Abstract
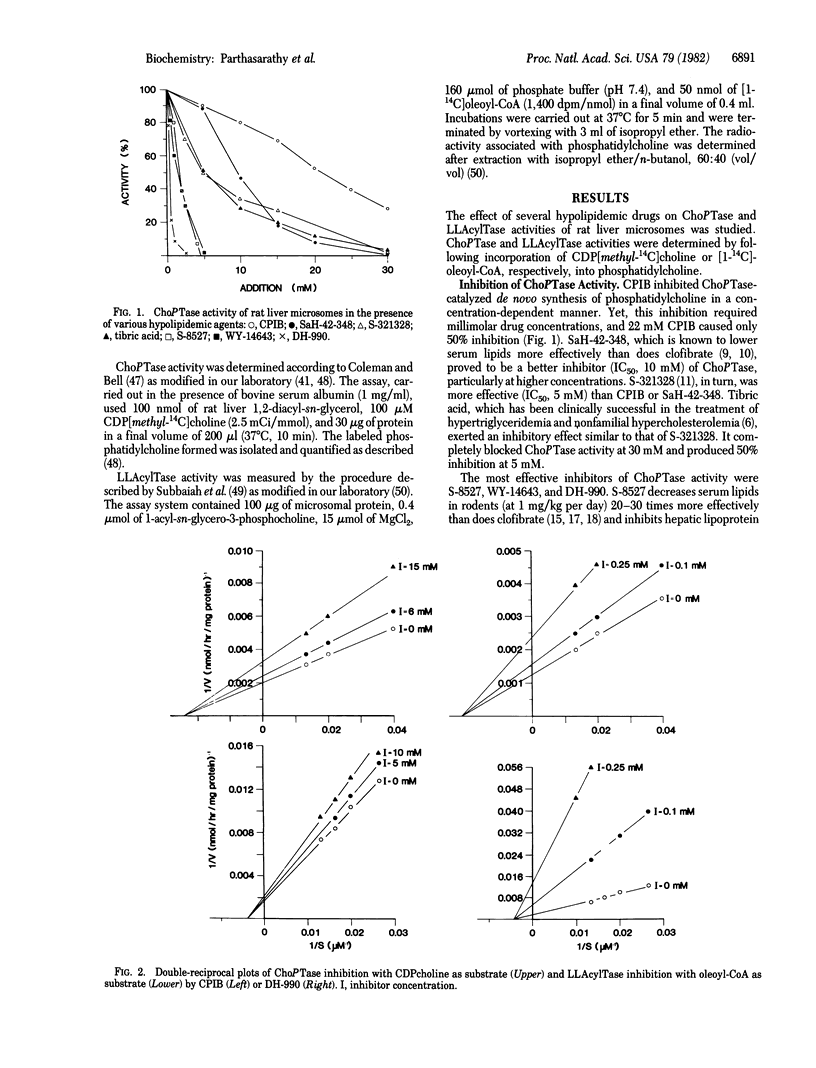
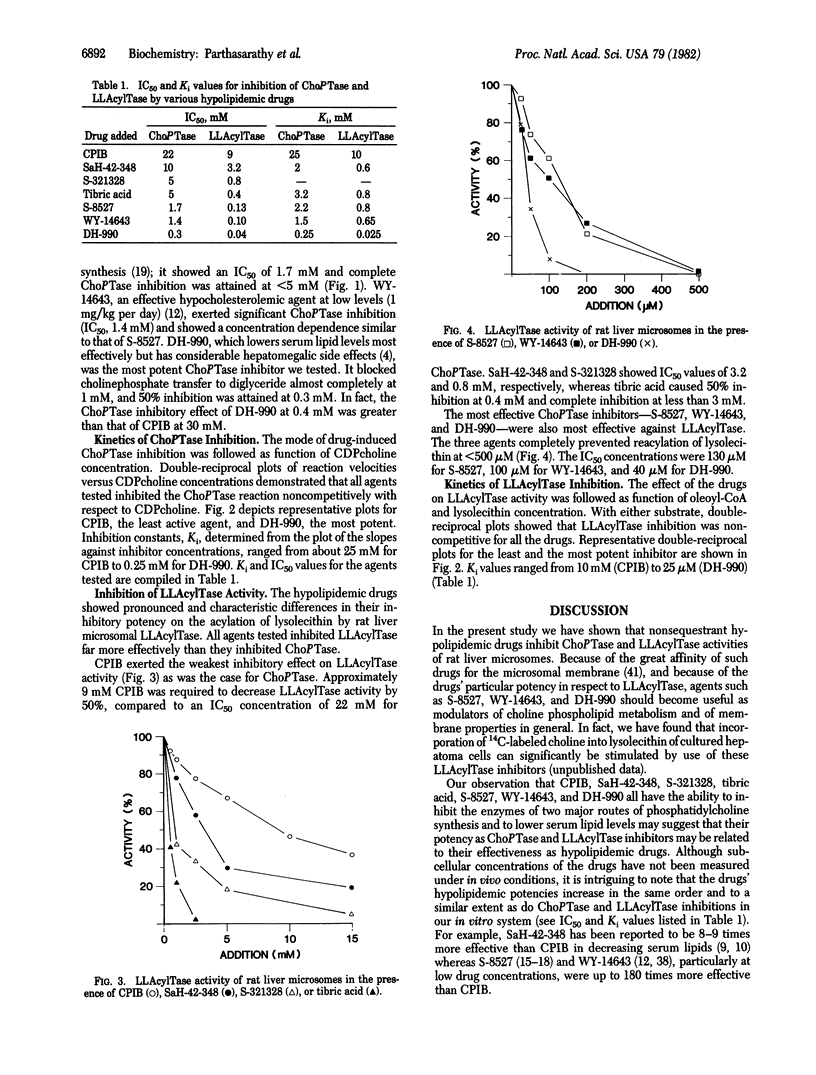
Clofibric acid (CPIB) and several other systemic hypolipidemic drugs are shown to block phosphatidylcholine synthesis by inhibiting cholinephosphotransferase (ChoPTase; CDPcholine:1,2-diacylglycerol cholinephosphotransferase, EC 2.7.8.2) and particularly lysolecithin acyltransferase (LLAcylTase; acyl-CoA:1-acylglycero-3-phosphocholine O-acyltransferase, EC 2.3.1.23) of rat liver microsomes. Whereas millimolar drug concentrations are required to affect de novo lecithin synthesis catalyzed by ChoPTase, reacylation of lysolecithin by LLAcylTase is inhibited at micromolar levels. Increasing effectiveness in ChoPTase inhibition is observed in the series CPIB, SaH-42-348, tibric acid, S-321328, WY-14643, S-8527, and DH-990, with IC50 ranging from 22 mM (CPIB) to 0.3 mM (DH-990). LLAcylTase inhibition by the hypolipidemic drugs follows the same general pattern, but IC50 concentrations range from 9 mM (CPIB) to 40 microM (DH-990). The agents inhibit ChoPTase (Ki, 25-0.25 mM) and LLAcylTase (Ki, 10-0.025 mM) noncompetitively. The data suggest that inhibition of phosphatidylcholine synthesis, particularly by the LLAcylTase pathway, may be related to a drug's effectiveness in decreasing serum triglyceride and cholesterol levels by blocking lipoprotein synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. L., Webb W. W., Fallon H. J. Inhibition of hepatic triglyceride formation by clofibrate. J Clin Invest. 1971 Nov;50(11):2339–2346. doi: 10.1172/JCI106732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball M. R., Gumaa K. A., McLean P. Effect of clofibrate on the CoA thioester profile in rat liver. Biochem Biophys Res Commun. 1979 Mar 30;87(2):489–496. doi: 10.1016/0006-291x(79)91822-9. [DOI] [PubMed] [Google Scholar]
- Berndt J., Gaumert R., Still J. Mode of action of the lipid-lowering agents, clofibrate and BM 15075, on cholesterol biosynthesis in rat liver. Atherosclerosis. 1978 Jun;30(2):147–152. doi: 10.1016/0021-9150(78)90057-6. [DOI] [PubMed] [Google Scholar]
- Brindley D. N., Bowley M. Drugs affecting the synthesis of glycerides and phospholipids in rat liver. The effects of clofibrate, halofenate, fenfluramine, amphetamine, cinchocaine, chlorpromazine, demethylimipramine, mepyramine and some of their derivatives. Biochem J. 1975 Jun;148(3):461–469. doi: 10.1042/bj1480461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuzzi D. M., Lackman R. D., Uberti M. O., Reed M. A. Stimulation of hepatic triglyceride synthesis and secretion by clofibrate. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1499–1508. doi: 10.1016/0006-291x(74)90367-2. [DOI] [PubMed] [Google Scholar]
- Cenedella R. J., Crouthamel W. G. Halofenate and clofibrate: mechanism of hypotriglyceridemic action in the rat. J Lipid Res. 1976 Mar;17(2):156–166. [PubMed] [Google Scholar]
- Cohen B. I., Raicht R. F., Shefer S., Mosbach E. H. Effects of clofibrate on sterol metabolism in the rat. Biochim Biophys Acta. 1974 Oct 16;369(1):79–85. doi: 10.1016/0005-2760(74)90194-5. [DOI] [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Phospholipid synthesis in isolated fat cells. Studies of microsomal diacylglycerol cholinephosphotransferase and diacylglycerol ethanolaminephosphotransferase activities. J Biol Chem. 1977 May 10;252(9):3050–3056. [PubMed] [Google Scholar]
- D'Atri G., Gomarasca P., Galimberti E., Sirtori C. R., Kritchevsky D. Clofibrate, pirinixil (BR 931) and WY-14,643 do not affect body cholesterol in Sprague-Dawley rats. Atherosclerosis. 1980 Nov;37(3):475–483. doi: 10.1016/0021-9150(80)90154-9. [DOI] [PubMed] [Google Scholar]
- Daae L. N., Aas M. Fatty acid activation and acyl transfer in rat liver during clofibrate feeding. Atherosclerosis. 1973 May-Jun;17(3):389–400. doi: 10.1016/0021-9150(73)90030-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G., Schreibman P. H., Nestel P. J. Mechanisms of action of clofibrate on cholesterol metabolism in patients with hyperlipidemia. J Lipid Res. 1972 Jul;13(4):531–551. [PubMed] [Google Scholar]
- Hayase K., Parthasarathy S., Eppler C. M., Baumann W. J. A rapid assay of acyl-coenzyme A:lysolecithin acyltransferase activity. J Lipid Res. 1980 May;21(4):484–488. [PubMed] [Google Scholar]
- Hürter P., Hüsing J., Kohl F. V., von Wichert P. Lung phospholipid content in CPIB-induced hypolipidemia. Respiration. 1973;30(3):285–293. doi: 10.1159/000193044. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Kritchevsky D. Newer hypolipidemic compounds. Adv Exp Med Biol. 1975;63:135–150. doi: 10.1007/978-1-4684-3258-9_9. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S. A. Effect of SaH 42-348, an analog of clofibrate, on lipid metabolism in rats. Arzneimittelforschung. 1973 Jun;23(6):858–859. [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S. A., Sallata P., Kabakjian J. R., Cristofalo V. J. Effect of the ethyl ester and sodium salt of alpha-p-chlorophenoxyisobutyric acid on cholesterol oxidation by rat liver mitochondria. Proc Soc Exp Biol Med. 1969 Oct;132(1):79–82. [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S. A., Story J. A. Influence of four agents (tibric acid, DH 990, oxandrolone and Sch 9122) on aspects of lipid metabolism in rats). Arzneimittelforschung. 1976;26(5):862–864. [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S. A., Story J. A. The effect of two substituted valeric acid derivatives on cholesterol metabolism in rats. Proc Soc Exp Biol Med. 1974 Jan;145(1):12–17. doi: 10.3181/00379727-145-37738. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S. A. The influence of 1,1-bis(4'-(1"-carboxy-1"-methylpropoxy)-phenyl)cyclohexane (a new aryloxy compound, S-8527) on cholesterol metabolism in rats. Atherosclerosis. 1973 Jul-Aug;18(1):93–99. doi: 10.1016/0021-9150(73)90120-2. [DOI] [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem. 1960 Aug;235:2233–2237. [PubMed] [Google Scholar]
- LONG C., PENNY I. F. The structure of the naturally occurring phosphoglycerides. III. Action of moccasin-venom phospholipase A on ovolecithin and related substances. Biochem J. 1957 Feb;65(2):382–389. doi: 10.1042/bj0650382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. Inhibition of monoacylglycerophosphate formation by chlorophenoxyisobutyrate and -benzalbutyrate. J Biol Chem. 1972 Feb 25;247(4):1281–1287. [PubMed] [Google Scholar]
- Lazarow P. B. Three hypolipidemic drugs increase hepatic palmitoyl-coenzyme A oxidation in the rat. Science. 1977 Aug 5;197(4303):580–581. doi: 10.1126/science.195342. [DOI] [PubMed] [Google Scholar]
- Macfarlane M. G., Knight B. C. The biochemistry of bacterial toxins: The lecithinase activity of Cl. welchii toxins. Biochem J. 1941 Sep;35(8-9):884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragoudakis M. E. Inhibition of hepatic acetyl coenzyme A carboxylase by hypolipidemic agents. J Biol Chem. 1969 Sep 25;244(18):5005–5013. [PubMed] [Google Scholar]
- Noseda G., Sirtori C. R. Klinische Prüfung von "Tibric Acid", einem neuen lipidsenkenden Stoff. Schweiz Med Wochenschr. 1974 Dec 21;104(51):1917–1922. [PubMed] [Google Scholar]
- Nozawa Y. Inhibition of lipid biosynthesis by p-chlorophenoxy-isobutyrate (CPIB) in Tetrahymena pyriformis. J Biochem. 1973 Dec;74(6):1157–1163. doi: 10.1093/oxfordjournals.jbchem.a130343. [DOI] [PubMed] [Google Scholar]
- O'Doherty P. J., Kakis G., Kuksis A. Role of luminal lecithin in intestinal fat absorption. Lipids. 1973 May;8(5):249–255. doi: 10.1007/BF02531899. [DOI] [PubMed] [Google Scholar]
- O'Doherty P. J. The importance of the steric configuration of lysophosphatidylcholine in the lymphatic transport of fat. Lipids. 1979 Jan;14(1):84–87. doi: 10.1007/BF02533574. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Baumann W. J. Lysolecithin as regulator of de novo lecithin synthesis in rat liver microsomes. Biochem Biophys Res Commun. 1979 Nov 28;91(2):637–642. doi: 10.1016/0006-291x(79)91569-9. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Cady R. K., Kraushaar D. S., Sladek N. E., Baumann W. J. Inhibition of diacylglycerol:CDPcholine cholinephosphotransferase activity by dimethylaminoethyl p-chlorophenoxyacetate. Lipids. 1978 Feb;13(2):161–164. doi: 10.1007/BF02533260. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., El-Rahman A., Baumann W. J. Modulation of phosphatidylcholine synthesis in vitro. Inhibition of diacylglycerol cholinephosphotransferase and lysophosphatidylcholine acyltransferase by centrophenoxine and neophenoxine. Biochim Biophys Acta. 1981 Aug 24;665(2):345–351. doi: 10.1016/0005-2760(81)90020-5. [DOI] [PubMed] [Google Scholar]
- Santilli A. A., Scotese A. C., Tomarelli R. M. A potent antihypercholesterolemic agent: (4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio) acetic acid (Wy-14643). Experientia. 1974 Oct 15;30(10):1110–1111. doi: 10.1007/BF01923636. [DOI] [PubMed] [Google Scholar]
- Skrede S., Halvorsen O. Increased biosynthesis of CoA in the liver of rats treated with clofibrate. Eur J Biochem. 1979 Jul;98(1):223–229. doi: 10.1111/j.1432-1033.1979.tb13180.x. [DOI] [PubMed] [Google Scholar]
- Steere M., Mansbach C. M., 2nd The contribution of serum phosphatidylcholine and lysophosphatidylcholine to lymph phosphatidylcholine. Biochim Biophys Acta. 1980 Dec 5;620(3):462–471. doi: 10.1016/0005-2760(80)90138-1. [DOI] [PubMed] [Google Scholar]
- Subbaiah P. V., Sastry P. S., Ganguly J. Acylation of lysolecithin in the intestinal mucosa of rats. Biochem J. 1970 Jun;118(2):241–246. doi: 10.1042/bj1180241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Aono S., Nakatani H. Hypolipidemic effect of a new aryloxy compound, S-8527, in experimental animals. Jpn J Pharmacol. 1974 Jun;24(3):407–414. doi: 10.1254/jjp.24.407. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Effects of S-8527 (1,1-bis(4'-(1'-carboxy-1'-methylpropoxy)phenyl) cyclohexane), a new hypolipidemic compound, on cholesterol and lipoprotein metabolism in rats. Biochem Pharmacol. 1976 Feb 1;25(3):325–328. doi: 10.1016/0006-2952(76)90222-7. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Effects of S-8527 (1,1-bis4'-(1"-carboxy'1"-methylpropoxy)-phenyl)-cyclohexane), a new hypolipidemic compound, on triglyceride metablolism in rats. Biochem Pharmacol. 1975 Jun 15;24(11-12):1203–1207. doi: 10.1016/0006-2952(75)90063-5. [DOI] [PubMed] [Google Scholar]
- THORP J. M., WARING W. S. Modification of metabolism and distribution of lipids by ethyl chlorophenoxyisobutyrate. Nature. 1962 Jun 9;194:948–949. doi: 10.1038/194948a0. [DOI] [PubMed] [Google Scholar]
- Timms A. R., Kelly L. A., Ho R. S., Trapold J. H. Laboratory studies of 1-methyl-4-piperidyl bis(p-chlorophenoxy) acetate (SaH 42-348)--a new hypolipidemic agent. Biochem Pharmacol. 1969 Aug;18(8):1861–1871. doi: 10.1016/0006-2952(69)90281-0. [DOI] [PubMed] [Google Scholar]
- Toki K., Nakamura Y., Agatsuma K., Nakatani H., Aono S. Hypolipidemic action of a new aryloxy compound (S-8527) in rats. Atherosclerosis. 1973 Jul-Aug;18(1):101–108. doi: 10.1016/0021-9150(73)90121-4. [DOI] [PubMed] [Google Scholar]
- Tomarelli R. M., Bauman L. M., Savini S. Effect of Wy-14,643 on cholesterol metabolism in normal and hypercholesterolemic rats. Atherosclerosis. 1978 Aug;30(4):301–311. doi: 10.1016/0021-9150(78)90123-5. [DOI] [PubMed] [Google Scholar]
- Tso P., Lam J., Simmonds W. J. The importance of the lysophosphatidylcholine and choline moiety of bile phosphatidylcholine in lymphatic transport of fat. Biochim Biophys Acta. 1978 Mar 30;528(3):364–372. doi: 10.1016/0005-2760(78)90025-5. [DOI] [PubMed] [Google Scholar]
- Voltti H., Savolainen M. J., Jauhonen V. P., Hassinen I. E. Clofibrate-induced increase in coenzyme A concentration in rat tissues. Biochem J. 1979 Jul 15;182(1):95–102. doi: 10.1042/bj1820095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L. W. Regulation of hepatic cholesterol biosynthesis by clofibrate administration. J Pharmacol Exp Ther. 1971 Aug;178(2):361–370. [PubMed] [Google Scholar]



