Abstract
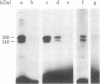
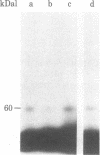
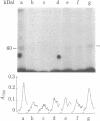
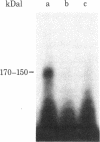
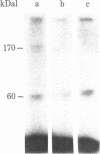
Membrane components that interact with epidermal growth factor (EGF) and transforming growth factors (TGFs) have been identified by covalent crosslinking to their respective 125I-labeled ligands. Under appropriate conditions, disuccinimidyl suberate or hydroxysuccinimidyl p-azidobenzoate cross-link receptor-bound 125I-labeled EGF to a 140- to 170-kilodalton (kDal) receptor species in membranes from both A431 human carcinoma cells and normal rat kidney cells. 125I-Labeled sarcoma growth factor (SGF), a TGF from virally transformed mouse 3T3 cells, also can be affinity-crosslinked to the 140- to 170-kDal EGF receptor species in membranes from A431 and rat kidney cells. The labeling of this receptor is inhibited when either excess unlabeled EGF or SGF is present during incubation of membranes with either 125I-labeled EGF or 125I-labeled SGF. In contrast, a second receptor species of 60 kDal is affinity-labeled with 125I-labeled SGF but not with 125I-labeled EGF in membranes from both A431 and rat kidney cells. SGF and a TGF from virally transformed rat embryo cells inhibit the labeling of the 60-kDal species when present in excess during incubation of membranes with 125I-labeled SGF, whereas EGF is completely ineffective in inhibiting the labeling of this receptor. The data suggest that a specific 60-kDal receptor that displays high affinity for TGFs but not for EGF may mediate induction of the transformed phenotype. In addition, SGF and other TGFs interact with the 140- to 170-kDal EGF receptor that appears to mediate normal cell growth effects.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Comens P. G., Simmer R. L., Baker J. B. Direct linkage of 125I-EGF to cell surface receptors. A useful artifact of chloramine-T treatment. J Biol Chem. 1982 Jan 10;257(1):42–45. [PubMed] [Google Scholar]
- De Larco J. E., Preston Y. A., Todaro G. J. Properties of a sarcoma-growth-factor-like peptide from cells transformed by a temperature-sensitive sarcoma virus. J Cell Physiol. 1981 Oct;109(1):143–152. doi: 10.1002/jcp.1041090116. [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Reynolds R., Carlberg K., Engle C., Todaro G. J. Sarcoma growth factor from mouse sarcoma virus-transformed cells. Purification by binding and elution from epidermal growth factor receptor-rich cells. J Biol Chem. 1980 Apr 25;255(8):3685–3690. [PubMed] [Google Scholar]
- De Larco J. E., Todaro G. J. Sarcoma growth factor (SGF): specific binding to epidermal growth factor (EGF) membrane receptors. J Cell Physiol. 1980 Feb;102(2):267–277. doi: 10.1002/jcp.1041020218. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Nexø E., Hollenberg M. D. Solubilization and isolation of the human placenta receptor for epidermal growth factor-urogastrone. J Biol Chem. 1980 Nov 25;255(22):10737–10743. [PubMed] [Google Scholar]
- Johnson G. L., MacAndrew V. I., Jr, Pilch P. F. Identification of the glucagon receptor in rat liver membranes by photoaffinity crosslinking. Proc Natl Acad Sci U S A. 1981 Feb;78(2):875–878. doi: 10.1073/pnas.78.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Fox C. F. Controlled proteolysis of EGF receptors: evidence for transmembrane distribution of the EGF binding and phosphate acceptor sites. J Supramol Struct. 1980;14(4):461–471. doi: 10.1002/jss.400140405. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J. Human transforming growth factor. Production by a melanoma cell line, purification, and initial characterization. J Biol Chem. 1982 May 10;257(9):5220–5225. [PubMed] [Google Scholar]
- Massague J., Guillette B. J., Czech M. P. Affinity labeling of multiplication stimulating activity receptors in membranes from rat and human tissues. J Biol Chem. 1981 Mar 10;256(5):2122–2125. [PubMed] [Google Scholar]
- Massague J., Guillette B. J., Czech M. P., Morgan C. J., Bradshaw R. A. Identification of a nerve growth factor receptor protein in sympathetic ganglia membranes by affinity labeling. J Biol Chem. 1981 Sep 25;256(18):9419–9424. [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. A unique proteolytic cleavage site on the beta subunit of the insulin receptor. J Biol Chem. 1981 Apr 10;256(7):3182–3190. [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Ozanne B., Fulton R. J., Kaplan P. L. Kirsten murine sarcoma virus transformed cell lines and a spontaneously transformed rat cell-line produce transforming factors. J Cell Physiol. 1980 Oct;105(1):163–180. doi: 10.1002/jcp.1041050118. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Interaction of cross-linking agents with the insulin effector system of isolated fat cells. Covalent linkage of 125I-insulin to a plasma membrane receptor protein of 140,000 daltons. J Biol Chem. 1979 May 10;254(9):3375–3381. [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Todaro G. J., Fryling C., Stephenson J. R. Human transforming growth factors induce tyrosine phosphorylation of EGF receptors. Nature. 1981 Jul 16;292(5820):259–262. doi: 10.1038/292259a0. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Frolik C. A., Marquardt H., Todaro G. J., Sporn M. B. Isolation from murine sarcoma cells of novel transforming growth factors potentiated by EGF. Nature. 1982 Feb 4;295(5848):417–419. doi: 10.1038/295417a0. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Fryling C., Johnson P. A., Sporn M. B. Transforming growth factors (TGFs): properties and possible mechanisms of action. J Supramol Struct Cell Biochem. 1981;15(3):287–301. doi: 10.1002/jsscb.1981.380150306. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]