SUMMARY
Cellular response to osmotic stress is critical for survival and involves volume control through the regulated transport of osmolytes [1–3]. Organelles may respond similarly to abrupt changes in cytoplasmic osmolarity [4–6]. The plastids of the Arabidopsis thaliana leaf epidermis provide a model system for the study of organellar response to osmotic stress within the context of the cell. An Arabidopsis mutant lacking two plastid-localized homologs of the bacteria mechanosensitive channel MscS (MscS-Like (MSL) 2 and 3) exhibits large round epidermal plastids that lack dynamic extensions known as stromules [7]. This phenotype is present under normal growth conditions and does not require exposure to extracellular osmotic stress. Here, we show that increasing cytoplasmic osmolarity through a genetic lesion known to produce elevated levels of soluble sugars, exogenously providing osmolytes in the growth media, or withholding water rescues the msl2-1 msl3-1 leaf epidermal plastid phenotype, producing plastids that resemble the wild type in shape and size. Furthermore, the epidermal plastids in msl2-1 msl3-1 leaves undergo rapid and reversible volume and shape changes in response to extracellular hypertonic or hypotonic challenges. We conclude that plastids are under hypoosmotic stress during normal plant growth and dynamic response to this stress requires MSL2 and MSL3.
RESULTS
In agreement with previously published results, confocal laser scanning microscopy (CLSM) of plants constitutively expressing a plastid-localized fluorophore (RecARED) revealed that the leaf epidermal plastids in wild type (WT) Columbia ecotype plants were small and ovoid, while those in msl2-1 msl3-1 double mutant plants were enlarged and spherical (Figure 1A, [7]). MSL2 and MSL3 are plastid-localized Arabidopsis homologs of E. coli MscS, one of several bacterial mechanosensitive (MS) channels that open in response to increased membrane tension, allowing the efflux of osmolytes and preventing cellular lysis during extreme osmotic downshock [8]. These results led us to hypothesize that the osmolarity of the epidermal plastid stroma is higher than that of the cytoplasm, favoring the influx of water into the plastid. In WT plastids the resulting increase in envelope membrane tension would gate the MSL2 and MSL3 channels, allowing the efflux of osmolytes and maintaining normal plastid shape. In msl2-1 msl3-1 plastids, osmolytes would not be released, water influx would be unrelieved, and large round plastids would result. If this hypothesis were correct, increasing the osmolarity of the cytoplasm relative to the plastid stroma should reduce water flux from the cytoplasm to the plastid stroma and suppress the round morphology of msl2-1 msl3-1 leaf epidermal plastids.
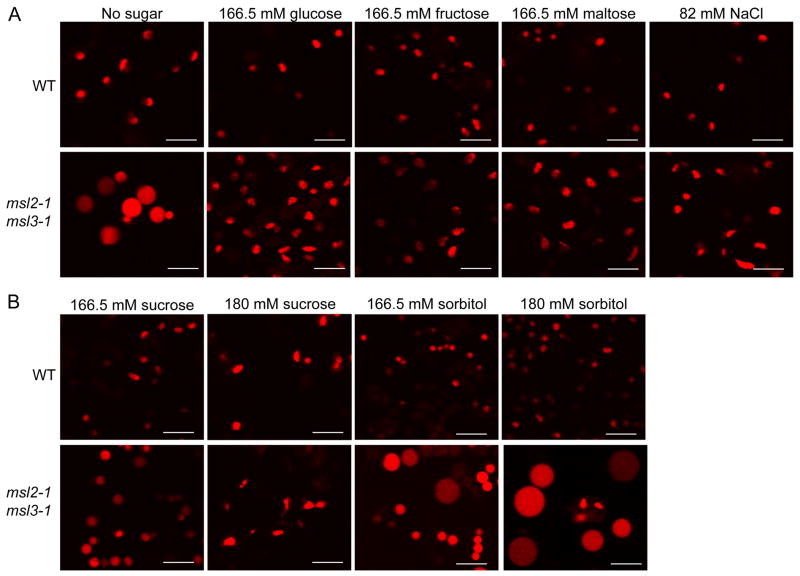
Figure 1. Supplementing growth media with osmolytes suppresses the leaf epidermal plastid phenotype of msl2-1 msl3-1 mutant plants.
Representative confocal laser scanning microscopy (CLSM) images taken from plants harboring the pRecARED plastid marker (pseudocolored red). Leaf epidermal plastids from 12-day old WT and msl2-1 msl3-1 seedlings were imaged after growth on solid media supplemented with the indicated amounts of (A) glucose, fructose, maltose, NaCl, or (B) sucrose or sorbitol. Images were taken from the lower right-hand quadrant of the first or second true leaf. Size bar is 10 microns.
Supplementing growth media with osmolytes suppresses the leaf epidermal plastid phenotype of the msl2-1 msl3-1 mutant
To test the hypothesis that msl2-1 msl3-1 leaf epidermal plastids are under hypoosmotic stress, we first investigated whether their large round phenotype could be suppressed by providing osmolytes to growing seedlings. Treatment of Arabidopsis seedlings with NaCl, sucrose, or mannitol has been demonstrated to decrease leaf osmotic potential two- to three-fold [9, 10]. WT and msl2-1 msl3-1 plants were grown on solid media containing 3% (166.5 mM) glucose or equiosmolar amounts of fructose, maltose, or NaCl and the morphology of leaf epidermal plastids examined by CLSM. While the leaf epidermal plastids of msl2-1 msl3-1 plants grown on media without sugar were large and round, they were small and ovoid when grown on media supplemented with sugars or with salt (Figure 1A). A slightly higher concentration of sucrose (180 mM) was required to produce msl2-1 msl3-1 leaf epidermal plastids that more closely resembled the WT in shape and size (Figure 1B). However, exogenous sorbitol (which cannot be taken up by plant roots and therefore should not affect the cytoplasmic osmolarity of leaf cells [11]) did not alter the large leaf epidermal plastid phenotype in msl2-1 msl3-1 plants, even when provided at 180 mM (Figure 1B). WT leaf epidermal plastid morphology appeared unaffected by these treatments.
The pgm-1 lesion suppresses the leaf epidermal plastid phenotypes of the msl2-1 msl3-1 mutant
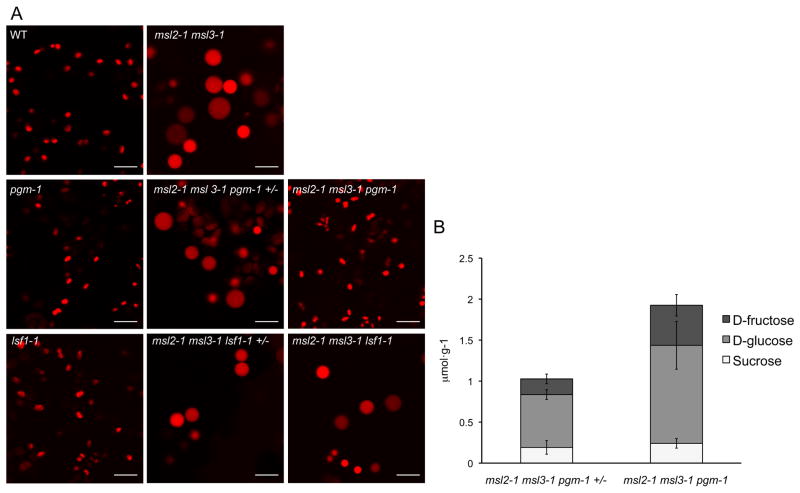
To further examine the role of cytoplasmic osmolarity in the round leaf epidermal plastid phenotype of msl2-1 msl3-1 plants, we investigated whether it could be suppressed by a genetic lesion known to produce high levels of soluble sugars. The PHOSPHOGLUCOMUTASE (PGM)/STARCH-FREE (STF1) locus encodes a plastid-localized enzyme required for the conversion of photosynthate into starch [12]. pgm/stf1 mutants contain very small amounts of plastidic starch [13–17] and exhibit high levels of soluble sugars in their leaves during the day [15, 17–24]. The concentration of soluble sugars in pgm-1 mutant cells is high enough to induce the expression of sugar-responsive genes [19, 20, 22, 25, 26].
The pgm-1 allele was introduced into the msl2-1 msl3-1 pRecARED line by crossing, and msl2-1 msl3-1 pgm-1 pRecARED and msl2-1 msl3-1 pgm-1 +/− pRecARED siblings were identified in the F3 generation (see Experimental Procedures). As expected, WT and pgm-1 plants exhibited small, ovoid leaf epidermal plastids, while msl2-1 msl3-1 and msl2-1 msl3-1 pgm-1 +/− mutant lines had large, round leaf epidermal plastids (Figure 2A). However, the triple homozygous msl2-1 msl3-1 pgm-1 mutant had small, oval-shaped leaf epidermal plastids that closely resembled the WT. Introduction of a like sex4 mutant allele (lsf1-1), which causes the accumulation of high levels of plastidic starch [27], did not alter the leaf epidermal plastid phenotype of the msl2-1 msl3-1 mutant.
Figure 2. The pgm-1 lesion leads to increased levels of soluble sugars and suppresses the leaf epidermal plastid phenotype of the msl2-1 msl3-1 mutant.
(A) Representative CLSM images of leaf epidermal plastids in plants of the indicated genotypes harboring the pRecARED plastid marker. Images were taken from the lower right-hand quadrant of fully expanded cauline leaves of 3-week old soil-grown plants. At least six plants from each genotype were characterized. Size bar is 10 microns. (B) Soluble sugar levels were significantly increased in msl2-1 msl3-1 pgm-1 plants compared to msl2-1 msl3-1 pgm-1 +/− siblings (P = 0.02, Student’s t test). Leaf tissue was collected at the end of a 16-hour day. Error bars indicate standard deviation between three biological replicates for each genotype.
To confirm that the pgm-1 allele resulted in the accumulation of high levels of soluble sugars, we measured the amounts of sucrose, D-glucose, and D-fructose in leaf tissue at the end of the light cycle using same plants used in Figure 2A. We observed increased levels of all three sugars in the homozygous triple msl2-1 msl3-1 pgm-1 mutant, with an overall 2-fold increase in soluble sugars compared to msl2-1 msl3-1 pgm-1 +/− siblings (Figure 2B), similar to that previously reported for the pgm-1 mutant under the same light conditions [20, 23].
msl2-1 msl3-1 leaf epidermal plastids exhibit a rapid and reversible change in morphology when intact leaves are subjected to extreme hyperosmotic shock
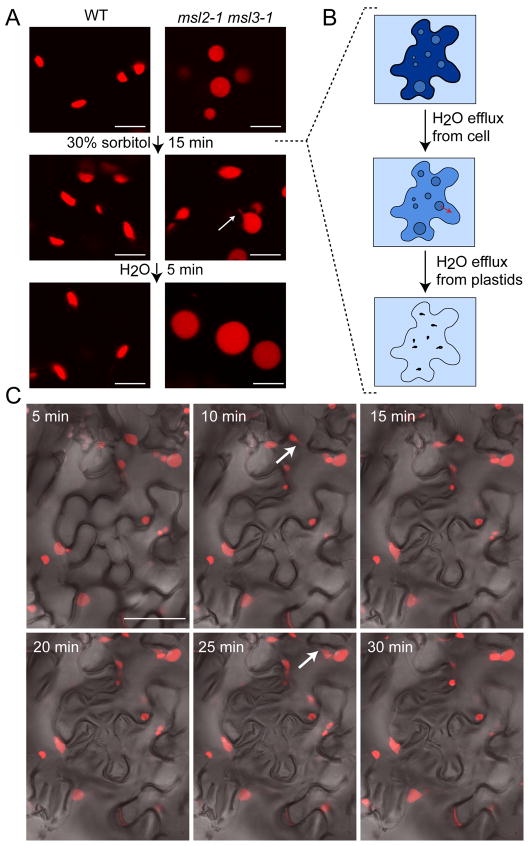
To characterize the dynamic nature of epidermal plastid morphology in the leaves of the msl2-1 msl3-1 mutant, we subjected excised leaf tissue to hyperosmotic shock (Figure 3). Epidermal plastids from the first or second leaves of 2 week-old seedlings were imaged before and after submersion of the excised leaves in 30% sorbitol. As shown in Figure 3A, msl2-1 msl3-1 leaf epidermal plastids lost their round shape and exhibited stromule-like extensions after 15 minutes of sorbitol treatment, but recovered a round shape and large size after a 5-minute incubation in distilled water. A time course revealed that msl2-1 msl3-1 epidermal plastids began to lose their smooth edges 5 minutes after excised leaves were mounted in sorbitol, and stromule-like projections were visible by 10 minutes of treatment (arrows, Figure 3C). WT leaf epidermal plastid morphology remained unchanged throughout these treatments (Figure 3A, Supplementary Figure 1).
Figure 3. Intact msl2-1 msl3-1 leaves exhibit rapid and reversible changes in epidermal plastid morphology when subjected to hyperosmotic shock.
CLSM images taken from plants harboring the pRecARED plastid marker. (A) Epidermal plastid morphology was imaged in WT and msl2-1 msl3-1 leaves mounted in water (top), after 15 minutes of treatment with 30% sorbitol (middle), and after washing and re-mounting in water (bottom). Size bar is 10 microns. (B) Cartoon depicting the immediate effects of immersing a single mutant epidermal leaf cell in 30% sorbitol. Red arrows indicate the flow of water from compartments with higher osmotic potential (dark blue) to compartments with lower osmotic potential (light blue). (C) Changes in msl2-1 msl3-1 leaf epidermal plastid morphology over the course of a 30-minute treatment with 30% sorbitol. Arrows indicate stromules. Size bar is 50 microns.
msl2-1 msl3-1 leaf epidermal plastid morphology dynamically responds to water availability in live plants
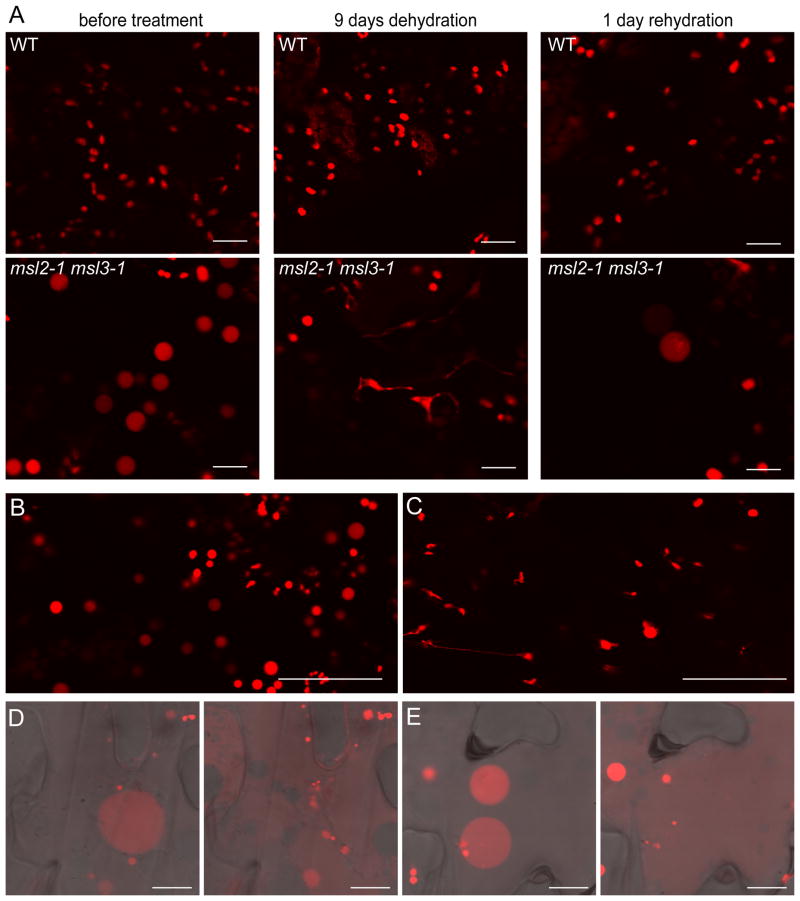
We also tested a physiological approach to increasing cytoplasmic osmolarity by withholding water from soil-grown plants, a treatment previously shown to decrease leaf osmotic potential two-fold [10]. In plants deprived of water for 9–12 days, msl2-1 msl3-1 leaf epidermal plastids were small and no longer round (Figure 4A). 24 hours after rewatering, msl2-1 msl3-1 leaf epidermal plastids were detected, but those that were present had recovered their characteristic round shape. WT leaf epidermal plastids remained small and ovular throughout the drought and rehydration treatments.
Figure 4. msl2-1 msl3-1 leaf epidermal plastids shrink in plants and intact leaves experiencing drought, then swell and lyse upon rehydration.
Representative CLSM images taken from plants harboring the pRecARED plastid marker. (A) WT and msl2-1 msl3-1 plants were grown in the same pot for three weeks and leaf epidermal plastids imaged before and after indicated dehydration and rehydration treatments. (B–C) Leaf epidermal plastid morphology was imaged before (B) and after (C) 60 minutes of dehydration on the bench top. (D) and (E) Epidermal cells from excised, dehydrated, and rehydrated leaves before (left-hand panels) and after (right-hand panels) plastid lysis. Size bars are 50 microns in (A–C) and 10 microns in (D–E).
msl2-1 msl3-1 leaf epidermal plastids lyse when subjected to extreme hypoosmotic shock
The low number of epidermal plastids in msl2-1 msl3-1 mutant leaves recovering from dehydration stress lead us to speculate that they were lysing upon rehydration of the tissue in the same way that E. coli cells lacking MS channels lyse under hypoosmotic stress [8]. Rosette leaves from msl2-1 msl3-1 plants were excised and allowed to dehydrate on the bench top for 45–60 minutes. This treatment resulted in the suppression of the large round plastid phenotype (Figure 4B and C). Dehydrated leaves were then mounted in water. Within a few minutes of rehydration, leaf epidermal plastids became large and round (Figure 4D and E, left-hand panels). After longer periods we frequently observed the lysis of leaf epidermal plastids and the subsequent appearance of dsRED signal in the cytoplasm; two examples are shown in the right-hand panels of Figure 4D and E. No lysis was observed in WT leaves subjected to the same regime.
DISCUSSION
Classic and contemporary studies have demonstrated that chloroplasts and nuclei are capable of volume regulation in response to extracellular osmotic challenges [4, 5, 28-31]. Here we add to these studies by addressing the extent to which organelles experience osmotic stress from within the cytoplasm during normal growth conditions, and by providing a molecular mechanism for volume control by organelles—the opening of MS ion channels under hypoosmotic stress. The large, round leaf epidermal plastid phenotype of msl2-1 msl3-1 plants was reliably and reversibly ameliorated by a number of treatments that increase cytoplasmic osmolarity: growth on high levels of actively transported osmotica (Figure 1), a genetic lesion known to increase levels of soluble sugars (Figure 2), dehydration of soil-grown plants or excised leaves (Figure 4), and the immersion of excised leaves in a hypertonic solution (Figure 3). The leaf epidermal plastids of WT plants were not appreciably changed in size or shape by any of these treatments. Furthermore, msl2-1 msl3-1 mutant plastids lysed inside leaf epidermal cells that were exposed to extreme hypoosmotic shock, while WT plastids were unaffected (Figure 4). Thus, the leaf epidermal plastid phenotype of the msl2-1 msl3-1 mutant can be attributed to an abnormally high stromal osmolarity, leading to the influx of water, subsequent plastid swelling, and plastidic sensitivity to hypoosomotic shock.
MSL2 and MSL3 are likely to directly mediate the efflux of osmolytes when the plastid inner envelope is under tension. They are evolutionarily related to the bacterial MS channel MscS [32–34], and MSL3 can provide osmotic shock protection to an E. coli strain lacking three major MS ion channels [7]. Taken together, these data support the conclusion that the stroma of leaf epidermal plastids has a lower osmotic potential (higher solute level) than the cytoplasm even under normal growth conditions and that MSL2 and MSL3 are required to relieve this hypoosmotic stress.
A high stromal solute level relative to the cytoplasm is likely to be generated through the active transport of energy and metabolites into leaf epidermal plastids [35]. Surprisingly, the chloroplasts found in the layer below the epidermis (the mesophyll) do not exhibit the same round phenotype as the plastids of the epidermis in msl2-1 msl3-1 mutant leaves [7]. This may be explained by differences in the metabolic activity and membrane transport complement between chloroplasts and the nonphotosynthetic plastids of the epidermis. For example, during the day, sucrose is transported into the epidermal plastid, while chloroplasts produce sucrose and either store it as starch or export it to the cytoplasm [35]. Perhaps chloroplasts use the export or the storage of sugar to modulate stromal osmolarity, making MS channels superfluous. Another possibility is that the internal thylakoid membranes, which are specific to chloroplasts, provide a mechanism for raising the osmotic potential of the stroma, as has been proposed for the cristae of mitochondria [36].
The changes in plastid volume and morphology shown in Figure 3A and C are rapid, and can be attributed largely to the movement of water across the plasma membrane and plastid envelope. Figure 3B diagrams the expected movement of water in a leaf epidermal cell immediately after exposure to 30% sorbitol. While the osmotic potential of Arabidopsis leaf cells is −0.8 to −1.0 MPa [9, 10], a 30% sorbitol solution has an osmotic potential of −4 MPa according to the van’t Hoff equation. Extracellular sorbitol treatment thus creates the rapid efflux of water from the cytoplasm of the epidermal cell to the extracellular sorbitol solution (from higher to lower osmotic potential). As a result, the osmotic potential of the cytoplasm decreases, allowing water to move from the plastid stroma to the cytoplasm, and thereby reducing plastid volume. The transport of water across the plastid envelope could be facilitated by aquaporins, water transport channels found in all kingdoms of life [37]. Or, as is proposed for the many microbial species for which no aquaporin has been found, passive water transport may suffice [38].
Summary
We have previously described an Arabidopsis mutant lacking two plastid-localized MS channels [7]; in this study we used this mutant as a sensitized background for the analysis of plastidic osmotic stress within the context of the cell cytoplasm. We used genetic, physiological, and media manipulations to show that under normal growth conditions the epidermal plastids of the leaf experience hypoosmotic stress. Furthermore, the effects of extreme osmotic stresses on mutant plastid morphology are rapid and reversible, occurring within a few minutes of treatment with hypertonic or hypotonic solutions. Finally, our data show that MS channels are critical for maintaining the wild-type responses of leaf epidermal plastids to changing cellular osmolarity, offering a likely molecular mechanism by which these endosymbiotic organelles respond to hypoosmotic stress. Given the evolutionary relationship between MSL2, MSL3, and MS channels found in bacteria, these results also provide an opportunity to study how this survival mechanism may allow other organelles and intracellular pathogens to respond to the dynamic osmotic challenges of the cytoplasm.
Supplementary Material
CLSM images taken from excised leaves of WT plants harboring the pRecARED plastid marker over the course of a 30-minute on-slide incubation in 30% sorbitol. Arrow indicates a stromule. Bar is 50 microns.
HIGHLIGHTS.
The cytoplasm provides a hypoosmotic environment for leaf epidermal plastids under normal growth conditions.
Plastid-targeted homologs of the bacterial mechanosensitive channel MscS are required to relieve this stress.
Acknowledgments
This work was supported by NSF MCB0816627 (to E.S.H). We acknowledge the Arabidopsis Biological Resource Center for pgm-1 and lsf1-1 mutant lines. We are also grateful to the Washington University in Saint Louis greenhouse staff for their assistance and David Braun for advice regarding soluble sugar quantification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pedersen SF, Kapus A, Hoffmann EK. Osmosensory mechanisms in cellular and systemic volume regulation. J Am Soc Nephrol. 2011;22:1587–1597. doi: 10.1681/ASN.2010121284. [DOI] [PubMed] [Google Scholar]
- 2.Hohmann S, Krantz M, Nordlander B. Yeast osmoregulation. Methods Enzymol. 2007;428:29–45. doi: 10.1016/S0076-6879(07)28002-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finan JD, Chalut KJ, Wax A, Guilak F. Nonlinear osmotic properties of the cell nucleus. Ann Biomed Eng. 2009;37:477–491. doi: 10.1007/s10439-008-9618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCain DC. Combined effects of light and water stress on chloroplast volume regulation. Biophysical Journal. 1995;69:1105–1110. doi: 10.1016/S0006-3495(95)79984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boustany NN, Drezek R, Thakor NV. Calcium-induced alterations in mitochondrial morphology quantified in situ with optical scatter imaging. Biophys J. 2002;83:1691–1700. doi: 10.1016/S0006-3495(02)73937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inan G, Zhang Q, Li P, Wang Z, Cao Z, Zhang H, Zhang C, Quist TM, Goodwin SM, Zhu J, et al. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol. 2004;135:1718–1737. doi: 10.1104/pp.104.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejardin A, Sokolov LN, Kleczkowski LA. Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem J. 1999;344(Pt 2):503–509. [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson SI. Plant sugar-response pathways. Part of a complex regulatory web. Plant Physiol. 2000;124:1532–1539. doi: 10.1104/pp.124.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeeman SC, Smith SM, Smith AM. The diurnal metabolism of leaf starch. Biochem J. 2007;401:13–28. doi: 10.1042/BJ20061393. [DOI] [PubMed] [Google Scholar]
- 13.Fettke J, Malinova I, Albrecht T, Hejazi M, Steup M. Glucose-1-phosphate transport into protoplasts and chloroplasts from leaves of Arabidopsis. Plant Physiol. 2010;155:1723–1734. doi: 10.1104/pp.110.168716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streb S, Egli B, Eicke S, Zeeman SC. The Debate on the Pathway of Starch Synthesis: A Closer Look at Low-Starch Mutants Lacking Plastidial Phosphoglucomutase Supports the Chloroplast-Localized Pathway. Plant Physiology. 2009;151:1769–1772. doi: 10.1104/pp.109.144931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspar T, Huber SC, Somerville C. Alterations in Growth, Photosynthesis, and Respiration in a Starchless Mutant of Arabidopsis thaliana (L.) Deficient in Chloroplast Phosphoglucomutase Activity. Plant Physiol. 1985;79:11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuhaus HE, Stitt M. Control analysis of photosynthate partitioning. Planta. 1990;182:445–454. doi: 10.1007/BF02411398. [DOI] [PubMed] [Google Scholar]
- 17.Kofler H, Hausler RE, Schulz B, Groner F, Flugge UI, Weber A. Molecular characterisation of a new mutant allele of the plastid phosphoglucomutase in Arabidopsis, and complementation of the mutant with the wild-type cDNA. Mol Gen Genet. 2000;263:978–986. doi: 10.1007/pl00008698. [DOI] [PubMed] [Google Scholar]
- 18.Corbesier L, Lejeune P, Bernier G. The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta. 1998;206:131–137. doi: 10.1007/s004250050383. [DOI] [PubMed] [Google Scholar]
- 19.Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006;140:637–646. doi: 10.1104/pp.105.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res. 2006;119:115–123. doi: 10.1007/s10265-005-0251-1. [DOI] [PubMed] [Google Scholar]
- 21.Antonio C, Larson T, Gilday A, Graham I, Bergstrom E, Thomas-Oates J. Quantification of sugars and sugar phosphates in Arabidopsis thaliana tissues using porous graphitic carbon liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A. 2007;1172:170–178. doi: 10.1016/j.chroma.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Blasing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell. 2005;17:3257–3281. doi: 10.1105/tpc.105.035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibon Y, Blasing OE, Palacios-Rojas N, Pankovic D, Hendriks JH, Fisahn J, Hohne M, Gunther M, Stitt M. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004;39:847–862. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 24.Lunn JE, Feil R, Hendriks JH, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J. 2006;397:139–148. doi: 10.1042/BJ20060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usadel B, Blasing OE, Gibon Y, Retzlaff K, Hohne M, Gunther M, Stitt M. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 2008;146:1834–1861. doi: 10.1104/pp.107.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibon Y, Blaesing OE, Hannemann J, Carillo P, Hohne M, Hendriks JH, Palacios N, Cross J, Selbig J, Stitt M. A Robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell. 2004;16:3304–3325. doi: 10.1105/tpc.104.025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comparot-Moss S, Kotting O, Stettler M, Edner C, Graf A, Weise SE, Streb S, Lue WL, MacLean D, Mahlow S, et al. A putative phosphatase, LSF1, is required for normal starch turnover in Arabidopsis leaves. Plant Physiol. 2010;152:685–697. doi: 10.1104/pp.109.148981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta AS, Berkowitz GA. Chloroplast osmotic adjustment and water stress effects on photosynthesis. Plant Physiol. 1988;88:200–206. doi: 10.1104/pp.88.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson SP. Osmotic Adjustment by Intact Isolated Chloroplasts in Response to Osmotic Stress and Its Effect on Photosynthesis and Chloroplast Volume. Plant Physiology. 1985;79:996–1002. doi: 10.1104/pp.79.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santakumari M, Berkowitz G. Chloroplast volume: cell water potential relationships and acclimation of photosynthesis to leaf water deficits. Photosynthesis Research. 1991;28:9–20. doi: 10.1007/BF00027172. [DOI] [PubMed] [Google Scholar]
- 31.McCain DC, Markley JL. In vivo study of chloroplast volume regulation. Biophys J. 1992;61:1207–1212. doi: 10.1016/S0006-3495(92)81930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, Saier MH., Jr Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haswell ES. MscS-Like Proteins in Plants. In: Hamill OP, editor. Mechanosensitive Ion Channels. Part A. Vol. 58 2007. [Google Scholar]
- 34.Balleza D, Gomez-Lagunas F. Conserved motifs in mechanosensitive channels MscL and MscS. Eur Biophys J. 2009;38:1013–1027. doi: 10.1007/s00249-009-0460-y. [DOI] [PubMed] [Google Scholar]
- 35.Weber AP, Linka N. Connecting the plastid: transporters of the plastid envelope and their role in linking plastidial with cytosolic metabolism. Annu Rev Plant Biol. 2011;62:53–77. doi: 10.1146/annurev-arplant-042110-103903. [DOI] [PubMed] [Google Scholar]
- 36.Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru II, Slepchenko B, Loew LM, Hsieh CE, Buttle K, Marko M. Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life. 2001;52:93–100. doi: 10.1080/15216540152845885. [DOI] [PubMed] [Google Scholar]
- 37.Gomes D, Agasse A, Thiebaud P, Delrot S, Geros H, Chaumont F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta. 2009;1788:1213–1228. doi: 10.1016/j.bbamem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Tanghe A, Van Dijck P, Thevelein JM. Why do microorganisms have aquaporins? Trends Microbiol. 2006;14:78–85. doi: 10.1016/j.tim.2005.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CLSM images taken from excised leaves of WT plants harboring the pRecARED plastid marker over the course of a 30-minute on-slide incubation in 30% sorbitol. Arrow indicates a stromule. Bar is 50 microns.