Abstract
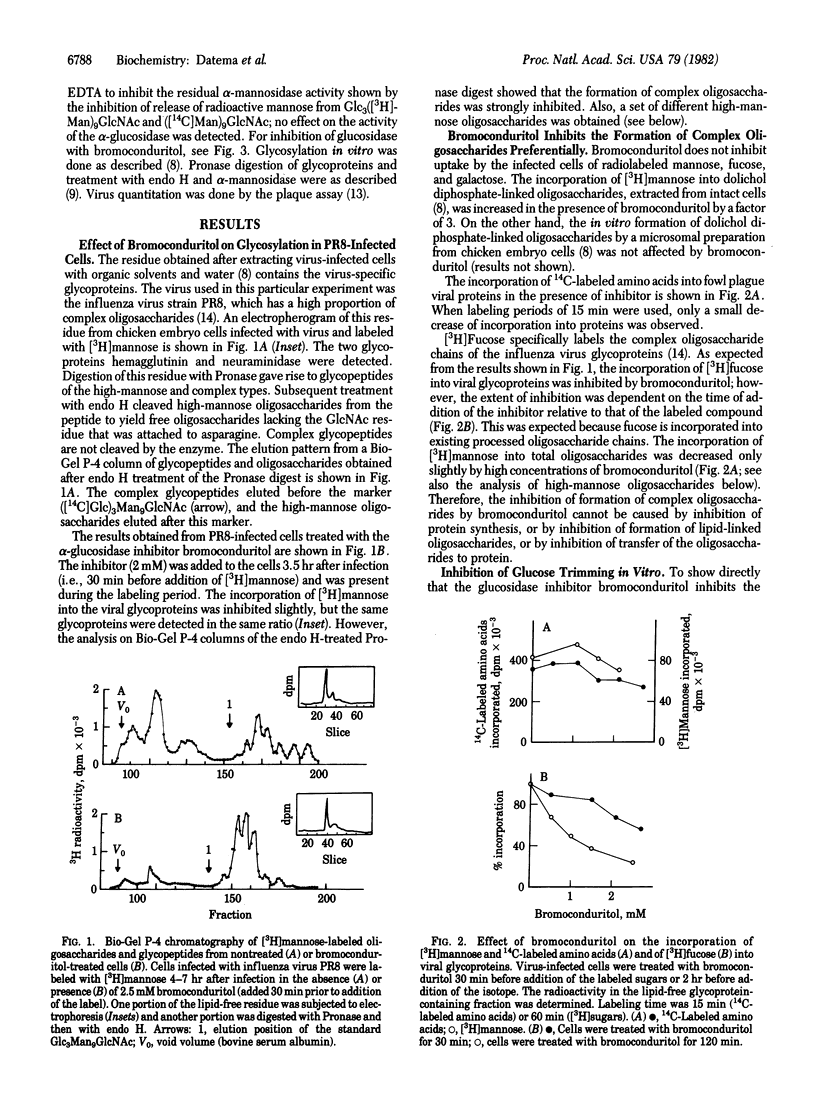
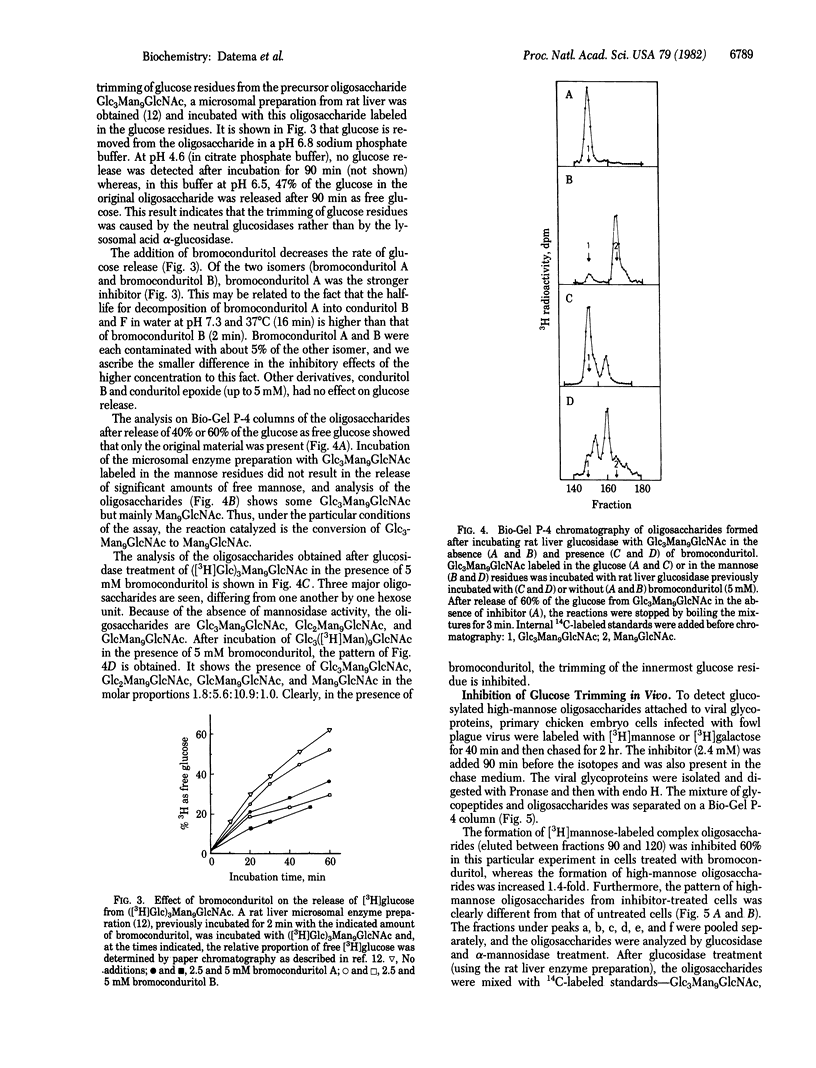
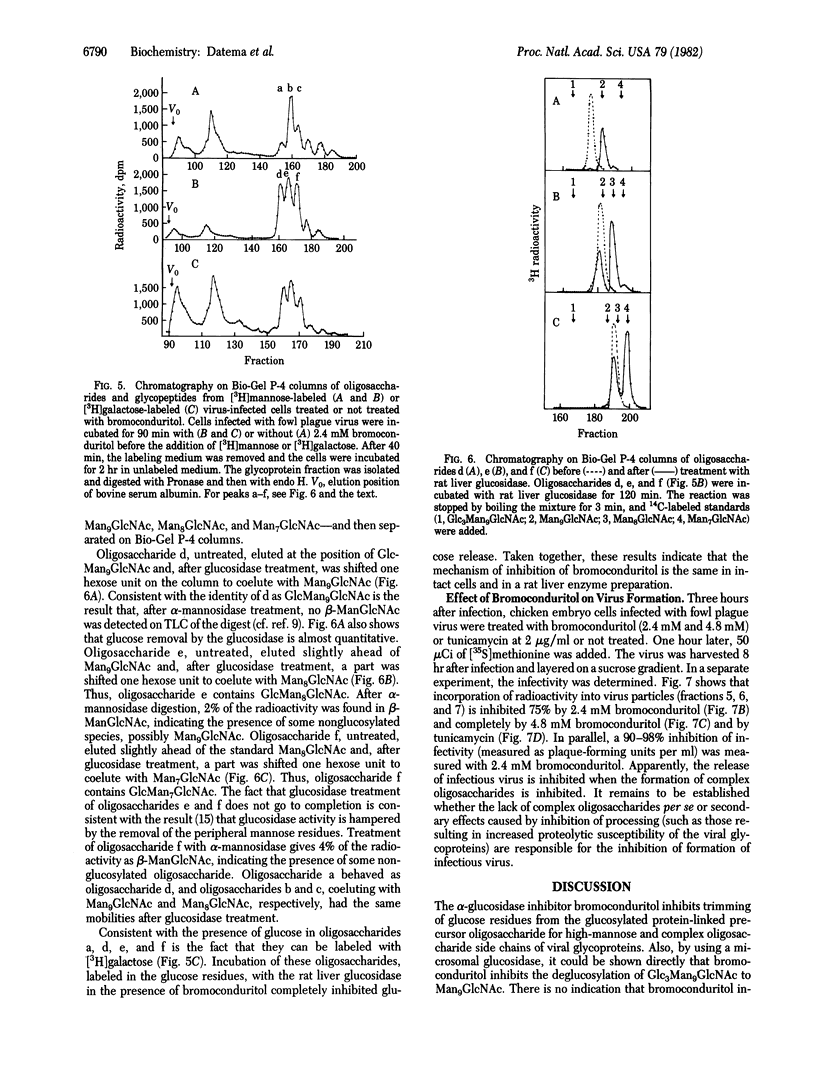
The alpha-glucosidase inhibitor bromoconduritol (6-bromo-3,4,5-trihydroxycyclohex-1-ene) inhibits trimming of the innermost glucose residue from the Glc3Man9GlcNAc2 precursor of high-mannose and complex oligosaccharides. This inhibition occurs both in intact cells and with a microsomal enzyme preparation. The formation of lipid-linked oligosaccharides was increased in glucosidase-inhibited cells. Inhibition of transfer of high-mannose oligosaccharides to protein was not observed. In bromoconduritol-treated virus-infected cells, trimming of mannose can occur despite incomplete removal of glucose. The glucosylated high-mannose oligosaccharides GlcMan9GlcNAc, GlcMan8GlcNAc, and GlcMan7GlcNAc were released from viral glycoproteins after digestion with Pronase and endo-beta-N-acetylglucosaminidase H. The formation of complex oligosaccharides was concomitantly inhibited. The release of infectious fowl plague virus particles (an influenza virus) was inhibited from bromoconduritol-treated infected chicken-embryo cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- DAVENPORT F. M., ROTT R., SCHAEFER W. Physical and biological properties of influenza virus components obtained after ether treatment. J Exp Med. 1960 Nov 1;112:765–782. doi: 10.1084/jem.112.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Schwarz R. T. Effect of energy depletion on the glycosylation of a viral glycoprotein. J Biol Chem. 1981 Nov 10;256(21):11191–11198. [PubMed] [Google Scholar]
- Datema R., Schwarz R. T., Jankowski A. W. Fluoroglucose-inhibition of protein glycosylation in vivo. Inhibition of mannose and glucose incorporation into lipid-linked oligosaccharides. Eur J Biochem. 1980 Aug;109(2):331–341. doi: 10.1111/j.1432-1033.1980.tb04799.x. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Legler G. Glucosidases. Methods Enzymol. 1977;46:368–381. doi: 10.1016/s0076-6879(77)46044-0. [DOI] [PubMed] [Google Scholar]
- Legler G., Lotz W. Untersuchungen zum Wirkungsmechanismus glykosidspaltender Enzyme. VII. Funktionelle Gruppen am aktiven Zentrum einer alpha-Glucosidase aus Saccharomyces cerevisiae. Hoppe Seylers Z Physiol Chem. 1973 Mar;354(3):243–254. [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. Inhibition of the dolichol pathway of protein glycosylation. Methods Enzymol. 1982;83:432–443. doi: 10.1016/0076-6879(82)83041-3. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Klenk H. D. Carbohydrates of influenza virus. IV. Strain-dependent variations. Virology. 1981 Sep;113(2):584–593. doi: 10.1016/0042-6822(81)90186-0. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J., Bhoyroo V. D. Processing of carbohydrate units of glycoproteins. Characterization of a thyroid glucosidase. J Biol Chem. 1979 Aug 25;254(16):7659–7667. [PubMed] [Google Scholar]
- Ugalde R. A., Staneloni R. J., Leloir L. F. Microsomal glucosidases of rat liver. Partial purification and inhibition by disaccharides. Eur J Biochem. 1980 Dec;113(1):97–103. doi: 10.1111/j.1432-1033.1980.tb06144.x. [DOI] [PubMed] [Google Scholar]



