Abstract
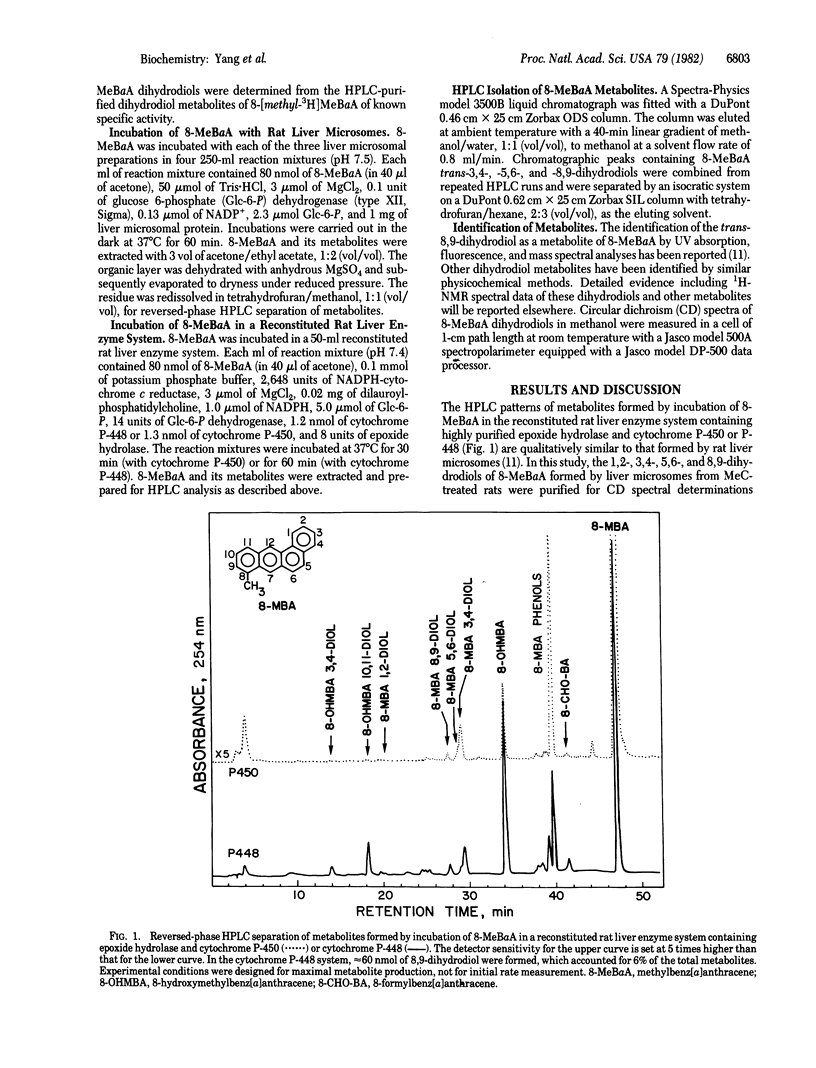
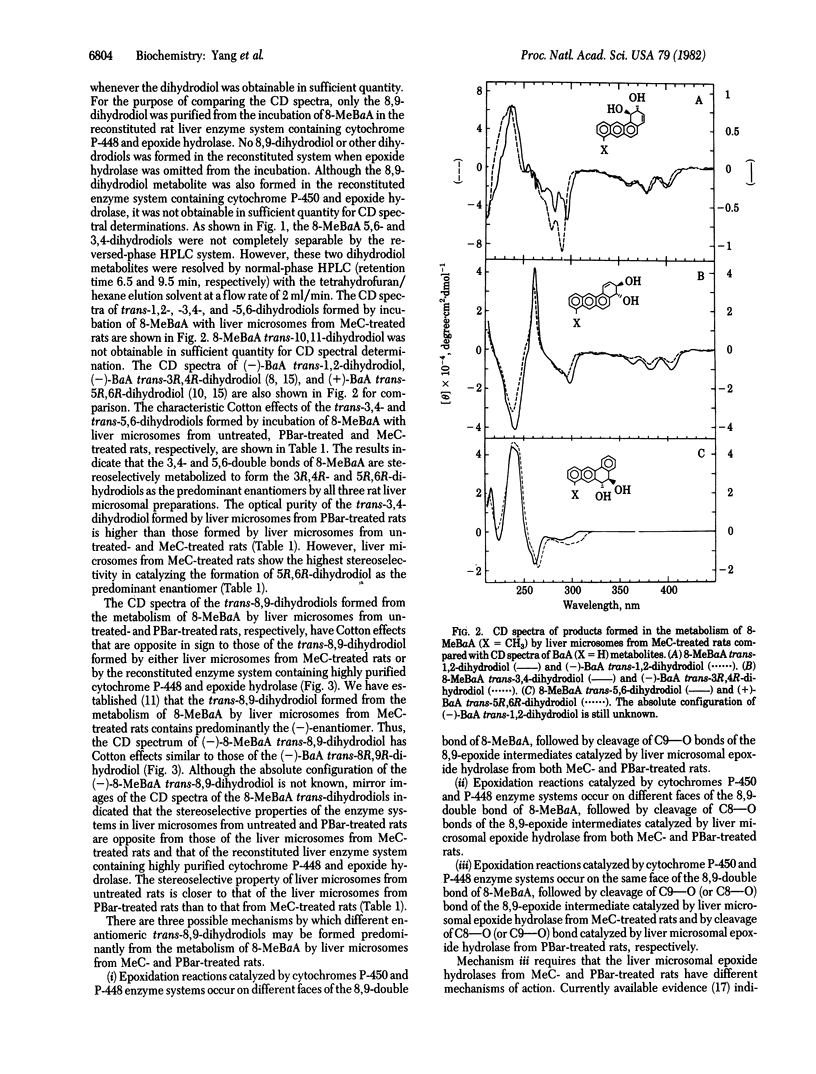
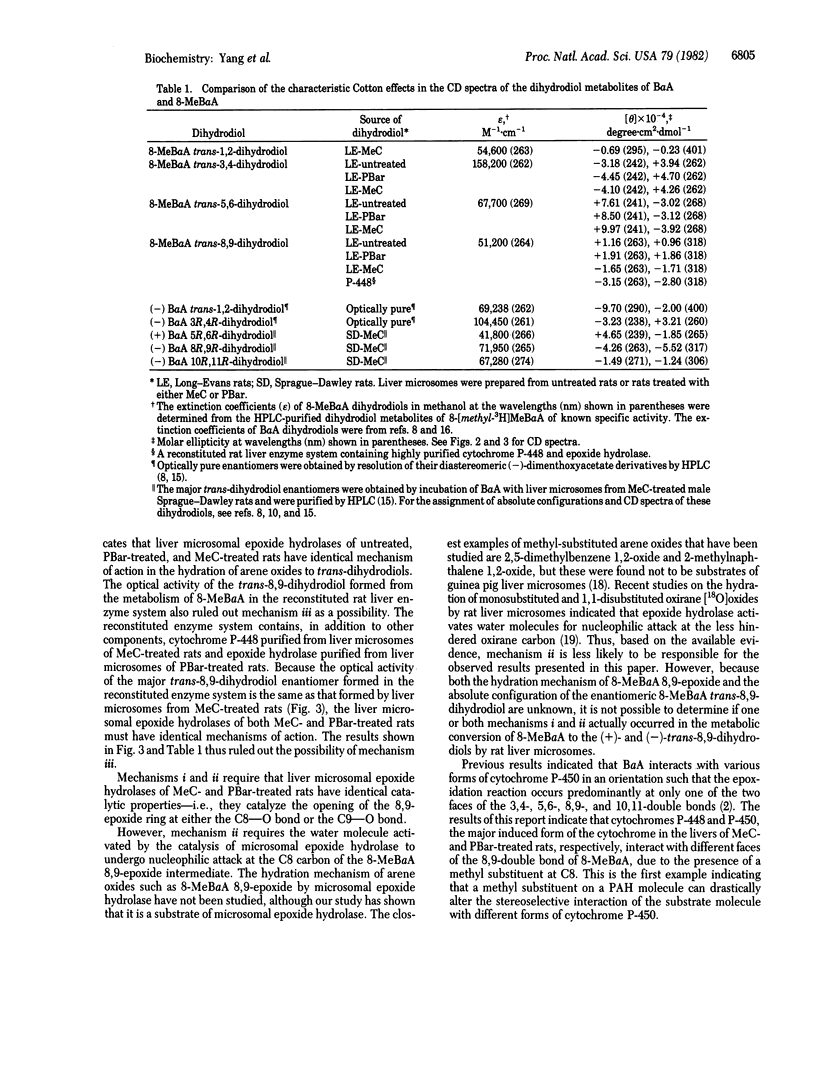
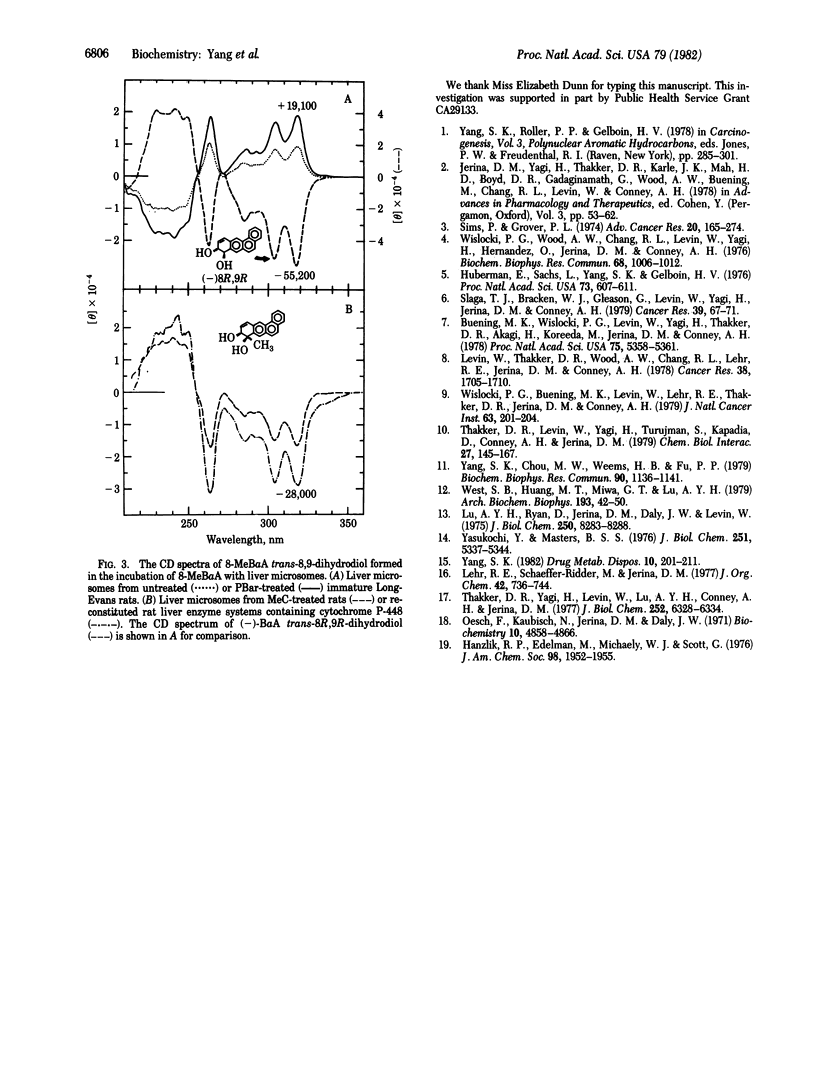
8-Methylbenz[a]anthracene (8-MeBaA) transdihydrodiol metabolites were isolated by reversed-phase and normal-phase HPLCs from incubations of 8-MeBaA with liver microsomes or a reconstituted system containing purified cytochrome P-448 and epoxide hydrolase. Regardless of the enzyme source, the metabolically formed 8-MeBaA trans-3,4- and -5,6-dihydrodiols were found to be enriched in one enantiomeric isomer and differed only in the degree of optical purity. The 8-MeBaA trans-8,9-dihydrodiol formed by liver microsomes from either untreated or phenobarbital-treated rats was enriched with the (+)-enantiomer. In contrast, the 8-MeBaA trans-8,9-dihydrodiol formed either by liver microsomes from 3-methylcholanthrene-treated rats or by the reconstituted rat liver enzyme system containing cytochrome P-448 and epoxide hydrolase was enriched with the (-)enantiomer. These results indicate that, in catalyzing the formation of 3,4- and 5,6-epoxide intermediates, the interaction with the unsubstituted 3,4- and 5,6-double bonds of 8-MeBaA by the different forms of cytochrome P-450 occur preferentially on the same face of the aromatic plane and they differ only in the degree of stereoselectivity. However, different forms of cytochrome P-450 may interact with different faces of the aromatic plane at the methyl-substituted 8,9-double bond of 8-MeBaA, resulting in the formation of trans-8,9-dihydrodiols enriched in different enantiomeric forms. This demonstrates that different forms of cytochrome P-450 may catalyze the epoxidation reaction preferentially at different sides of the methyl-substituted double bond of a planar polycyclic hydrocarbon molecule. These properties may be used to further classify and to understand the enzyme-substrate interactions of the different forms of cytochrome P-450 in the drug-metabolizing enzyme systems.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buening M. K., Wislocki P. G., Levin W., Yagi H., Thakker D. R., Akagi H., Koreeda M., Jerina D. M., Conney A. H. Tumorigenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides in newborn mice: exceptional activity of (+)-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5358–5361. doi: 10.1073/pnas.75.11.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlik R. P., Edelman M., Michaely W. J., Scott G. Enzymatic hydration of (18O)epoxides. Role of nucleophilic mechanisms. J Am Chem Soc. 1976 Mar 31;98(7):1952–1955. doi: 10.1021/ja00423a050. [DOI] [PubMed] [Google Scholar]
- Huberman E., Sachs L., Yang S. K., Gelboin V. Identification of mutagenic metabolites of benzo(a)pyrene in mammalian cells. Proc Natl Acad Sci U S A. 1976 Feb;73(2):607–611. doi: 10.1073/pnas.73.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin W., Thakker D. R., Wood A. W., Chang R. L., Lehr R. E., Jerina D. M., Conney A. H. Evidence that benzo(a)anthracene 3,4-diol-1,2-epoxide is an ultimate carcinogen on mouse skin. Cancer Res. 1978 Jun;38(6):1705–1710. [PubMed] [Google Scholar]
- Lu A. Y., Ryan D., Jerina D. M., Daly J. W., Levin W. Liver microsomal expoxide hydrase. Solubilization, purification, and characterization. J Biol Chem. 1975 Oct 25;250(20):8283–8288. [PubMed] [Google Scholar]
- Oesch F., Kaubisch N., Jerina D. M., Daly J. W. Hepatic epoxide hydrase. Structure-activity relationships for substrates and inhibitors. Biochemistry. 1971 Dec 21;10(26):4858–4866. doi: 10.1021/bi00802a005. [DOI] [PubMed] [Google Scholar]
- Sims P., Grover P. L. Epoxides in polycyclic aromatic hydrocarbon metabolism and carcinogenesis. Adv Cancer Res. 1974;20:165–274. doi: 10.1016/s0065-230x(08)60111-6. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Bracken W. J., Gleason G., Levin W., Yagi H., Jerina D. M., Conney A. H. Marked differences in the skin tumor-initiating activities of the optical enantiomers of the diastereomeric benzo(a)pyrene 7,8-diol-9,10-epoxides. Cancer Res. 1979 Jan;39(1):67–71. [PubMed] [Google Scholar]
- Thakker D. R., Levin W., Yagi H., Turujman S., Kapadia D., Conney A. H., Jerina D. M. Absolute stereochemistry of the trans-dihydrodiols formed from benzo[a]anthracene by liver microsomes. Chem Biol Interact. 1979 Oct;27(2-3):145–161. doi: 10.1016/0009-2797(79)90122-4. [DOI] [PubMed] [Google Scholar]
- Thakker D. R., Yagi H., Levin W., Lu A. Y., Conney A. H., Jerina D. M. Stereospecificity of microsomal and purified epoxide hydrase from rat liver. Hydration of arene oxides of polycyclic hydrocarbons. J Biol Chem. 1977 Sep 25;252(18):6328–6334. [PubMed] [Google Scholar]
- West S. B., Huang M. T., Miwa G. T., Lu A. Y. A simple and rapid procedure for the purification of phenobarbital-inducible cytochrome P-450 from rat liver microsomes. Arch Biochem Biophys. 1979 Mar;193(1):42–50. doi: 10.1016/0003-9861(79)90006-7. [DOI] [PubMed] [Google Scholar]
- Wislocki P. G., Buening M. K., Levin W., Lehr R. E., Thakker D. R., Jerina D. M., Conney A. H. Tumorigenicity of the diastereomeric benz[a]anthracene 3,4-diol-1,2-epoxides and the (+)- and (-)-enantiomers of benz[a]anthracene 3,4-dihydrodiol in newborn mice. J Natl Cancer Inst. 1979 Jul;63(1):201–204. [PubMed] [Google Scholar]
- Wislocki P. G., Wood A. W., Chang R. L., Levin W., Yagi H., Hernandez O., Jerina D. M., Conney A. H. High mutagenicity and toxicity of a diol epoxide derived from benzo(a)pyrene. Biochem Biophys Res Commun. 1976 Feb 9;68(3):1006–1012. doi: 10.1016/0006-291x(76)91246-8. [DOI] [PubMed] [Google Scholar]
- Yang S. K., Chou M. W., Weems H. B., Fu P. P. Enzymatic formation of an 8,9-diol from 8-methylbenz[a]anthracene. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1136–1141. doi: 10.1016/0006-291x(79)91154-9. [DOI] [PubMed] [Google Scholar]
- Yang S. K. The absolute stereochemistry of the major trans-dihydrodiol enantiomers formed from 11-methylbenz[a]anthracene by rat liver microsomes. Drug Metab Dispos. 1982 May-Jun;10(3):205–211. [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]


