci. 2004;7:217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Chao HH, Luo X, Chang JL, Li CS. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time--an intra-subject analysis. BMC Neurosci. 2009;10:75. doi: 10.1186/1471-2202-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci. 2009;29:15870–7. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage. 2012;59(2):1912–23. doi: 10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Hutchison KE. Neural Mechanisms of Risk Taking and Relationships with Hazardous Drinking. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2011.01694.x. in press. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–86. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–70. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61:502–10. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. J Exp Psychol Hum Percept Perform. 1990;16:164–82. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- Delisle J, Braun CM. A context for normalizing impulsiveness at work for adults with attention deficit/hyperactivity disorder. Arch Clin Neuropsychol. 2011;26:602–13. doi: 10.1093/arclin/acr043. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ. Neural mechanisms underlying trait impulsivity in non-clinical adults: stop-signal performance and event-related potentials. Prog Neuropsychopharmacol Biol Psychiatry. 2006;31:443–54. doi: 10.1016/j.pnpbp.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ, Clarke AR. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: event-related potentials in the stop-signal paradigm. Biol Psychiatry. 2003;54:1345–54. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. Journal of Neuroscience. 2009;29:10171–9. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. 2. New York, NY: Springer Verlag; 1999. [Google Scholar]
- Egner T, Monti JM, Trittschuh EH, Wieneke CA, Hirsch J, Mesulam MM. Neural integration of top-down spatial and feature-based information in visual search. J Neurosci. 2008;28:6141–6151. doi: 10.1523/JNEUROSCI.1262-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2010(2):251–77. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality and Individual Differences. 1985;6:21–29. [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–9. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Attentional Modulation of Primary Interoceptive and Exteroceptive Cortices. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Simoes-Franklin C, Murphy K, Hester R, Meaney J, Robertson IH, Garavan H. The role of a right fronto-parietal network in cognitive control—common activations for “cues-to-attend” and response inhibition. J Psychophysiol. 2006;20:286–296. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Polone J-B, Heather JD, et al. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;2:165–189. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. Neuroimage. 2000;12:466–77. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–9. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Barnes GR, Sahani M, Dolan RJ. Functional evidence for a dual route to amygdala. Current Biology. 2012;22:129–134. doi: 10.1016/j.cub.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbing DW, Ahadi SA, Patton JH. Toward a conceptualization of impulsivity: Components across the behavioral and self-report domains. Multivariate Behavioral Research. 1987;22:357–379. doi: 10.1207/s15327906mbr2203_6. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, De Beurs E, Van Den Brink W. The role of self-reported impulsivity and reward sensitivity versus neurocognitive measures of disinhibition and decision-making in the prediction of relapse in pathological gamblers. Psychological Medicine. 2008;38:41–50. doi: 10.1017/S0033291707000694. [DOI] [PubMed] [Google Scholar]
- Greening SG, Finger EC, Mitchell DG. Parsing decision making processes in prefrontal cortex: response inhibition, overcoming learned avoidance, and reversal learning. Neuroimage. 2011;54:1432–41. doi: 10.1016/j.neuroimage.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition-Emotion Integration in the Anterior Insular Cortex. Cereb Cortex. doi: 10.1093/cercor/bhr367. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Champerlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug Alcohol Depend. 2008;96:99–110. doi: 10.1016/j.drugalcdep.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick OM, Luo XR, Li CS. Saliency processing and obesity: an fMRI study of the stop signal task. Obesity. 2011 doi: 10.1038/oby.2011.180. Electonic Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick OM, Ide JS, Luo XR, Li CS. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PLoS ONE. 2010;5(10):e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci. 2004;16:776–85. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Hinvest NS, Elliott R, McKie S, Anderson IM. Neural correlates of choice behavior related to impulsivity and venturesomeness. Neuropsychologia. 2011;49:2311–20. doi: 10.1016/j.neuropsychologia.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Holzschneider K, Mulert C. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci. 2011;13(4):453–61. doi: 10.31887/DCNS.2011.13.4/kholzschneider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li CS. Human Brain Mapping. 2011. Neural processes of preparatory control for stop signal inhibition. Electronic Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chao HH, Winkler AD, Li CS. The effects of age on cerebral activations: internally versus externally driven processes. Frontiers Aging Neuroscience. 2012;4:4. doi: 10.3389/fnagi.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage. 2011a;54:455–64. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS. Error-related functional connectivity of the habenula in humans. Frontiers Human Neuroscience. 2011b;16:25. doi: 10.3389/fnhum.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob GA, Gutz L, Bader K, Lieb K, Tuscher O, Stahl C. Impulsivity in borderline personality disorder: Impairment in self-report measures, but not behavioral inhibition. Psychopathology. 2010;43:180–188. doi: 10.1159/000304174. [DOI] [PubMed] [Google Scholar]
- Jacob GA, Gutz L, Bader K, Lieb K, Tüscher O, Stahl C. Impulsivity in borderline personality disorder: impairment in self-report measures, but not behavioral inhibition. Psychopathology. 2010;43:180–8. doi: 10.1159/000304174. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Hughes M, Fulham WR, Michie PT, Karayanidis F. The spatial and temporal dynamics of anticipatory preparation and response inhibition in task-switching. Neuroimage. 2010;51:432–49. doi: 10.1016/j.neuroimage.2010.01.090. [DOI] [PubMed] [Google Scholar]
- Jepma M, Verdonschot RG, van Steenbergen H, Rombouts SA, Nieuwenhuis S. Neural mechanisms underlying the induction and relief of perceptual curiosity. Front Behav Neurosci. 2012;6:5. doi: 10.3389/fnbeh.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota H, Sekiguchi H, Takeuchi S, Miyazaki M, Kohno Y, Nakajima Y. The role of the dorsolateral prefrontal cortex in the inhibition of stereotyped responses. Exp Brain Res. 2010;203:593–600. doi: 10.1007/s00221-010-2269-4. [DOI] [PubMed] [Google Scholar]
- Kagan J, Pearson L, Welch L. Conceptual impulsivity and inductive reasoning. Child Dev. 1966;37:583–94. doi: 10.1111/j.1467-8624.1966.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Rosman BL, Day D, Albert J, Philips W. Information Processing in the child: significance of analytic and reflective attitudes. Psychological Monographs. 1964:78. [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P. Impulsivity and neural correlates of response inhibition in schizophrenia. Psychological Medicine. 2011;41:291–9. doi: 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–61. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H. Functional anatomy of go/no-go discrimination and response selection- a PET study in man. Brain Res. 1996;728:79–89. [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89(1):273–6. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical Neuroscience. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol. 1998;79:1535–48. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1970;49:467–477. [PubMed] [Google Scholar]
- Li CS, Chen SH, Lin WH, Yang YY. Attentional blink in adolescents with varying levels of impulsivity. J Psychiatr Res. 2005;39:197–205. doi: 10.1016/j.jpsychires.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Chen S-H. Obsessive-compulsiveness and impulsivity in a non-clinical population of adolescent males and females. Psychiatr Res. 2006;149:129–138. doi: 10.1016/j.psychres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2007;33:1798–806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008a;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Chao HH, Sinha R, Paliwal P, Constable RT, Zhang S, Lee TW. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience. 2008b;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Chao HH, Lee TW. Neural correlates of speeded as compared with delayed responses in a stop signal task: an indirect analog of risk taking and association with an anxiety trait. Cerebral Cortex. 2009;19:839–48. doi: 10.1093/cercor/bhn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Bekker EM, Quik EH, Bakker J, Kenemans JL, Verbaten MN. Differences between low and high trait impulsivity are not associated with differences in inhibitory motor control. Journal of Attention Disorders. 2004;8:25–32. doi: 10.1177/108705470400800104. [DOI] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cereb Cortex. 2011;21:95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a user’s guide to the stop signal paradigm. In: Dagenbach D, Carr T, editors. Inhibitory Processes in Attention, Memory, and Language. Academic Press; San Diego, CA: 1994. pp. 189–240. [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;102:271–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Luengo MA, Carillo-de-la-Pena MT, Otero JM, Romero E. A short-term longitudinal study of impulsivity and antisocial behavior. Journal of Personality and Social Psychology. 1991;66:542–548. doi: 10.1037//0022-3514.66.3.542. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19:287–99. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Marcantoni WS, Lepage M, Beaudoin G, Bourgouin P, Richer F. Neural correlates of dual task interference in rapid visual streams: an fMRI study. Brain Cogn. 2003;53:318–21. doi: 10.1016/s0278-2626(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. Neural correlates of the attentional blink. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Marsh DM, Dougherty DM, Mathias CW, Moeller FG, Hicks LR. Comparisons of women with high and low trait impulsivity using behavioral models of response-disinhibition and reward choice. Personality and Individual Differences. 2002;33:1291–1310. [Google Scholar]
- McLaren D, Ries M, Xu G, Fitzgerald M, Kastman E, et al. A Method for Improved Sensitivity and Flexibility of Psychophysiological Interactions in Event-Related FMRI Experiments. Annual Meeting of the Organzization for Human Brain Mapping.2008. [Google Scholar]
- Meyer ML, Masten CL, Ma Y, Wang C, Shi Z, Eisenberger NI, Han S. Empathy for the Social Suffering of Friends and Strangers Recruits Distinct Patterns of Brain Activation. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nss019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk R, Bowden-Jones H, Verdejo-Garcia A, Clark L. Impulsivity and cognitive distortions in pathological gamblers attending the UK National Problem Gambling Clinic: a preliminary report. Psychological Medicine. 2011 doi: 10.1017/S003329171100095X. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neurosci Biobehav Rev. 2012 Jan;36(1):459–78. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158(11):1783–93. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, Toma K, Nakamura K, Hanakawa T, Konishi J, Fukuyama H, Shibasaki H. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10:193–9. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- Nakata H, Sakamoto K, Ferretti A, Gianni Perrucci M, Del Gratta C, Kakigi R, Luca Romani G. Somato-motor inhibitory processing in humans: an event-related functional MRI study. Neuroimage. 2008;39:1858–66. doi: 10.1016/j.neuroimage.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–33. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Chamberlain SR, Kim SW, Schreiber LR, Grant JE. A neurocognitive comparison of cognitive flexibility and response inhibition in gamblers with varying degrees of clinical severity. Psychological Medicine. 2011;41:2111–9. doi: 10.1017/S0033291711000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Heslenfeld DJ, Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage. 2004;22(2):822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Penny W, Holmes AP. Random-effects analysis. In: Frackowiak, et al., editors. Human Brain Function. Elsevier; San Diego: 2004. pp. 843–850. [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry. 2000;48:238–46. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR. Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Res. 2006;1105:143–54. doi: 10.1016/j.brainres.2006.02.091. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fornells A, Lorenzo-Seva U, Andrés-Pueyo A. Are high-impulsives and high risk-taking people more motor disinhibited in the presence of incentive? Personality and Individual Differences. 2002;32:661–683. [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52:47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–8. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Russo PM, De Pascalis V, Varriale V, Barratt ES. Impulsivity, intelligence and P300 wave: an empirical study. Int J Psychophysiol. 2008;69:112–8. doi: 10.1016/j.ijpsycho.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Schuermann B, Kathmann N, Stiglmayr C, Renneberg B, Endrass T. Impaired decision making and feedback evaluation in borderline personality disorder. Psychological Medicine. 2011;41:1917–27. doi: 10.1017/S003329171000262X. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Linenweber M, Petersen SE, Corbetta M. Multiple neural correlates of detection in the human brain. Proc Natl Acad Sci USA. 2001;98:313–318. doi: 10.1073/pnas.021381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. NeuroImage. 2003;19:1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. NeuroReport. 2000;11:271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- Spinella M. Normative data and a short form of the Barratt Impulsiveness Scale. Int J Neurosci. 2007;117:359–68. doi: 10.1080/00207450600588881. [DOI] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J Cogn Neurosci. 2003;15:785–99. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–94. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Tang GS, van den Bos W, Andrade EB, McClure SM. Social Anxiety Modulates Risk Sensitivity through Activity in the Anterior Insula. Front Neurosci. 2011;5:142. doi: 10.3389/fnins.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Douglas D, Aschenbrenner K, Gitelman DR, Small DM. The anterior insular cortex represents breaches of taste identity expectation. J Neurosci. 2011 Oct 12;31(41):14735–44. doi: 10.1523/JNEUROSCI.1502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord. 2012 Nov;26(13):2396–403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord. 2012 Nov;26(13):2396–403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience. 2003;6:624–31. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol. 2003;11:210–7. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- Wilk HA, Ezekiel F, Morton JB. Brain regions associated with moment-to-moment adjustments in control and stable task-set maintenance. Neuroimage. 2012;59(2):1960–7. doi: 10.1016/j.neuroimage.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–95. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, LI C-SR. Functional networks for cognitive control in a stop signal task: independent component analysis. Human Brain Mapp. 2012a;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012b;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]