Abstract
Chronic serum sickness leads to the formation of glomerular immune complexes; however, C57BL/6 mice do not develop glomerulonephritis unless complement factor H (CFH) is absent from the plasma. Here we studied the role for C5a receptor (R) in this setting. The exaggerated humoral immune response in CFH−/− mice was normalized in CFH−/−C5aR−/− double knockout mice, highlighting the C5aR dependence. The CFH knockout mice developed proliferative glomerulonephritis with endocapillary F4/80+ macrophage infiltration, a process reduced in the double knockout mice. There was no interstitial inflammation by histologic criteria or flow cytometry for F4/80+Ly6ChiCCR2hi inflammatory macrophages. There were, however, more interstitial CD3+CD4+ T lymphocytes in CFH knockout mice with chronic serum sickness, while double knockout mice had greater than 5-fold more Ly6CloCCR2lo anti-inflammatory macrophages compared to the CFH knockout mice. Mice lacking C5aR were significantly protected from functional renal disease as assessed by blood urea nitrogen levels. Thus, IgG- and iC3b-containing immune complexes are not inflammatory in C57BL/6 mice. Yet when these mice lack CFH, sufficient C3b persists in glomeruli to generate C5a and activate C5aR.
Keywords: complement, glomerulonephritis, macrophages
The complement system provides a first line of defense against some microorganisms and participates in innate and adaptive immune responses to many others. More than 30 plasma and cell-associated proteins in three activation pathways converge on C3 and C5 to generate C3a, C3b, C5a, and C5b-9, each of which has biological activity. The C3a and C5a anaphylatoxins have specific rhodopsin family seven-span transmembrane receptors (R). The three extracellular loops of C3aR and C5aR confer ligand-binding specificity, whereas their activation is transduced through various αβγ G-proteins at the internal portion of the plasma membrane.1, 2 Although traditionally considered proinflammatory on bone marrow–derived cells, it is clear that C3aR and C5aR have a more widespread distribution with a variety of effects attributable to their activation.3, 4
The ability to surmount infectious challenges is presumed to have driven evolution of an active complement system. This does appear to have its price, with diseases such as atypical hemolytic uremic syndrome and age-related macular degeneration attributable to mutations conferring gain of function to activators and/or loss of function to regulators within the complement system.5, 6, 7 A relatively proactivating state of complement also appears relevant in a group of human glomerular diseases with membranoproliferative histopathological features and predominant C3 deposition, collectively termed C3 glomerulopathy.8 To date, most frequently implicated in these various diseases are genetic abnormalities in the important fluid-phase complement regulator, complement factor H (CFH).9, 10
CFH is also an important complement regulator in mice; CFH−/− mice of mixed genetic background develop glomerulonephritis (GN) spontaneously, which leads to the late death of ∼25% of mice.11, 12 This GN occurring in 1-year-old DBA/2 and C3H/HeN background CFH−/− mice requires C5 but not C6.11, 12 The genetic background is relevant, as C57BL/6 mice appear resistant to GN occurring spontaneously in CFH deficiency, as well as that occurring with immune complex deposition in chronic serum sickness (CSS).13 In contrast to wild-type C57BL/6 mice, C57BL/6 CFH−/− mice are uniformly susceptible to CSS with development of diffuse proliferative GN.14
Pharmacologically targeting the complement system is now a clinical reality. Because of the nature of complement activation, many inhibitors directed against a given complement protein also block successive steps in the activation pathway(s). For example, the anti-C5 antibody eculizumab prevents formation of C5a and C5b-9; the latter accounts for its therapeutic efficacy and now is in routine clinical use in paroxysmal nocturnal hemoglobinuria, as well as increased risks of Neisserial infections.15 Eculizumab is highly effective, but it is also the only complement inhibitor in clinical use. Thus, it has been used in a number of past and present clinical trials, including in membranous nephropathy, lupus nephritis, atypical and Shiga toxin–associated hemolytic uremic syndrome, C3GN, and anti-neutrophil autoantibody-mediated GN,16, 17, 18 diseases with a considerable range in phenotype. Identifying which among the limited number of active complement products are pathogenic in a given disease process is not a trivial point. Blocking those that are not directly relevant to the disease will needlessly impair normal immunity and potential counterregulatory (anti-inflammatory) signals, for which there is growing evidence.3
Here we addressed the role for C5aR in CSS-induced GN occurring in CFH−/− mice. Activation through C5aR appears to have several effects in this model, the net of which is necessary for disease.
RESULTS
C5aR-dependent acute kidney injury in CFH−/− mice with CSS
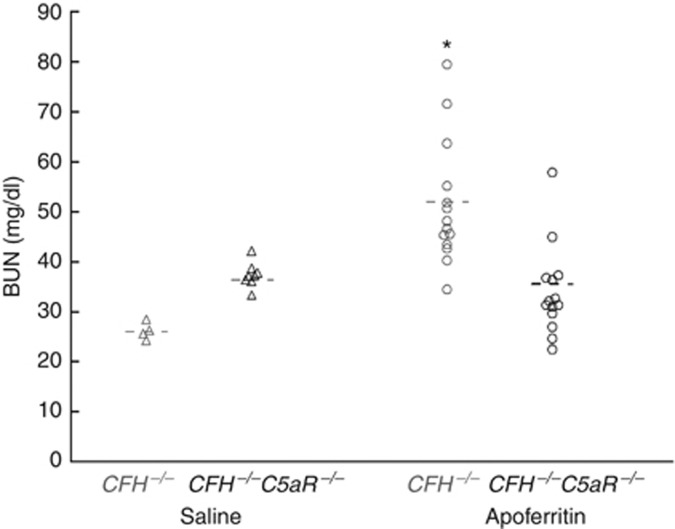
Here we used the CSS model induced with heterologous (apo)ferritin as originally described by Stilmant et al.19 and modified by Iskandar et al.20, 21 C57BL/6 CFH−/− and CFH−/−C5aR−/− mice (n=14 each) were actively immunized with daily intraperitoneal horse spleen apoferritin. As controls, CFH−/− (n=4) and CFH−/−C5aR−/− (n=8) mice were treated identically, except that apoferritin was omitted from the injections. As shown in Figure 1, control CFH−/− mice had normal renal function at the end of the 5-week experimental protocol as assessed by blood urea nitrogen (BUN) levels. In contrast, CFH−/− mice with CSS had impaired renal function (BUN=51.4±12.4 mg/dl), which was prevented in CFH−/−C5aR−/− mice (BUN=34.0±8.9 mg/dl; P<0.001 vs. CFH−/− mice). Thus, functional renal insufficiency in the CSS model requires both the absence of CFH and the presence of C5aR.
Figure 1.
C5aR-dependent impairment of renal function in CFH−/− mice with CSS. Blood urea nitrogen (BUN) levels were measured after 5 weeks of active immunization of CFH−/− and CFH−/−C5aR−/− mice with apoferritin or saline as controls. Data from each group were normally distributed. Shown are individual values from all mice studied, with the mean value in each group as a horizontal line. Groups were significantly different by analysis of variance (P<0.001). *P<0.001 vs. CFH−/−C5aR−/− plus apoferritin. CFH, complement factor H; CSS, chronic serum sickness.
C5aR dependence of renal inflammation in CFH−/− mice with CSS
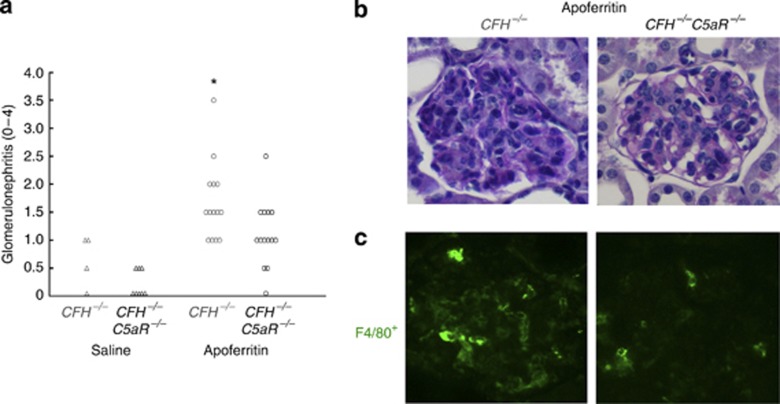
Histopathological features of GN were evaluated at the end of the 5-week experimental protocol. As in past studies,11, 14 some control CFH−/− mice had GN scores of 0.5–1.0, as was also true of control CFH−/−C5aR−/− mice (Figure 2a). CFH−/− mice with CSS developed GN, which was significantly reduced in CFH−/−C5aR−/− mice (Figure 2a and b; P=0.024). The primary histopathological feature was diffuse hypercellularity of the glomerular tufts (Figure 2b). The cellular composition of the observed GN was due, at least in part, to F4/80+ monocytic cells (Figure 2c). Thus, endocapillary diffuse proliferative GN in CSS appears to require absent complement regulation from CFH allowing signals through C5aR.
Figure 2.
Chronic serum sickness (CSS) leads to C5aR-dependent glomerulonephritis (GN) with macrophage infiltration in CFH−/− mice. Histopathological features of disease were determined after 5 weeks of active immunization of CFH−/− and CFH−/−C5aR−/− mice with apoferritin or saline as controls. (a) Semiquantitative GN scores from each mouse were compiled. Shown are individual values from all mice studied. Groups were significantly different by Kruskal–Wallis testing (P<0.001). *P=0.024 vs. CFH−/−C5aR−/− plus apoferritin. Representative periodic acid–Schiff staining (b) and immunofluorescence staining for F4/80+ cells (c) is shown for CFH−/− and CFH−/−C5aR−/− mice with CSS. Original magnification, × 400.
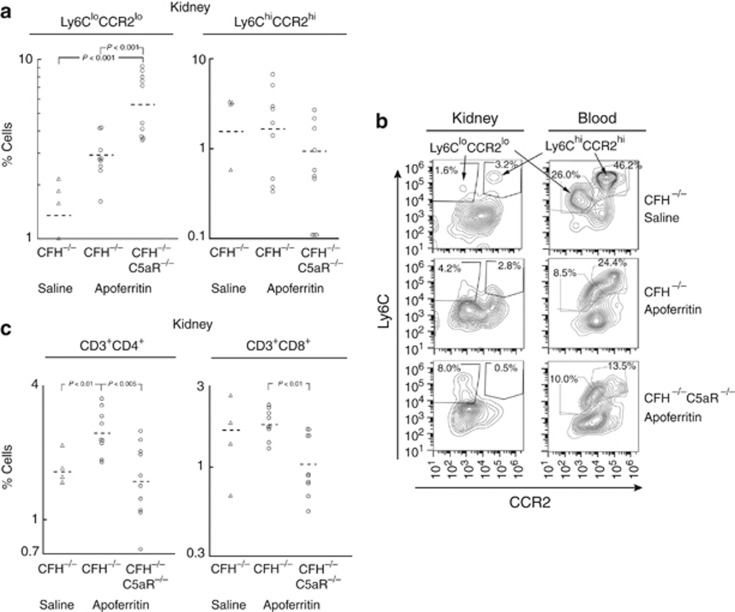
As typical for this model,14 there was no interstitial nephritis by histopathological criteria.22 To evaluate whether there were more subtle changes in mononuclear and lymphocytic cell populations within the interstitium, a separate group of animals was studied with CSS followed by flow cytometry. F4/80+Ly6C+ cells in tissue sites are considered macrophages, and can be divided by expression levels of Ly6C and CCR2 into inflammatory M1 (Ly6ChiCCR2hi) and anti-inflammatory (or ‘alternative') M2 (Ly6CloCCR2lo) macrophages.23, 24, 25 Saline-treated controls had few Ly6ChiCCR2hi cells in kidneys, which was also true for both groups of animals with CSS (Figure 3a and b). CFH−/−C5aR−/− mice with CSS had considerably increased intrarenal Ly6CloCCR2lo cells relative to CFH−/− control and CSS groups (Figure 3a and b). Consistent with an M2 macrophage phenotype, these cells were CD115+F4/80loCD11b+CD11clo as well; they were clearly distinct from CD115−F4/80hiCD11b+CD11c+ dendritic cells (Supplementary Information online).
Figure 3.
Role of C5aR in interstitial macrophage (a, b) and T lymphocyte (c) numbers in CFH−/− mice after 5 weeks of active immunization with apoferritin or saline as controls. The percentage of F4/80+ kidney cells that were Ly6ChiCCR2hi or Ly6CloCCR2lo is shown graphically in a with representative flow cytometry histograms shown in b. Peripheral blood mononuclear cells were studied in parallel, allowing comparisons with kidney cells. Representative data from kidney and blood are from the same animal. Similarly, the percentage of total isolated kidney cells that were CD3+CD4+ or CD3+CD8+ was determined (there was no CD3+CD4+CD8+ population; c). All log-transformed data were normally distributed and used in statistical analyses. Individual values from all mice studied are shown (a, c), with y-axes as log scales and means as horizontal lines. All data are derived from the same pool of cells and are directly comparable, whereas the y-axis scales were varied based on data. All groups but Ly6ChiCCR2hi cells had significantly different variances by analysis of variance (P<0.015). Significant differences in follow-up statistical comparisons are shown individually. CFH, complement factor H.
Peripheral blood F4/80+ cells from the same animals were also studied. Control CFH−/− mice receiving saline had distinct Ly6CloCCR2lo and Ly6ChiCCR2hi populations (Figure 3b, right panel), allowing comparison with those in the kidney (Figure 3b, left panel). Interestingly, both were reduced in CFH−/− mice with CSS, along with apparent shift of the Ly6CloCCR2lo population toward higher expression of both Ly6C and CCR2. As occurred in kidneys, CFH−/−C5aR−/− mice had fewer Ly6ChiCCR2hi cells, but greater numbers of Ly6CloCCR2lo cells, than CFH−/− mice with CSS.
The percentage of intrarenal CD19+ B lymphocytes tended to be increased in CSS, with values of 9.6 (6.0–13.5), 14.8 (11.0–18.8), and 14.6 (13.1–23.5) in CFH−/− controls, and CFH−/− and CFH−/−C5aR−/− mice with CSS, respectively (NS). Relative to controls, there were increased CD3+ T lymphocytes in kidneys from CFH−/− mice with CSS, accounted for by CD4+ cells (Figure 3c). In contrast, there was a decrease in CD8+ cells only in kidneys of CFH−/−C5aR−/− mice with CSS (Figure 3c). Thus, infiltration with both CD3+CD4+ and CD3+CD8+ T lymphocytes appears to have a C5aR dependence in the CSS model, with both being lower in kidneys of CFH−/−C5aR−/− mice compared with CFH−/− mice.
Effects of C5aR and altered humoral immune response on renal disease in CFH−/− mice with CSS
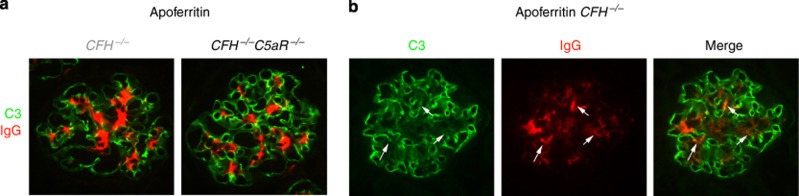
Glomeruli of CFH−/− mice with CSS had immunoglobulin G (IgG) within mesangial regions with some extension to peripheral capillary walls, which was significantly reduced in CFH−/−C5aR−/− mice with CSS (stained red in Figure 4); respective median staining scores were 3.0 (2.0–3.0) and 2.0 (1.5–2.5; P<0.015). As anticipated, glomeruli from CFH−/− mice had linear glomerular capillary wall staining for C3 (stained green in Figure 4). As we have seen in prior studies,14, 26, 27 the baseline bright C3 staining was reduced in areas of IgG staining (Figure 4b, white arrows). Besides immunofluorescence microscopy, our past studies have shown that glomerular capillary wall complement regulation is insufficient when plasma CFH is absent; this allows ongoing C3 activation by immune complexes.26
Figure 4.
Representative immunofluorescence staining for C3 (green) and immunoglobulin G (IgG, red) in glomeruli of CFH−/− and CFH−/−C5aR−/− mice with chronic serum sickness. (a) Linear glomerular capillary wall C3 staining typical for unmanipulated CFH−/− mice was present in both groups. Mesangial IgG was present in both groups, but in greater relative amounts and with more extension to the peripheral capillary wall in CFH−/− mice compared with CFH−/−C5aR−/− mice. (b) Individual staining for C3 and IgG are shown, along with the merged image. The arrows depict regions with colocalized C3 and IgG. CFH, complement factor H.
In the 12 control mice receiving saline, measured anti-horse apoferritin IgG levels were 0.1±0.03 U/ml (all ⩽0.3 U/ml), consistent with the specificity of the technique. Active immunization did lead to generation of anti-apoferritin antibodies, which were greater in actively immunized CFH−/− mice compared with CFH−/−C5aR−/− mice (5.3±0.1 and 3.9±0.1 U/ml, respectively; P<0.001). Consistent with our past studies,14 the anti-apoferritin humoral immune response was exaggerated in CFH−/− mice, as shown by comparison with anti-apoferritin IgG levels of 3.5±0.2 U/ml in five wild-type C57BL/6 mice studied as controls. Thus, excessive production of anti-apoferritin IgG in CFH−/− mice, which we presumed to have a complement dependence, is specifically C5aR dependent.
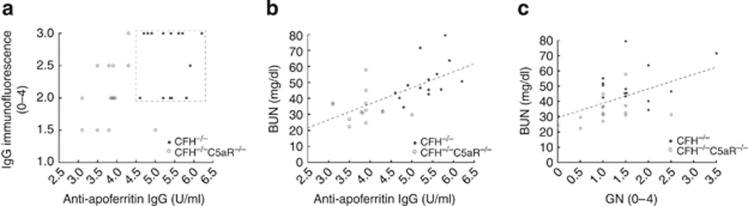
Measured anti-apoferritin IgG, glomerular IgG deposits, GN scores, and BUN values were all decreased in CSS in CFH−/−C5aR−/− mice relative to CFH−/− mice. We were interested to determine whether these variables were related; in particular, whether the observed effects of C5aR deficiency on renal disease could be attributed to reduction of anti-apoferritin IgG to wild-type levels. Glomerular IgG deposition positively correlated with serum anti-apoferritin IgG (r=0.46, P=0.013), with the two experimental groups being completely distinct in this analysis (as shown by the box in Figure 5a). Neither serum anti-apoferritin nor glomerular IgG had any relation to GN scores (r=0.27–0.37). BUN levels positively correlated with both anti-apoferritin IgG values (Figure 5b; r=0.65, P<0.001) and the extent of GN (Figure 5c; r=0.48, P=0.010). Each had an independent contribution to BUN levels, as shown by the equation [BUN]=−6.2 + 17.3 anti-apoferritin/2 + 6.5 GN (r=0.72, P<0.001); even though anti-apoferritin was weighted in half (to be comparable to GN scores), it remained the principal determinant of BUN. Thus, C5aR-dependent excessive production of anti-apoferritin IgG antibodies in CFH−/− mice tracks strongly with several end-organ disease features.
Figure 5.
Relationship between measured variables in CFH−/− (•) and CFH−/−C5aR−/− mice (○) after 5 weeks of chronic serum sickness. (a) Semiquantitative scores for glomerular immunoglobulin G (IgG; y-axis) were plotted against anti-apoferritin IgG levels (x-axis). The box enclosed by dashed lines separates the two groups completely. Measured blood urea nitrogen (BUN) concentrations (y-axes) were plotted against anti-apoferritin IgG levels (b) and semiquantitative glomerulonephritis scores (c, x-axes). The dashed lines are best-fit regression lines for data from both groups. CFH, complement factor H.
DISCUSSION
There is a body of evidence supporting the relevance of complement activation in immune complex GN.20, 28, 29 In the experimental CSS model, C57BL/6 mice are completely resistant to glomerular inflammation unless they lack CFH, in which case they are uniformly susceptible to GN.14 This is attributable to plasma-derived CFH facilitating inactivation of C3b in glomerular-bound immune complexes, which occurs even with large immune complex burdens.26 In these instances, despite the abundance of IgG and iC3b in glomeruli, which are respective ligands for inflammatory cell FcγRs and β2-integrins, there is no glomerular inflammation. Thus, inflammatory cell recruitment to glomeruli appears to require an additional ‘signal;' on the basis of considerable circumstantial evidence, we felt this likely to be a complement activation product. Here we confirm this premise and show that C5a acting through its cognate receptor C5aR is required for this immune complex GN model.
The relevance of C5a generation and C5aR signaling in acute glomerular neutrophil infiltration has been illustrated by C5-dependent, C6-independent nephrotoxic serum nephritis in CFH−/− mice12 and C5aR-dependent experimental anti-neutrophil autoantibody GN.30 Lipopolysaccharide is a potent inducer of C5aR expression in a variety of cells including those in the kidney,31, 32 which is attributable to CCAAT and CP2 sites in the C5aR promoter.33 Matsuo colleagues34 administered lipopolysaccharide along with nephrotoxic serum to generate a thrombotic microangiopathy model, which required complement activation and signals through C5aR. This model has features in common with experimental anti-phospholipid antibody syndrome, in which complement activation led to C5aR-dependent placental infiltration with neutrophils and subsequent fetal loss.35 Here, CFH−/− mice lacking C5aR had reduced functional (i.e., BUN) and histopathological features of GN. Compared with these acute models of passive antibody administration, CSS is a relatively chronic disease process. Thus, rather than neutrophils, C5aR signals were responsible for endocapillary GN with infiltrating F4/80+ macrophages.
The CSS model is induced through repetitive immunization with apoferritin alone (i.e., without adjuvant) to generate an active humoral immune response. As in our prior studies,14 CFH−/− mice with CSS had increased serum levels of anti-apoferritin IgG compared with wild-type controls. We can now attribute this to excessive generation and signaling through C5aR, given equivalent anti-apoferritin IgG titers among wild-type and CFH−/−C5aR−/− mice. It has become clear that C5aR signals can affect adaptive immune responses in a variety of ways. C5aR can promote Th1 responses in T cells36 and inhibit effects of interferon-γ in macrophages.37 Thus, Wenderfer et al.38 showed that relative to control MRL/Faslpr lupus mice C5aR−/−MRL/Faslpr mice had diminished production of interferon-γ and interleukin-12, reduced CD4+ T-cell responses, and 15-fold less anti-double-stranded DNA IgG2a antibodies, together with lessened glomerular endo- and extracapillary inflammation and protection from renal failure.
In CSS and MRL/Faslpr models of GN, antibodies to exogenous or endogenous antigens are formed. These generate complement-activating immune complexes that deposit in glomeruli from the circulation and/or are formed directly in situ. The fact that C3 and C4 are key in immune complex processing is well established,39 including in these two model systems.13, 27, 40 The potential relevance for C5aR signals in glomerular immune complex processing in CSS was supported by studies by Falk and Jennette,20 in which C5-deficient mice had less glomerular immune complexes independent from the anti-apoferritin IgG response. There are also lower quantities of glomerular immune complexes in C5aR-deficient MRL/Faslpr lupus mice compared with C5aR-sufficient controls.38 Consistent with these two studies are our findings that C5aR-deficient CFH−/− mice had lower serum anti-apoferritin and glomerular-deposited IgG, which were independent from each other. An elegant paradigm advanced and supported experimentally by Gessner and colleagues41 is that C5aR activation of Gαi2 and phosphoinositide 3-kinase p110γ in mononuclear phagocytes leads to a transcriptional increase in FcγRIII and decrease in FcγRIIB through respective gene promoter ‘C5a-inductive' and ‘C5a-suppressive' regions.41, 42, 43 This phenomenon is relevant in experimental disease models of liver, lung, and kidney (i.e., mesangial cells);44, 45, 46 clinically relevant is the fact that blocking C5aR signals may underlie the therapeutic efficacy of intravenous immunoglobulin.47 Thus, by altering the balance between activating and inhibitory FcγRs on monocytic cells, C5aR can have a considerable impact on immune complex processing and its downstream effects.
In studies by Welch et al.48 using the CSS model, mice given lipopolysaccharide contemporaneously developed C5aR-dependent tubulointerstitial nephritis. Although CFH is a critical glomerular capillary wall complement regulator, it appears to be dispensable in the tubulointerstitium.49, 50 Thus, in our studies described here, there was little interstitial inflammation and few Ly6ChiCCR2hi (M1) macrophages in all groups. The fact that CFH−/− but not CFH−/−C5aR−/− mice with CSS have increased interstitial CD3+CD4+ T lymphocytes relative to controls illustrates that this occurs in a C5aR-dependent manner, which is also true in MRL/Faslpr lupus mice.38 The fate of interstitial CD3+CD8+ T lymphocytes also appears to rely on C5aR, given that the reduction occurs only in CFH−/−C5aR−/− mice with CSS. M2 macrophages are alternative to M1 macrophages, as they tend to limit inflammation and promote repair within tissues.23, 24, 51 There were considerably more Ly6CloCCR2lo cells in the peripheral blood and renal interstitium of CFH−/−C5aR−/− mice with CSS, indicating a blocking effect of signals through C5aR. Our ability to distinguish M1 from M2 macrophages in glomeruli is limited; as best we can determine based on F4/80 staining, glomerular inflammation is due to M1 macrophages, whereas M2 macrophages are restricted to the renal interstitium. Thus, although M2 macrophage numbers correlate with protection from clinicopathological features of GN in this model, their role(s) in this disease is not clear. Theoretically, when complement activation and C5aR signaling cease, the accumulation of M2 macrophages could be important in preserving renal function in inflammatory diseases of the different renal compartments.52, 53, 54
Taking the current data together with our past work, glomeruli of wild-type C57BL/6 mice with CSS are normal despite the presence of abundant IgG- and iC3b-containing immune complexes. In the absence of plasma CFH, there can be active C3b to form C5 convertases. These generate sufficient quantities of C5a to provide necessary proinflammatory signals through C5aR to result in glomerular inflammation and the clinicopathological picture of GN. Downstream effects of C5aR include increasing FcγRIII quantities43 and αMβ2 integrin (complement receptor 3) ligand avidity,55, 56 allowing recognition of and activation by IgG/iC3b-bearing immune complexes in the glomerulus. Our data presented here suggest that the effects of C5aR in the active inflammatory CSS model extend beyond promoting glomerular infiltration with F4/80+ macrophages. Dissecting the individual effects of C5aR signals on immune and inflammatory responses, and how each impacts renal disease, does have its experimental impediments; in particular, cells of both afferent and efferent immune systems originate from bone marrow stem cells, making chimeric studies problematic. Our ongoing research is attempting to surmount these obstacles.
MATERIALS AND METHODS
Animals
CFH−/− and C5aR−/− mice were generated and kindly provided by Drs Matthew Pickering and Marina Botto (Imperial College of London)11 and Drs Allison Humbles and Craig Gerard (Harvard Medical School),57 respectively. Animals were backcrossed at least 10 generations onto normal C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). Genotyping within and around CFH and C5aR alleles was performed using PCR-based approaches. All studies were approved by the University of Chicago Institutional Animal Care and Use Committee.
Two separate sets of experiments were performed with a total of 63 mice equally divided between CFH−/− (n=31) and CFH−/−C5aR−/− (n=32) groups. Male mice at 8 weeks of age were studied. In each experiment, littermate animals of comparable ages were studied. CSS was induced with daily intraperitoneal administration of 4 mg horse spleen apoferritin (Calzyme Laboratories, San Luis Obispo, CA).14, 19, 20, 21 Controls (n=8 from each group) were treated identically, except that apoferritin was omitted from the saline vehicle. After 5 weeks, animals were killed, and blood and tissues were collected. BUN concentrations were measured with a Beckman Autoanalyzer (Fullerton, CA).
Measurements from renal tissue
Tissues were fixed in 10% buffered formalin and embedded in paraffin, from which 4-μm-thick sections were cut and stained with periodic acid–Schiff. Slides were scored in a blinded manner by a renal pathologist (AC) for the extent of GN and interstitial nephritis using scales of 0 to 4 (in increments of 0.5) according to the schema of Passwell et al.22 as described previously.14, 26
Sections (4 μm) from frozen mouse kidneys were fixed in ethanol/ether (1:1) for 10 min followed by 95% ethanol for 20 min, washed with phosphate-buffered saline, and stained with fluorescein anti-mouse C3 and rhodamine anti-mouse IgG (Cappel, MP Biomedicals, Solon, OH). For staining with Alexa 488 anti-mouse F4/80 (AbD Serotec, Raleigh, NC), sections were fixed twice with cold acetone (10 min, 4 °C) with intervening air-drying. Slides were viewed with an Olympus BX-60 IF microscope (Carter Valley, PA). Representative photomicrographs were taken at identical settings with a Hamamatsu EM-CCD camera (Bridgewater, NJ).
Renal-infiltrating cells and peripheral blood mononuclear cells were isolated as described previously.58 In brief, mouse kidneys were minced and digested at 37 °C for 25 min with gentle agitation with collagenase I (2 mg/ml) and DNAse I (100 mg/ml) in Hank's buffered salt solution/1% (vol/vol) bovine serum albumin (all from Sigma/Aldrich, St Louis, MO). Erythrocytes were lysed with NH4Cl, and the cell suspension was passed through a 40-μm cell strainer (BD Biosciences, Franklin Lakes, NJ). Isolated cells (∼106) from each mouse kidney and peripheral blood were blocked with mAb 24.G2 and stained with monoclonal antibodies (with labeled fluor in parentheses) to CD3 (brilliant violet), F4/80 (PE/Cy7), Ly6C (Alexa 488) (Biolegend, San Diego, CA), CD4 (fluorescein isothiocyanate), CD8 (allophycocyanin) (AbDSerotec), and CCR2 (phycoerythrin) (RnD Systems, Minneapolis, MN). Flow cytometry was performed with a BD Biosciences LSR II and analyzed with the FlowJo software (Tree Star, Ashland, OR).
Statistical methods
Data were analyzed using the Minitab statistical software (v. 16, College Park, MD). Data sets were first analyzed with the Anderson–Darling normality test and considered parametric with Hα>0.05. Data from flow cytometry were log-transformed. Parametric and nonparametric data are presented as mean±s.e.m. and median (Q1–Q3), respectively. All data presented graphically are from individual animals. Parametric data were analyzed by one-way analysis of variance with comparisons among groups using Fisher's method. Nonparametric data sets were analyzed comparably by Kruskal–Wallis and Mann–Whitney testing. Potential correlations among variables were first examined by calculating the Pearson product moment; those significant were further examined using the least-squares method.
Acknowledgments
This work was supported by National Institutes of Health grant R01DK041873 to RJQ and by a grant from Kidneeds to JJA.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1. Renal M2 macrophages in CSS.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Klco JM, Wiegand CB, Narzinski K, et al. Essential role for the second extracellular loop in C5a receptor activation. Nat Struct Mol Biol. 2005;12:320–326. doi: 10.1038/nsmb913. [DOI] [PubMed] [Google Scholar]
- Klco JM, Nikiforovich GV, Baranski TJ. Genetic analysis of the first and third extracellular loops of the C5a receptor reveals an essential WXFG motif in the first loop. J Biol Chem. 2006;281:12010–12019. doi: 10.1074/jbc.M600548200. [DOI] [PubMed] [Google Scholar]
- Sacks S. Complement fragments C3a and C5a: the salt and pepper of the immune response. Eur J Immunol. 2010;40:668–670. doi: 10.1002/eji.201040355. [DOI] [PubMed] [Google Scholar]
- Klos A, Tenner AJ, Johswich KO, et al. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich M, Martinez-Barricarte R, Francis NJ, et al. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc Natl Acad Sci USA. 2011;108:8761–8766. doi: 10.1073/pnas.1019338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de CS, Harris CL, Morgan BP, et al. Lessons from functional and structural analyses of disease-associated genetic variants in the complement alternative pathway. Biochim Biophys Acta. 2011;1812:12–22. doi: 10.1016/j.bbadis.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Martinez-Barricarte R, Heurich M, Valdes-Canedo F, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120:3702–3712. doi: 10.1172/JCI43343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri F, Fremeaux-Bacchi V, Noel LH, et al. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010;6:494–499. doi: 10.1038/nrneph.2010.85. [DOI] [PubMed] [Google Scholar]
- Saunders RE, barrategui-Garrido C, Fremeaux-Bacchi V, et al. The interactive Factor H-atypical hemolytic uremic syndrome mutation database and website: update and integration of membrane cofactor protein and Factor I mutations with structural models. Hum Mutat. 2007;28:222–234. doi: 10.1002/humu.20435. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- Pickering MC, Warren J, Rose KL, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg RJ, Lim A, Haas M, et al. Immune complex glomerulonephritis in C4- and C3-deficient mice. Kidney Int. 1998;53:320–330. doi: 10.1046/j.1523-1755.1998.00723.x. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Pickering MC, Haas M, et al. Complement factor H limits immune complex deposition and prevents inflammation and scarring in glomeruli of mice with chronic serum sickness. J Am Soc Nephrol. 2005;16:52–57. doi: 10.1681/ASN.2004090778. [DOI] [PubMed] [Google Scholar]
- Parker C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet. 2009;373:759–767. doi: 10.1016/S0140-6736(09)60001-5. [DOI] [PubMed] [Google Scholar]
- Mache CJ, Cham-Roschitz B, Fremeaux-Bacchi V, et al. Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:1312–1316. doi: 10.2215/CJN.01090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364:2561–2563. doi: 10.1056/NEJMc1100859. [DOI] [PubMed] [Google Scholar]
- Salant DJ. Targeting complement C5 in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2011;22:7–9. doi: 10.1681/ASN.2010111145. [DOI] [PubMed] [Google Scholar]
- Stilmant MM, Couser WG, Cotran RS. Experimental glomerulonephritis in the mouse associated with mesangial deposition of autologous ferritin immune complexes. Lab Invest. 1975;32:746–756. [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. Immune complex induced glomerular lesions in C5 sufficient and deficient mice. Kidney Int. 1986;30:678–686. doi: 10.1038/ki.1986.240. [DOI] [PubMed] [Google Scholar]
- Iskandar SS, Jennette JC, Wilkman AS, et al. Interstrain variations in nephritogenicity of heterologous protein in mice. Lab Invest. 1982;46:344–351. [PubMed] [Google Scholar]
- Passwell J, Schreiner GF, Nonaka M, et al. Local extrahepatic expression of complement genes C3, factor B, C2 and C4 is increased in murine lupus nephritis. J Clin Invest. 1988;82:1676–1684. doi: 10.1172/JCI113780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll MA, Platt AM, Potteaux S, et al. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt AM, Bain CC, Bordon Y, et al. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 2010;184:6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Aneziokoro OGB, Chang A, et al. Distinct and separable roles of the complement system in factor H-deficient bone marrow chimeric mice with immune complex disease. J Am Soc Nephrol. 2006;17:1354–1361. doi: 10.1681/ASN.2006020138. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Wang Y, Chang A, et al. Mouse podocyte complement factor H—the functional analogue to human complement receptor 1. J Am Soc Nephrol. 2007;18:1157–1166. doi: 10.1681/ASN.2006101125. [DOI] [PubMed] [Google Scholar]
- Couser WG, Johnson RJ, Young BA, et al. The effects of soluble recombinant complement receptor 1 on complement-mediated experimental glomerulonephritis. J Am Soc Nephrol. 1995;5:1888–1894. doi: 10.1681/ASN.V5111888. [DOI] [PubMed] [Google Scholar]
- Puri TS, Quigg RJ. The many effects of complement C3- and C5-binding proteins in renal injury. Semin Nephrol. 2007;27:321–337. doi: 10.1016/j.semnephrol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Schreiber A, Xiao H, Jennette JC, et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviland DL, McCoy RL, Whitehead WT, et al. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–1869. [PubMed] [Google Scholar]
- Drouin SM, Kildsgaard J, Haviland J, et al. Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol. 2001;166:2025–2032. doi: 10.4049/jimmunol.166.3.2025. [DOI] [PubMed] [Google Scholar]
- Hunt JR, Martin CB, Martin BK. Transcriptional regulation of the murine C5a receptor gene: NF-Y is required for basal and LPS induced expression in macrophages and endothelial cells. Mol Immunol. 2005;42:1405–1415. doi: 10.1016/j.molimm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Kondo C, Mizuno M, Nishikawa K, et al. The role of C5a in the development of thrombotic glomerulonephritis in rats. Clin Exp Immunol. 2001;124:323–329. doi: 10.1046/j.1365-2249.2001.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon JE, Girardi G. The role of complement in the antiphospholipid syndrome. Curr Dir Autoimmun. 2004;7:133–148. doi: 10.1159/000075690. [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Hawlisch H, Belkaid Y, Baelder R, et al. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wenderfer SE, Ke B, Hollmann TJ, et al. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol. 2005;16:3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
- Hebert LA. The clearance of immune complexes from the circulation of man and other primates. Am J Kidney Dis. 1991;27:352–361. doi: 10.1016/s0272-6386(12)80488-4. [DOI] [PubMed] [Google Scholar]
- Sekine H, Reilly CM, Molano ID, et al. Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J Immunol. 2001;166:6444–6451. doi: 10.4049/jimmunol.166.10.6444. [DOI] [PubMed] [Google Scholar]
- Konrad S, Ali SR, Wiege K, et al. Phosphoinositide 3-kinases gamma and delta, linkers of coordinate C5a receptor-Fcgamma receptor activation and immune complex-induced inflammation. J Biol Chem. 2008;283:33296–33303. doi: 10.1074/jbc.M804617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad S, Engling L, Schmidt RE, et al. Characterization of the murine IgG Fc receptor III and IIB gene promoters: a single two-nucleotide difference determines their inverse responsiveness to C5a. J Biol Chem. 2007;282:37906–37912. doi: 10.1074/jbc.M707937200. [DOI] [PubMed] [Google Scholar]
- Skokowa J, Ali SR, Felda O, et al. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J Immunol. 2005;174:3041–3050. doi: 10.4049/jimmunol.174.5.3041. [DOI] [PubMed] [Google Scholar]
- Kumar V, Ali SR, Konrad S, et al. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke HH, Janssen-Graalfs I, Sowa EN, et al. Opposite regulation of type II and III receptors for immunoglobulin G in mouse glomerular mesangial cells and in the induction of anti-glomerular basement membrane (GBM) nephritis. J Biol Chem. 2002;277:27535–27544. doi: 10.1074/jbc.M200419200. [DOI] [PubMed] [Google Scholar]
- Shushakova N, Skokowa J, Schulman J, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgRs in immune complex-induced lung disease. J Clin Invest. 2002;110:1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad S, Baumann U, Schmidt RE, et al. Intravenous immunoglobulin (IVIG)-mediated neutralisation of C5a: a direct mechanism of IVIG in the maintenance of a high Fc gammaRIIB to Fc gammaRIII expression ratio on macrophages. Br J Haematol. 2006;134:345–347. doi: 10.1111/j.1365-2141.2006.06185.x. [DOI] [PubMed] [Google Scholar]
- Welch TR, Frenzke M, Witte D, et al. C5a is important in the tubulointerstitial component of experimental immune complex glomerulonephritis. Clin Exp Immunol. 2002;13:43–48. doi: 10.1046/j.1365-2249.2002.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Wang Y, Chang A, et al. Unrestricted C3 activation occurs in Crry-deficient kidneys which rapidly leads to chronic renal failure. J Am Soc Nephrol. 2007;18:811–822. doi: 10.1681/ASN.2006101176. [DOI] [PubMed] [Google Scholar]
- Bao L, Wang Y, Haas M, et al. Distinct roles for C3a and C5a in complement-induced tubulointerstitial injury. Kidney Int. 2011;80:524–534. doi: 10.1038/ki.2011.158. [DOI] [PubMed] [Google Scholar]
- Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- Ricardo SD, van GH, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Harris DC. Macrophages in renal disease. J Am Soc Nephrol. 2011;22:21–27. doi: 10.1681/ASN.2010030269. [DOI] [PubMed] [Google Scholar]
- Berger M, Budhu S, Lu E, et al. Different G(i)-coupled chemoattractant receptors signal qualitatively different functions in human neutrophils. J Leukoc Biol. 2002;71:798–806. [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- Hopken UE, Lu B, Gerard NP, et al. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J Exp Med. 1997;186:749–756. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Haas M, Pippin J, et al. Focal and segmental glomerulosclerosis induced in mice lacking decay-accelerating factor in T cells. J Clin Invest. 2009;119:1264–1274. doi: 10.1172/JCI36000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.