Abstract
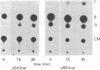
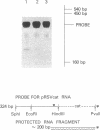
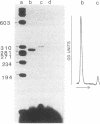
We characterized the transcriptional activity of the long terminal repeat (LTR) of Rous sarcoma virus by constructing a recombinant plasmid, pRSVcat, in which bacterial chloramphenicol acetyltransferase (CAT; acetyl-CoA:chloramphenicol 3-O-acetyltransferase, EC 2.3.1.28) coding sequences are placed under LTR control. We find that the LTR directs relatively high levels of CAT synthesis within 48 hr after calcium phosphate-mediated introduction of this plasmid into CV-1 monkey kidney cells, chicken embryo fibroblasts, Chinese hamster ovary cells, HeLa cells, or mouse NIH/3T3 cells. The level of CAT synthesis is 3-fold higher in CV-1 cells and up to 10-fold higher in HeLa and mouse NIH/3T3 cells than after transfection with a related vector, pSV2cat, carrying CAT sequences under control of the simian virus 40 early promoter. We have shown, by primer extension, that the amounts of CAT-specific mRNAs encoded by pRSVcat and pSV2cat correlate with the levels of CAT enzyme activity. By both S1 nuclease mapping and primer extension, we have demonstrated that the start site for RNA transcription within the LTR of pRSVcat corresponds to previous mapping data. We estimated transfection efficiencies by monitoring immunofluorescence induced by a rhodamine-labeled CAT antibody. Our results indicate that the Rous sarcoma virus LTR can direct synthesis of high levels of functional mRNA and has a wide expression range. The observed high transcriptional activity of the LTR is significant because it has been postulated that this LTR promotes activity of adjacent cellular oncogenes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M., Wood T. G., McClements W. L., Fischinger P. J., Vande Woude G. G. Activation of the transforming potential of a normal cell sequence: a molecular model for oncogenesis. Science. 1981 May 22;212(4497):941–943. doi: 10.1126/science.7233190. [DOI] [PubMed] [Google Scholar]
- Boettiger D. Virogenic nontransformed cells isolated following infection of normal rat kidney cells with B77 strain Rous sarcoma virus. Cell. 1974 Sep;3(1):71–76. doi: 10.1016/0092-8674(74)90042-7. [DOI] [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman S. A., Van Beveren C., Verma I. M. Identification of a RNA polymerase II initiation site in the long terminal repeat of Moloney murine leukemia viral DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5411–5415. doi: 10.1073/pnas.78.9.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Joyner A., Yamamoto Y., Bernstein A. Retrovirus long terminal repeats activate expression of coding sequences for the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1573–1577. doi: 10.1073/pnas.79.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Tyagi J. S., de Crombrugghe B., Pastan I. Transcription of the chicken alpha 2 (Type I) collagen gene by homologous cell-free extracts. J Biol Chem. 1982 Jun 25;257(12):7254–7261. [PubMed] [Google Scholar]
- Merlino G. T., Vogeli G., Yamamoto T., de Crombrugghe B., Pastan I. Accurate in vitro transcriptional initiation of the chick alpha 2 (Type I) collagen gene. J Biol Chem. 1981 Nov 10;256(21):11251–11258. [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Ostrowski M. C., Berard D., Hager G. L. Specific transcriptional initiation in vitro on murine type C retrovirus promoters. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4485–4489. doi: 10.1073/pnas.78.7.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Roth C. W., Singh T., Pastan I., Gottesman M. M. Rous sarcoma virus transformed cells are resistant to cyclic AMP. J Cell Physiol. 1982 Apr;111(1):42–48. doi: 10.1002/jcp.1041110108. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Function of the retrovirus long terminal repeat. Cell. 1982 Jan;28(1):3–5. doi: 10.1016/0092-8674(82)90367-1. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 1981 Nov;27(1 Pt 2):1–3. doi: 10.1016/0092-8674(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli G., Ohkubo H., Sobel M. E., Yamada Y., Pastan I., de Crombrugghe B. Structure of the promoter for chicken alpha 2 type I collagen gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5334–5338. doi: 10.1073/pnas.78.9.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Quade K. Infection of rat cells by avian sarcoma virus: factors affecting transformation and subsequent reversion. Virology. 1980 Oct 30;106(2):217–233. doi: 10.1016/0042-6822(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Jay G., Pastan I. Unusual features in the nucleotide sequence of a cDNA clone derived from the common region of avian sarcoma virus messenger RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):176–180. doi: 10.1073/pnas.77.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]