Abstract
Lateral mobility of an active fluorescent derivative of cytochrome c on the membranes of giant mitochondria was measured by fluorescence redistribution after photobleaching. A diffusion coefficient of 1.6 X 10(-10) cm2/sec was determined for the labeled cytochrome c on inner mitochondrial membranes under conditions where succinate oxidase activity was demonstrated. This relatively low rate of diffusion, together with results of other investigators, is explained in terms of a model involving a dynamic equilibrium between freely diffusing and associated forms of electron-transfer components.
Full text
PDF
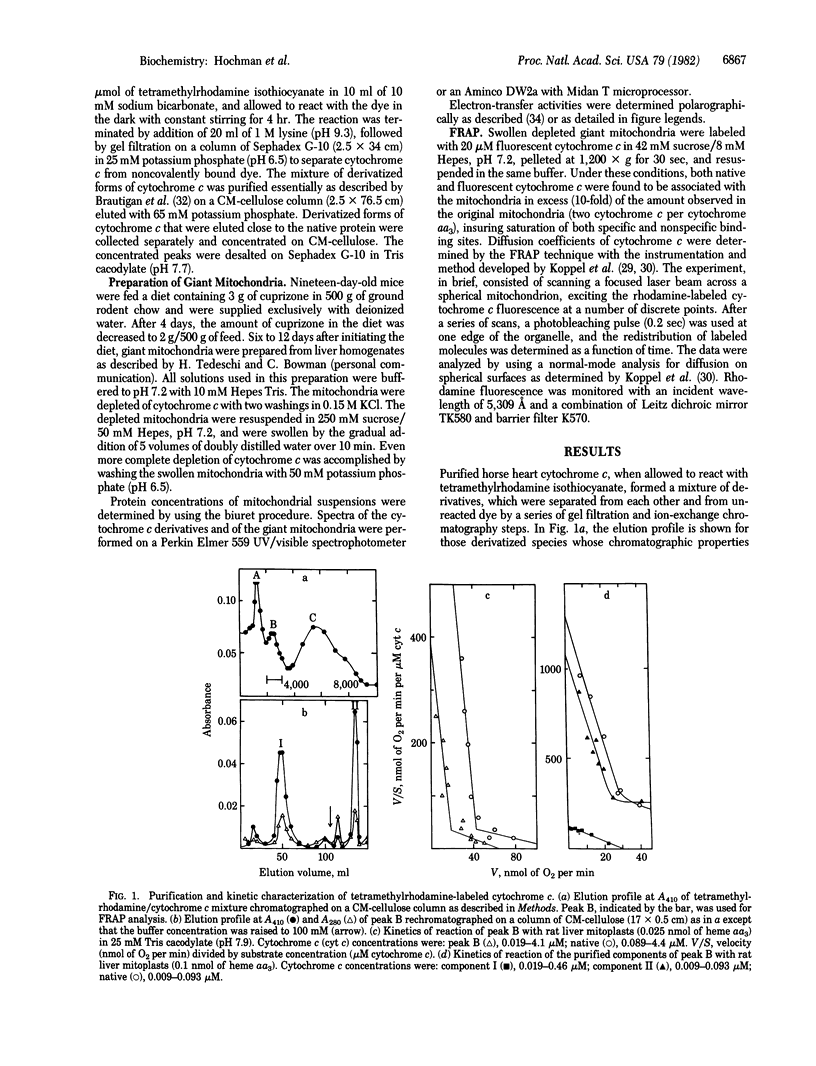
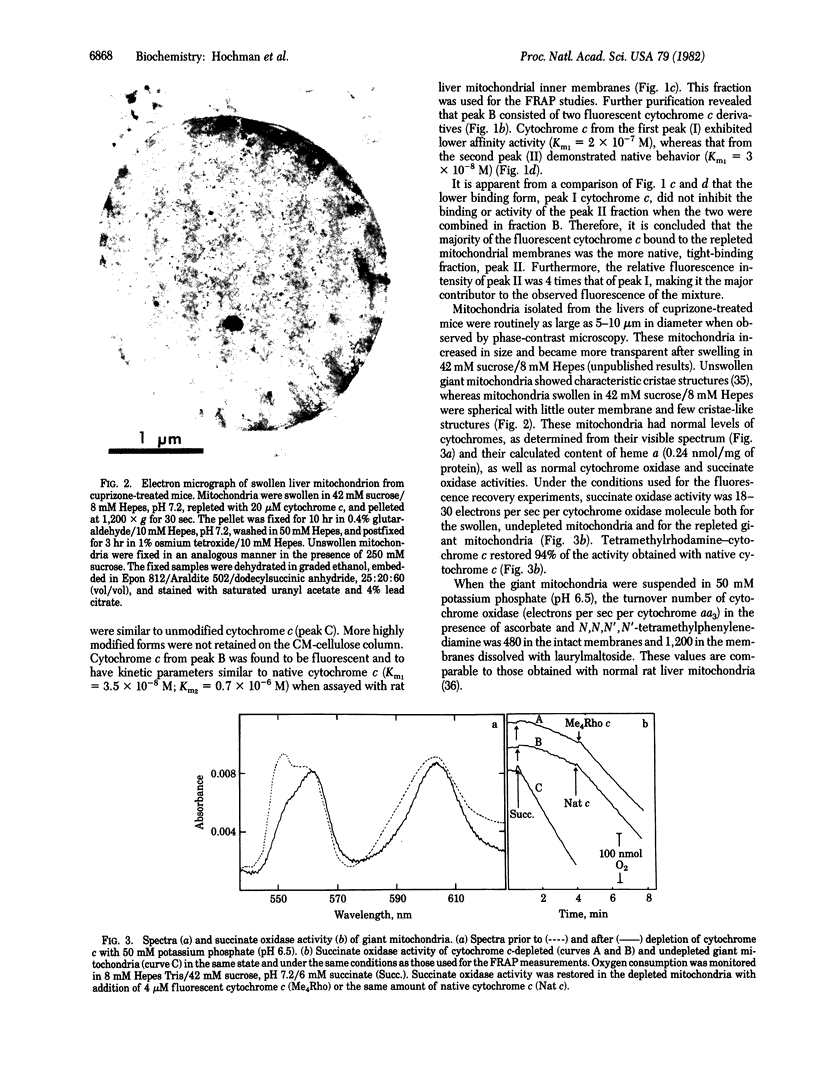
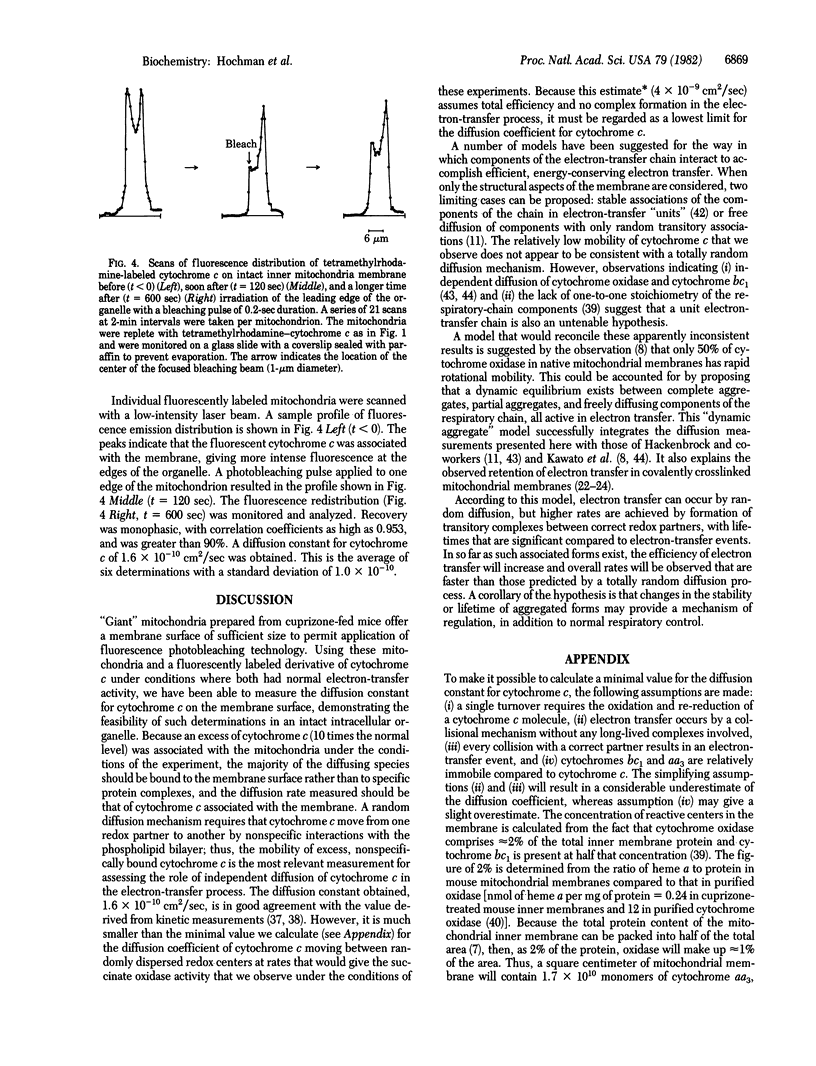

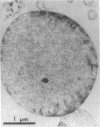
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. J., Smith H. T., Smith M. B., Millett F. S. Effect of specific lysine modification on the reduction of cytochrome c by succinate-cytochrome c reductase. Biochemistry. 1978 Jun 27;17(13):2479–2483. doi: 10.1021/bi00606a003. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson R., Gutweniger H., Montecucco C., Colonna R., Zanotti A., Azzi A. Covalent binding of arylazido derivatives of cytochrome c to cytochrome oxidase. FEBS Lett. 1977 Sep 1;81(1):147–150. doi: 10.1016/0014-5793(77)80948-4. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Definition of cytochrome c binding domains by chemical modification. I. Reaction with 4-chloro-3,5-dinitrobenzoate and chromatographic separation of singly substituted derivatives. J Biol Chem. 1978 Jan 10;253(1):130–139. [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–164. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Tarr G. E., Margoliash E. Definition of cytochrome c binding domains by chemical modification. II. Identification and properties of singly substituted carboxydinitrophenyl cytochromes c at lysines 8, 13, 22, 27, 39, 60, 72, 87, and 99. J Biol Chem. 1978 Jan 10;253(1):140–148. [PubMed] [Google Scholar]
- Chance B. The function of cytochrome c. Ann N Y Acad Sci. 1974 Feb 18;227:613–626. doi: 10.1111/j.1749-6632.1974.tb14425.x. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Vanderkooi J. M., Wilson D. F. Cytochrome c interactions with membranes. A photoaffinity-labeled cytochrome c. Arch Biochem Biophys. 1975 Nov;171(1):108–116. doi: 10.1016/0003-9861(75)90013-2. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Fuller S. D., Capaldi R. A., Henderson R. Structure of cytochrome c oxidase in deoxycholate-drived two-dimensional crystals. J Mol Biol. 1979 Oct 25;134(2):305–327. doi: 10.1016/0022-2836(79)90037-8. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Darley-Usmar V. M., Capaldi R. A. Covalent complex between yeast cytochrome c and beef heart cytochrome c oxidase which is active in electron transfer. Biochemistry. 1981 Nov 24;20(24):7046–7053. doi: 10.1021/bi00527a043. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., FOWLER L. R., GRIFFITHS D. E. Studies on the electron transfer system. XLII. Reconstitution of the electron transfer system. J Biol Chem. 1962 Aug;237:2661–2669. [PubMed] [Google Scholar]
- Hanski E., Rimon G., Levitzki A. Adenylate cyclase activation by the beta-adrenergic receptors as a diffusion-controlled process. Biochemistry. 1979 Mar 6;18(5):846–853. doi: 10.1021/bi00572a017. [DOI] [PubMed] [Google Scholar]
- Kawato S., Sigel E., Carafoli E., Cherry R. J. Cytochrome oxidase rotates in the inner membrane of intact mitochondria and submitochondrial particles. J Biol Chem. 1980 Jun 25;255(12):5508–5510. [PubMed] [Google Scholar]
- Kawato S., Sigel E., Carafoli E., Cherry R. J. Rotation of cytochrome oxidase in phospholipid vesicles. Investigations of interactions between cytochrome oxidases and between cytochrome oxidase and cytochrome bc1 complex. J Biol Chem. 1981 Jul 25;256(14):7518–7527. [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Lateral diffusion in biological membranes. A normal-mode analysis of diffusion on a spherical surface. Biophys J. 1980 Apr;30(1):187–192. doi: 10.1016/S0006-3495(80)85087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol W. H., Margoliash E. The asymmetric distribution of charges on the surface of horse cytochrome c. Functional implications. J Biol Chem. 1982 Apr 25;257(8):4426–4437. [PubMed] [Google Scholar]
- Nicholls P. Cytochrome c binding to enzymes and membranes. Biochim Biophys Acta. 1974 Dec 30;346(3-4):261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- Overfield R. E., Wraight C. A. Oxidation of cytochromes c and c2 by bacterial photosynthetic reaction centers in phospholipid vesicles. 1. Studies with neutral membranes. Biochemistry. 1980 Jul 8;19(14):3322–3327. doi: 10.1021/bi00555a034. [DOI] [PubMed] [Google Scholar]
- Overfield R. E., Wraight C. A. Oxidation of cytochromes c and c2 by bacterial photosynthetic reaction centers in phospholipid vesicles. 2. Studies with negative membranes. Biochemistry. 1980 Jul 8;19(14):3328–3334. doi: 10.1021/bi00555a035. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. Cytochrome bc1 and cytochrome oxidase can bind to the same surface domain of the cytochrome c molecule. FEBS Lett. 1978 Aug 15;92(2):223–226. doi: 10.1016/0014-5793(78)80759-5. [DOI] [PubMed] [Google Scholar]
- Roberts H., Hess B. Kinetics of cytochrome c oxidase from yeast. Membrane-facilitated electrostatic binding of cytochrone c showing a specific interaction with cytochrome c oxidase and inhibition by ATP. Biochim Biophys Acta. 1977 Oct 12;462(1):215–234. doi: 10.1016/0005-2728(77)90204-3. [DOI] [PubMed] [Google Scholar]
- Rosevear P., VanAken T., Baxter J., Ferguson-Miller S. Alkyl glycoside detergents: a simpler synthesis and their effects on kinetic and physical properties of cytochrome c oxidase. Biochemistry. 1980 Aug 19;19(17):4108–4115. doi: 10.1021/bi00558a032. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M. Transfer of glucagon receptor from liver membranes to a foreign adenylate cyclase by a membrane fusion procedure. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1174–1178. doi: 10.1073/pnas.76.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A. E., Hackenbrock C. R. Rate of lateral diffusion of intramembrane particles: measurement by electrophoretic displacement and rerandomization. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6246–6250. doi: 10.1073/pnas.78.10.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Ferguson-Miller S., Osheroff N., Margoliash E. Definition of cytochrome c binding domains by chemical modification: kinetics of reaction with beef mitochondrial reductase and functional organization of the respiratory chain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):155–159. doi: 10.1073/pnas.76.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandler B., Hoppel C. L. Division of giant mitochondria during recovery from cuprizone intoxication. J Cell Biol. 1973 Jan;56(1):266–272. doi: 10.1083/jcb.56.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. A., Suárez-Villafañe M., Ferguson-Miller S. The active form of cytochrome c oxidase: effects of detergent, the intact membrane, and radiation inactivation. Biophys J. 1982 Jan;37(1):285–293. doi: 10.1016/S0006-3495(82)84677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi K., Packer L. Glutaraldehyde-fixed mitochondria. I. Enzyme activity, ion translocation, and conformational changes. Arch Biochem Biophys. 1967 Sep;121(3):633–640. doi: 10.1016/0003-9861(67)90048-3. [DOI] [PubMed] [Google Scholar]
- Waring A., Davis J. S., Chance B., Erecińska M. Low temperature kinetic studies on rat liver mitochondria containing covalently linked derivatives of cytochrome c. J Biol Chem. 1980 Jul 10;255(13):6212–6218. [PubMed] [Google Scholar]