Abstract
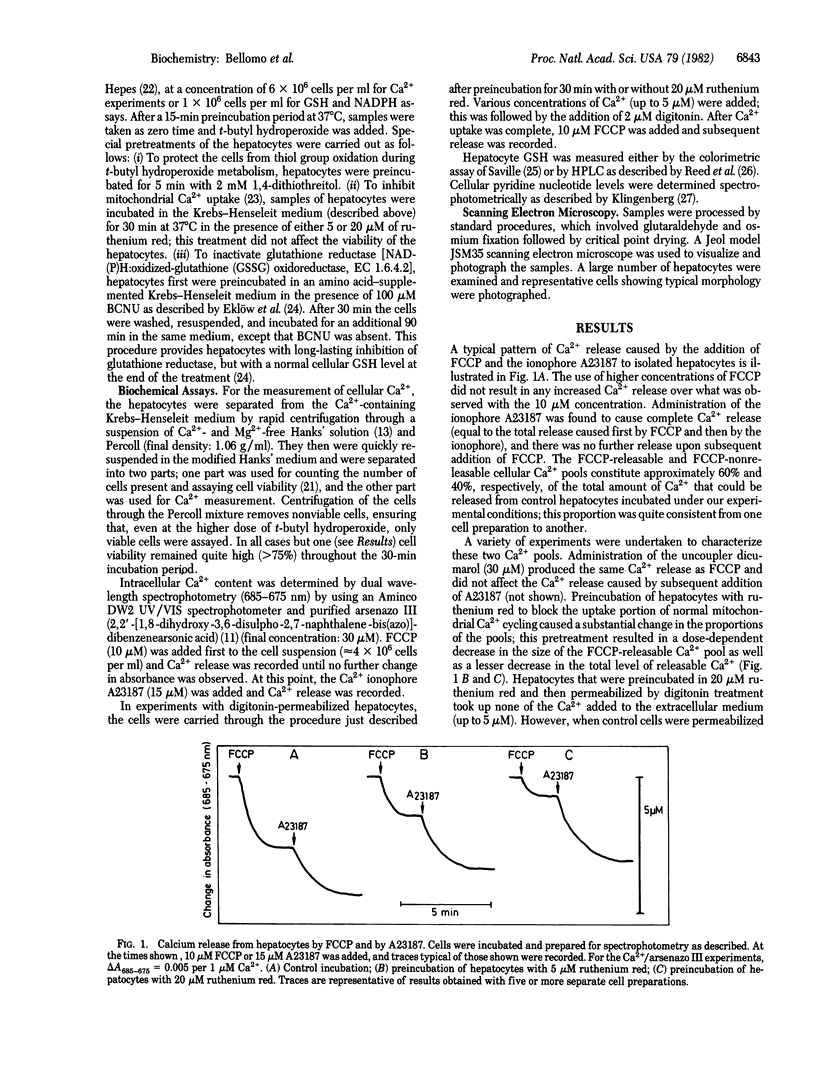
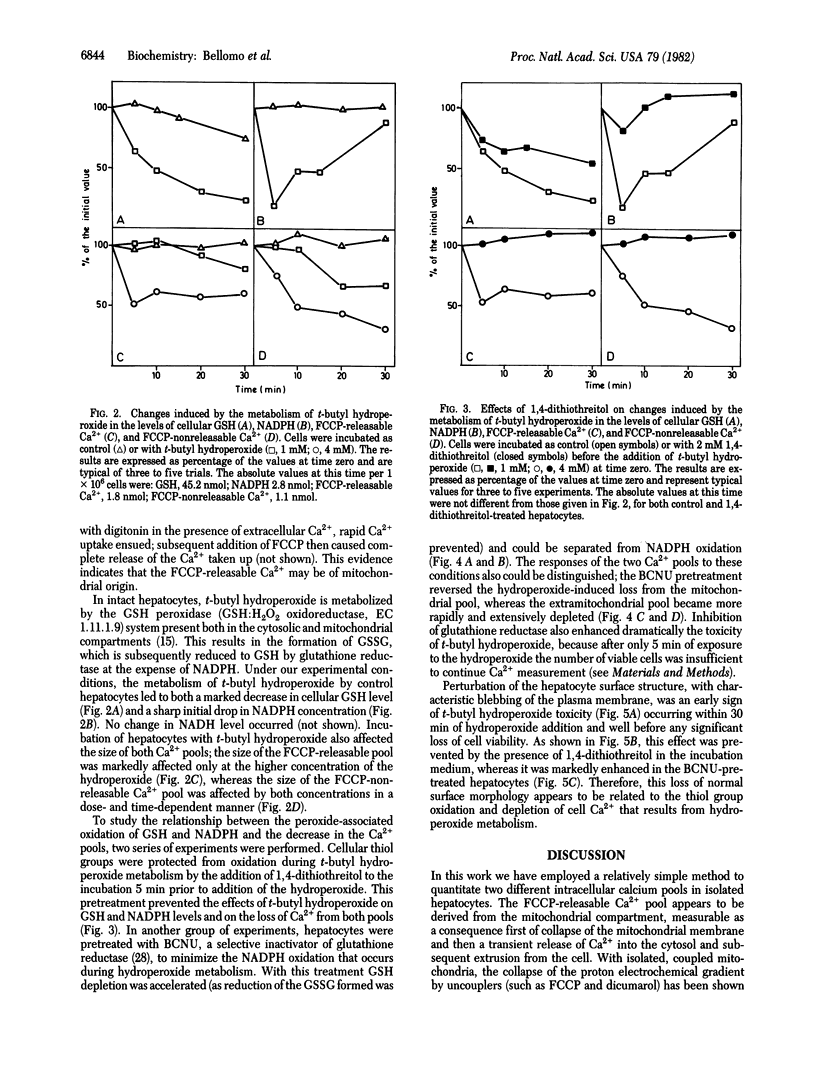
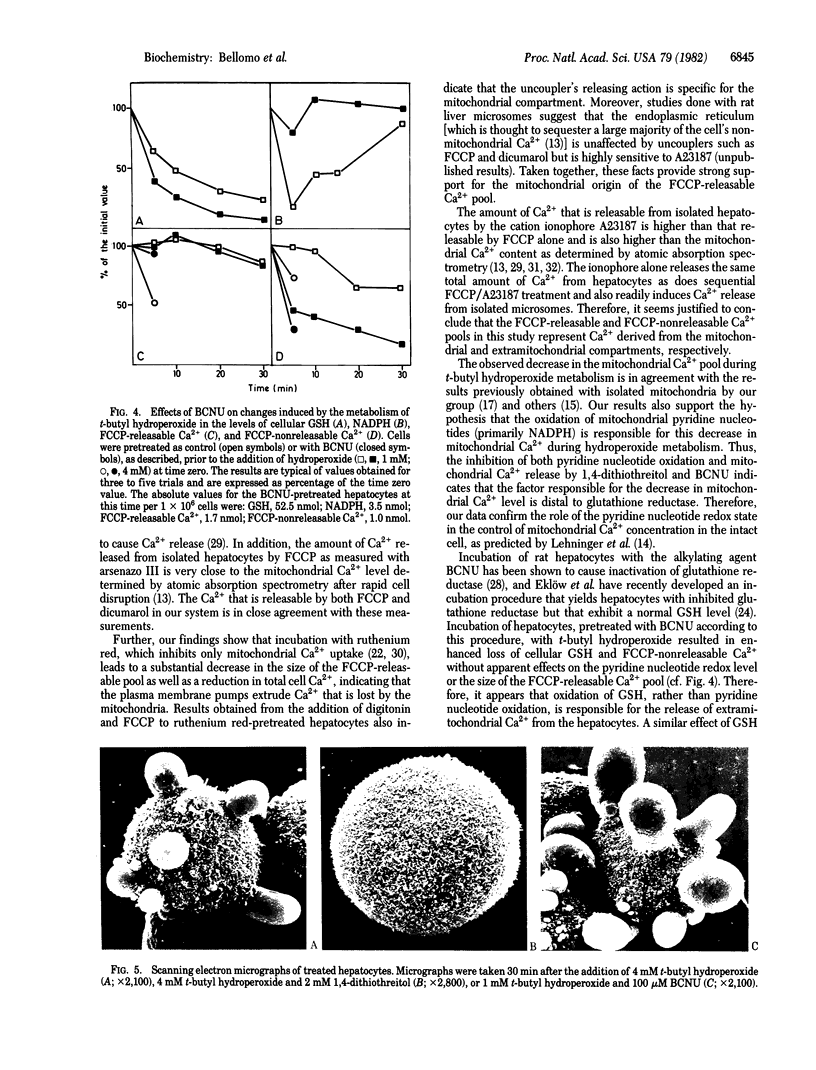
In suspensions of isolated hepatocytes, two intracellular Ca2+ pools were distinguished in the presence of the metallochrome indicator arsenazo III, first by treatment with the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) and then with the Ca2+ ionophore A23187. The available evidence indicates that the two pools are of mitochondrial and extramitochondrial origin. Metabolism of t-butyl hydroperoxide by hepatocytes caused release of Ca2+ from both compartments concomitant with oxidation of cellular glutathione and NADPH, which was followed by characteristic alterations in cell surface structure. When NADPH oxidation was prevented by selective inactivation of glutathione reductase, t-butyl hydroperoxide metabolism was without effect on the mitochondrial Ca2+ pool, whereas the loss from the extramitochondrial pool was accelerated. Our results suggest that different regulatory mechanisms modulate mitochondrial (NADPH-dependent) and extramitochondrial (thiol-dependent) Ca2+ compartmentation and that disturbance of normal Ca2+ homeostasis may be critical in peroxide-induced cytotoxicity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Bygrave F. L. Mitochondria and the control of intracellular calcium. Biol Rev Camb Philos Soc. 1978 Feb;53(1):43–79. doi: 10.1111/j.1469-185x.1978.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Bygrave F. L. Properties of energy-dependent calcium transport by rat liver microsomal fraction as revealed by initial-rate measurements. Biochem J. 1978 Jan 15;170(1):87–91. doi: 10.1042/bj1700087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret-Berthon B., Claret M., Mazet J. L. Fluxes and distribution of calcium in rat liver cells: kinetic analysis and identification of pools. J Physiol. 1977 Nov;272(3):529–552. doi: 10.1113/jphysiol.1977.sp012058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky W. P., Cockrell R. S. Ca2+ transport across plasma and mitochondrial membranes of isolated hepatocytes. FEBS Lett. 1975 Nov 1;59(1):39–43. doi: 10.1016/0014-5793(75)80336-x. [DOI] [PubMed] [Google Scholar]
- Högberg J., Kristoferson A. A correlation between glutathione levels and cellular damage in isolated hepatocytes. Eur J Biochem. 1977 Mar 15;74(1):77–82. doi: 10.1111/j.1432-1033.1977.tb11368.x. [DOI] [PubMed] [Google Scholar]
- Jewell S. A., Bellomo G., Thor H., Orrenius S., Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982 Sep 24;217(4566):1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- Kendrick N. C., Ratzlaff R. W., Blaustein M. P. Arsenazo III as an indicator for ionized calcium in physiological salt solutions: its use for determination of the CaATP dissociation constant. Anal Biochem. 1977 Dec;83(2):433–450. doi: 10.1016/0003-2697(77)90052-5. [DOI] [PubMed] [Google Scholar]
- Kleineke J., Stratman F. W. Calcium transport in isolated rat hepatocytes. FEBS Lett. 1974 Jul 1;43(1):75–80. doi: 10.1016/0014-5793(74)81109-9. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Vercesi A., Bababunmi E. A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxides can modulate the redox state of pyridine nucleotides and the calcium balance in rat liver mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4340–4344. doi: 10.1073/pnas.76.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Axelsson K., Larson K. Thioltransferase. Methods Enzymol. 1981;77:281–285. doi: 10.1016/s0076-6879(81)77038-1. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Moore L., Chen T., Knapp H. R., Jr, Landon E. J. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975 Jun 25;250(12):4562–4568. [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Orrenius S., Thor H., Eklöw L., Moldéus P., Jones D. P. Drug-stimulated H2O2 formation in hepatocytes. Possible toxicological implications. Adv Exp Med Biol. 1981;136(Pt A):395–405. doi: 10.1007/978-1-4757-0674-1_28. [DOI] [PubMed] [Google Scholar]
- Prpić V., Bygrave F. L. On the inter-relationship between glucagon action, the oxidation-reduction state of pyridine nucleotides, and calcium retention by rat liver mitochondria. J Biol Chem. 1980 Jul 10;255(13):6193–6199. [PubMed] [Google Scholar]
- Reed D. J., Babson J. R., Beatty P. W., Brodie A. E., Ellis W. W., Potter D. W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- Sies H., Graf P., Estrela J. M. Hepatic calcium efflux during cytochrome P-450-dependent drug oxidations at the endoplasmic reticulum in intact liver. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3358–3362. doi: 10.1073/pnas.78.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Oshino N., Chance B., Silver I. A. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys. 1978 Nov;191(1):8–22. doi: 10.1016/0003-9861(78)90062-0. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Chemical modification studies on the Ca2+-dependent protein modulator: the role of methionine residues in the activation of cyclic nucleotide phosphodiesterase. Biochemistry. 1978 Sep 19;17(19):3924–3928. [PubMed] [Google Scholar]




