Abstract
Episodic memory deficits are proposed as a potential intermediate phenotype of schizophrenia. We examined deficits in visual episodic memory and associated brain activation differences among early course schizophrenia (n=22), first-degree relatives (n=16) and healthy controls without personal or family history of psychotic disorders (n=28).
Study participants underwent functional magnetic resonance imaging on a 3T scanner while performing visual episodic memory encoding and retrieval task. We examined in-scanner behavioral performance evaluating response time and accuracy of performance. Whole-brain BOLD response differences were analyzed using SPM5 correcting for multiple comparisons.
There was an incremental increase in response time among the study groups (Healthy Controls<First-degree relatives<Schizophrenia) with no differences in accuracy for encoding. Response time for retrieval was significantly increased in schizophrenia subjects compared to healthy controls with no difference in accuracy. Although there were no significant differences in BOLD responses for the encoding task, we noted increased BOLD response to retrieval in the prefrontal regions (Brodmann areas 9 and 8), thalamus and insula among the schizophrenia subjects compared to healthy controls, and the first-degree relatives.
Familial risk for schizophrenia may be associated with qualitatively similar but quantitatively milder abnormalities in visual episodic memory retrieval but not for encoding in the prefrontal cortex and thalamus.
Keywords: Cognition, Episodic Memory, Schizophrenia, functional neuroimaging, cognitive neuroscience
1. BACKGROUND
Cognitive deficits are observed in nearly 80% of schizophrenia subjects (Goldstein, 1990; Holthausen et al., 2002; Palmer et al., 1997). Even those performing within the normal range show evidence of decline from premorbid level of cognitive functioning (Joyce and Roiser, 2007) suggesting that cognitive deficits are related to the primary disease process (Goldberg et al., 1990) and may be a core feature of schizophrenia (Elvevag and Goldberg, 2000). They often appear before the emergence of psychotic symptoms (Cannon et al., 1994), limit long-term functional outcome (Green, 1996), and are minimally responsive to antipsychotics (Meltzer and McGurk, 1999). Specifically, deficits in working memory, episodic memory and executive control are the most pronounced cognitive impairments in schizophrenia (Joyce and Roiser, 2007; Reichenberg and Harvey, 2007). Further, schizophrenia subjects manifest pronounced episodic memory deficits that cannot be explained by demographic or clinical variables (Aleman et al., 1999). Genetic underpinnings of episodic memory deficits are suggested by the presence of impaired episodic memory in unaffected first-degree relatives at elevated risk for developing schizophrenia (Keefe et al., 1994) (Reichenberg et al., 2000). Determining the neural correlates of memory impairments in schizophrenia is essential to elucidate the neurobiology and genetic risk for schizophrenia (Reichenberg and Harvey, 2007) that may lead to the development of novel therapeutic agents.
While the neural mechanisms underlying impaired episodic memory in schizophrenia remain uncertain (Weiss and Heckers, 2001), most studies have implicated dysfunction in the prefrontal cortex (PFC) and medial temporal lobe (MTL) structures such as the hippocampus and the parahippocampal gyrus (Scoville and Milner, 1957). However, episodic memory dysfunction in schizophrenia is characteristically different from that observed following injury or ablation of MTL structures by the absence of amnesia (Gold et al., 1992; Paulsen et al., 1995) and other behavioral deficits. Within the MTL, the hippocampus may reinforce newly encoded information by binding it with associations during encoding (Achim and Lepage, 2005b; Vandenberghe et al., 1996) and may be responsible for consciously recalling during retrieval (Yonelinas and Levy, 2002). Functional imaging studies have found abnormal hippocampal activation in schizophrenia during episodic memory encoding and retrieval (Heckers et al., 1998; Jessen et al., 2003; Weiss and Heckers, 2001) that may be affected by specific genetic variations (Goldberg et al., 2006).
Difficulty in spontaneous organization of information, a component of memory function dependent on the frontal lobes, has long been hypothesized to play a critical role in memory dysfunction in schizophrenia (Achim and Lepage, 2005a; Ragland et al., 2009). Lesion studies of PFC closely resemble episodic memory deficits much like those experienced by schizophrenia subjects. For example, individuals with schizophrenia do not self-initiate semantic clustering (Achim and Lepage, 2005b; Gold et al., 1992; Paulsen et al., 1995) but greatly improve when encoding strategies are provided (Brebion et al., 1997; Heckers, 2001). Both decreased and increased blood oxygenation level dependent (BOLD) response, compared to healthy subjects, has been observed in the PFC during encoding and retrieval (Hofer et al., 2003; Weiss et al., 2003).
While genetic factors have been implicated in the risk for schizophrenia (Gottesman and Shields, 1966), specific genetic variations are yet to be identified. Endophenotypes can be helpful in identifying the genetic factors of complex psychiatric diseases (Gottesman and Gould, 2003). Thus, neural correlates of selected neuropsychological impairments in specific domains that are consistently demonstrated can be helpful endophenotypes (Braff et al., 2007). Earlier studies have shown such deficits in working memory, episodic memory and executive control (Joyce and Roiser, 2007; Reichenberg and Harvey, 2007). Within the episodic memory, both verbal and visual episodic memory are impaired in schizophrenia (Snitz et al., 2006). Verbal episodic memory deficits are observed in high-risk relatives, while the presence of visual episodic memory impairments in high-risk populations remains inconsistent (Skelley et al., 2008), possibly because visual episodic memory has been less well investigated in unaffected first-degree relatives of schizophrenia subjects (Seidman et al., 2003).
The present study investigated neural correlates of visual episodic memory in individuals with schizophrenia and first-degree relatives of schizophrenia in comparison with healthy controls. It was hypothesized that episodic memory performance would be impaired in subjects with schizophrenia compared to healthy controls. The performance of first-degree relatives of schizophrenia was predicted to be intermediate to that of schizophrenia and healthy controls. In addition, we hypothesized that the BOLD responses to the visual episodic memory tasks would be decreased in schizophrenia subjects compared to healthy controls in the hippocampal and parahippocampal regions during encoding, and in the PFC during retrieval. First-degree relatives were predicted to show intermediate activation between schizophrenia and healthy controls during both encoding and retrieval in the regions mentioned.
2. METHODS
2.1. Clinical Evaluations
We recruited 82 subjects between 18 and 50 years of age. Subjects were administered the Structured Clinical Interview for DSM-IV (SCID) (First, 1997) for diagnosis and Hollingshead Index for parental socioeconomic status (SES) (Hollingshead, 1975). A consensus diagnosis of schizophrenia or schizoaffective disorder was assigned by senior diagnosticians after pooling the SCID data, clinical observations from the professionals who provided direct care and a review of medical records. First-degree relatives were required to have at least one parent or sibling with schizophrenia or schizoaffective disorder per DSM-IV. Significant substance use was an exclusion criterion as were the presence of mental retardation, medical or neurological illness (e.g. epilepsy, history of encephalitis or meningitis), and head injury with significant loss of consciousness.
2.2. Imaging procedures
We, first, obtained structural MRI on Siemens 3T Tim Trio system using the MPRAGE sequence to co-register fMRI data, and to overlay the BOLD responses and identify regions of activation. After obtaining sagittal scout images, high-resolution axial slices were acquired along the anterior commissure-posterior commissure (AC-PC) plane. Scanning parameters were: TE 3.52 ms, TR 2300 ms, flip angle 9, thickness 0.8 mm, and number of slices 192.
In the same scanning session, subjects performed an episodic memory encoding and retrieval task and whole-brain BOLD responses were collected using gradient-echo echo-planar imaging (EPI). Scanning parameters for the fMRI were: TE=31 ms, TR=2000ms, 36 axial/oblique slices of 4 mm thickness, FOV 240 mm, flip angle 80° and matrix size=64×64.
The paradigm consisted of 4 blocks each of encoding and retrieval, and 9 blocks of rest (Hariri et al., 2003). Task performance data (accuracy and response time) were collected for both encoding and retrieval to examine in-scanner cognitive performance. Encoding blocks were shown first, followed by the retrieval blocks. The blocks started with 2s instruction followed by 6 stimuli of 3s each and then by 20s of rest. Stimuli consisted of indoor/outdoor emotionally neutral pictures from the International Affective Picture System (IAPS) collection (Lang and Ohman, 1988). During encoding, subjects were asked to identify if the pictures were indoor or outdoor. Accuracy of encoding was correct identification of indoor/outdoor context of picture and the response time was calculated as the duration between the stimulus presentation and button press response identifying indoor/outdoor context of the pictures. During retrieval, the subjects identified if the picture was new or was shown in one of the encoding blocks. Each retrieval block consisted of 3 new pictures and 3 pictures from the encoding block. Similar to encoding, accuracy and response times were correct identification of new/old and the time from stimulus presentation to button press response, respectively. These blocks were presented randomly. Each picture in retrieval was shown only once. Subjects viewed the task through a rear projection system and responded through the Fiber Optic Button Response System (http://www.pstnet.com/products/BrainLogics/).
2.3. Image processing, Quality Control and Plan of Analysis
Functional MRI data was analyzed using the SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Images were realigned and normalized to a standard EPI template and smoothed with 8×8×8 Gaussian kernel. Scans with motion artefacts, magnetic field inhomogeneity, incomplete coverage of brain (n=16) were not included in the analysis. Thus, the final sample with good quality fMRI data consisted of 66 subjects (schizophrenia, n=22; first-degree relatives, n=16; healthy controls, n=28).
Full-factorial random effects model was implemented within SPM5 including age, sex and SES as covariates. Intensity and extent threshold significance were determined a priori for whole-brain analysis after running 10,000 Monte Carlo simulations on AlphaSim (Ward, 2000) with a minimum voxel-wise threshold set to p<0.005. The simulation showed that an extent threshold of 93 contiguous voxels provided a corrected significance of p=0.048. Task performance data were analyzed using t tests for response time and the Kruskal-Wallis test for accuracy. Percentage BOLD signal changes were extracted from the regional masks of Brodmann areas (BA) defined by the WFU PickAtlas program (Maldjian et al., 2003) that contained the peak intensity voxel. These values were examined using ANOVA using SPSS 18.
3. RESULTS
3.1. Demographic and clinical characteristics
Study groups differed in age (ANOVA, F (2, 65)=2.69, p=0.08). Posthoc tests t-tests showed that the first-degree relatives were younger (22.96±5.10 years) than schizophrenia subjects (28.35±8.76 years; t=2.38, p=0.023) and healthy controls (26.92±6.91 years; t=1.99, p=0.052). Schizophrenia subjects and controls did not differ in age (t=0.65, p=0.52). Gender distribution did not differ across the study groups (SZ, M=11, F=11; HR, M=6, F=10; HC, M=9, F=19, χ2=1.68, df=3, p=0.43). Parental SES differed across the study groups (F(2, 65)=11.65, p=0.00005). Post hoc t-tests found that the healthy controls had higher parental SES than subjects with schizophrenia (t=4.67, p=0.00002) and first-degree relatives (t= 3.53, p=0.001). Schizophrenia subjects and first-degree relatives did not differ in SES (t=0.19, p=0.85).
Mean age at onset of first psychotic symptom was 24.07±8.08 years. Mean duration of illness of schizophrenia subjects from the onset of first psychotic symptom was 4.28±3.53 years. Total PANSS score at the time of cognitive testing and scanning was 50.09±37.04 (mean PANSS negative symptoms scores 14.59±10.68; positive symptoms 11.36±8.82) among cases. All subjects were on stable doses of either second or first generation antipsychotics. Of the 22 patients, 7 were diagnosed with schizoaffective disorder and 15 with schizophrenia.
3.2. Episodic Memory Task Performance
During encoding, there was a progressive increase in response time for indoor/outdoor judgments across the study groups (Healthy Controls<First-degree relatives<Schizophrenia) (Fig 1). Healthy controls completed the task with shortest response time (954.77±106.78 ms), followed by the first-degree relatives (1044.76±134.94 ms) (F=11.72, df=2, 58; p=0.0001) and then by subjects with schizophrenia (1164.87±184.07 ms). Post-hoc between-group comparisons revealed that each of these groups were significantly different in response time (Healthy controls vs Schizophrenia, F(1, 43)=22.65, p<0.001; First-degree relatives vs Schizophrenia, F(1, 33)=4.47, p=0.04; Healthy controls vs First-degree relatives, F(1, 39)=5.46, p=0.02).
Fig 1.
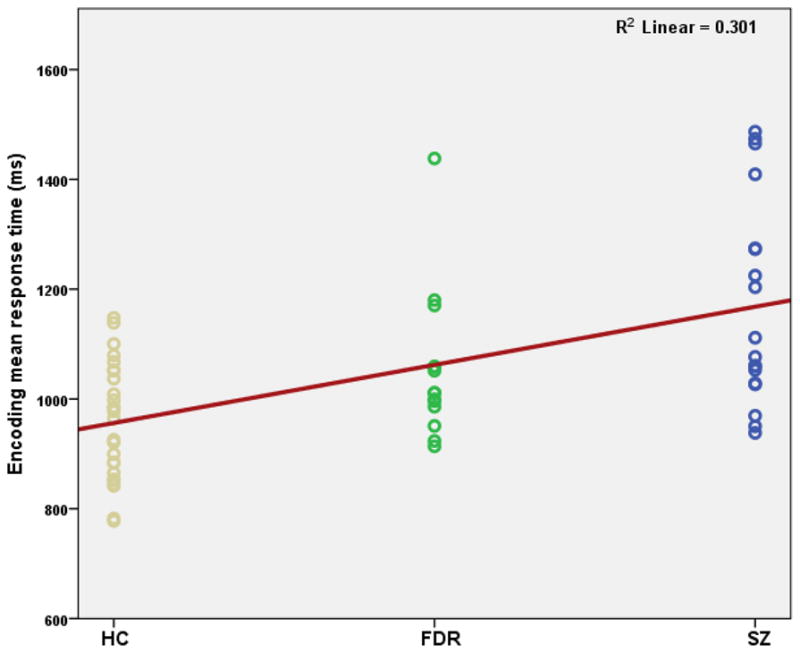
Mean response time for encoding of visual episodic memory among the study groups (HC=Healthy control subjects; FDR=First-degree relatives; SZ=Schizophrenia subjects).
We noted a trend for differences in response time to old/new judgments during retrieval (Healthy Controls, 1176.94±181.47 ms; First-degree relatives, 1200.81±137.98 ms; Schizophrenia, 1289.20±180.59 ms) (F=2.43, df=2, 58; p=0.098). Post-hoc between-group comparisons revealed the differences in retrieval time was significant between schizophrenia subjects and healthy controls (F(1, 43)=4.14, p=0.048) but not between first-degree relatives and schizophrenia (F(1, 33)=2.46, p=0.13) or healthy controls and first-degree relatives (F(1, 39)=0.19, p=0.27). Further, partial correlations controlling for age and SES revealed that the encoding and retrieval response times were positively correlated in schizophrenia (r=0.58, p=0.019) and healthy subjects (r=0.54, p=0.007) but not in first-degree relatives (r=0.45, p=0.14) (Supplemental figures).
There were no significant differences in accuracy for either encoding or retrieval components of the task across the study groups.
3.3. BOLD response differences
3.3.1. Main effect of study group and the task
Task main effect was noted in the middle and medial frontal gyri (BA 10), superior parietal lobule (BA 7), precuneus and the precentral gyrus (BA 6). Post hoc tests revealed main effects of encoding in the medial frontal gyrus (BA 10), cuneus (BA 19) and precentral gyrus (BA 6). Main effects of retrieval were noted in the BA 10, ventral posterior cingulate gyrus (BA 23), precuneus (BA 7) and precentral gyrus (BA 6). Group main effect was observed in the lingual gyrus (BA 18), parahippocampal gyrus, putamen, dorsal posterior cingulate cortex (BA 31), supramarginal gyrus (BA 40), insula (BA 13), superior parietal lobule (BA 7) and the declive of cerebellum.
3.3.2. Encoding
There were no significant group differences in BOLD response during encoding although there was main effect of encoding in BA 10, 19 and 6.
3.3.3. Retrieval
3.3.3.1. Schizophrenia subjects compared to healthy controls
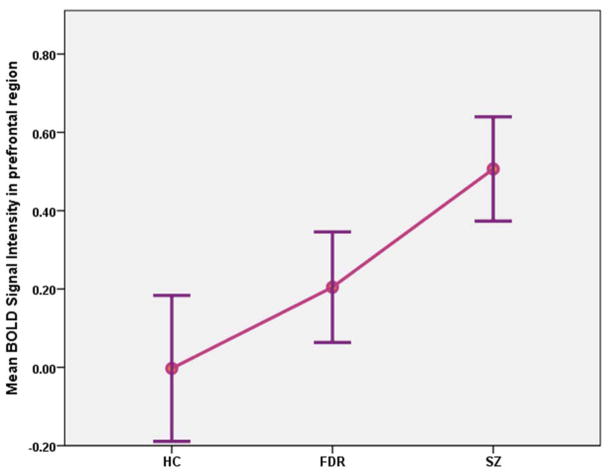
Schizophrenia subjects displayed increased BOLD response compared to healthy controls in the right middle frontal gyrus (BA 8 and 9), the left precentral gyrus (BA 6), bilateral thalami (left thalamic pulvinar region and the left ventral posterior lateral thalamus, and the right posterior lateral thalamus) and bilateral insula (BA 13) (Fig 2). Percentage BOLD signal changes extracted using the masks that encompassed the above Brodmann areas showed significant increase in BOLD signals in all of the above mentioned regions (Table 1). Fig 3 shows the percent BOLD signal changes for the prefrontal regions that included BA 8, 9 and 6. Schizophrenia subjects did not show any regions with decreased BOLD responses to retrieval compared to healthy subjects.
Fig 2.
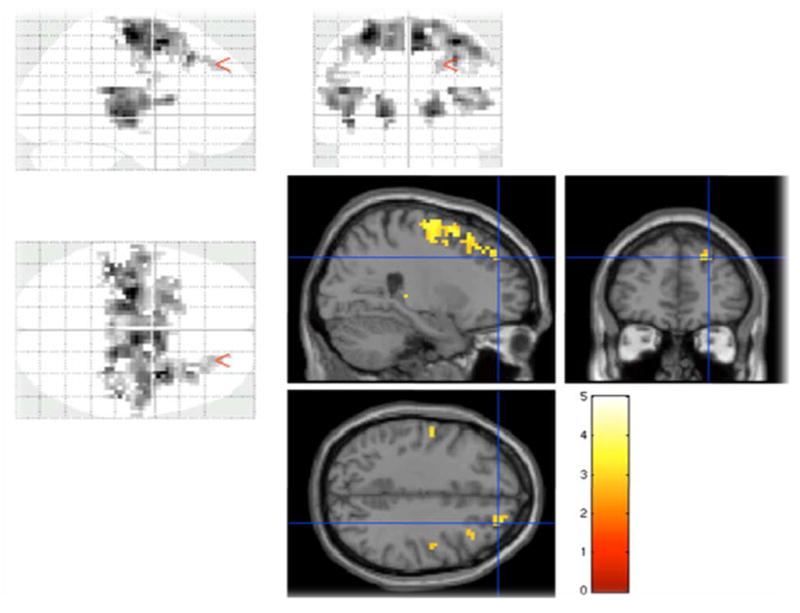
Schizophrenia subjects showing increased activation compared to healthy control subjects in the prefrontal (Brodmann areas 9, 8 and 6) regions and thalamus
Table 1.
Significant BOLD response differences between the study groups
| Contrasts with significant differences in BOLD responses | Talairach Coordinates | Brain Region | Brodmann Area | ke | t | Corrected significance (p) | Significance differences in percent BOLD signal changes between groups | ||
|---|---|---|---|---|---|---|---|---|---|
| Retrieval SZ > HC | 33 | 6 | 57 | Right middle frontal gyrus | BA 8 and 9 | 730 | 5.01 | 5.0 × 10−6 | 0.00006 |
| −27 | −15 | 63 | Left precentral gyrus | BA 6 | 620 | 4.89 | 7.3 × 10−6 | 0.0002 | |
| −18 | −21 | 0 | Left thalamus (pulvinar & posterior lateral) | 158 | 4.65 | 1.6 × 10−5 | 0.0005 | ||
| 22 | −19 | 14 | Right ventral posterior lateral thalamus | 154 | 4.16 | 5.1 × 10−6 | 0.0005 | ||
| −27 | −24 | 21 | Left insula | BA 13 | 217 | 4.50 | 1.6 × 10−5 | 0.0004 | |
| 36 | 9 | 12 | Right insula | BA 13 | 170 | 3.85 | 5.8 × 10−5 | 0.0005 | |
| Retrieval SZ > FDR | −51 | −9 | 21 | Right pre and post central gyri | BA 43 & 6 | 912 | 5.28 | 4.8 × 10−6 | 0.00002 |
| 18 | 9 | 69 | Right superior frontal gyrus | BA 8, 9 & 6 | 784 | 5.27 | 5.1 × 10−6 | 0.0009 | |
| 54 | −39 | 24 | Right insula | BA 13 | 106 | 3.58 | 1.7 × 10−4 | 0.0006 | |
| 24 | −24 | 3 | Right thalamus | 104 | 3.69 | 4.3 × 10−4 | 0.02 | ||
| Retrieval FDR > HC | −45 | −48 | 21 | Left Inferior Parietal Lobule | BA 40 | 90 | 3.42 × 10−4 | 0.019 | |
| 0 | −87 | 27 | Left Cuneus | BA 19 | 256 | 4.88 | 6.46 × 10−6 | 0.012 | |
Note: SZ=Schizophrenia; HC=Healthy Control Subjects; FDR=First-degree relatives; BA=Brodmann area
Fig 3.
Bold signal changes in subjects with schizophrenia (SZ), first-degree relatives (FDR) and healthy controls (HC) from a region-of-interest mask that included the Brodmann areas 9, 8 and 6 during visual episodic memory retreival.
3.3.3.2. Schizophrenia subjects compared to first-degree relatives
During retrieval, subjects with schizophrenia displayed increased BOLD response compared to the first-degree relatives in the right superior frontal gyrus (BA 8, 9 and 6), the right insula (BA 13), the right thalamus, and the right precentral and postcentral gyri (BA 43 and 6) (Table 1) (Fig 4). Correlation between response time for retrieval and BOLD signal changes in BA 8, 9 and 6 was not significant after controlling for age and SES (r=On reversing the contrast, we did not note decreased BOLD responses in any brain region.
Fig 4.
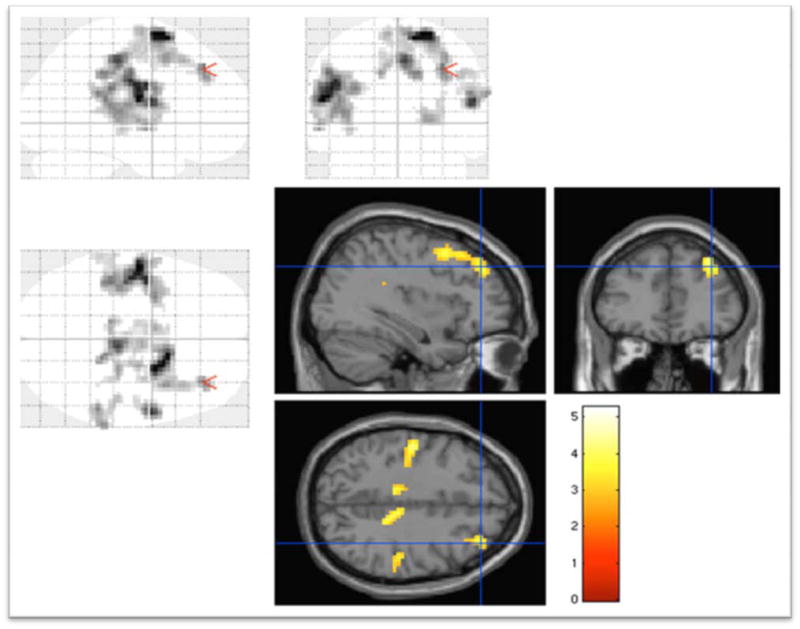
Schizophrenia subjects showing increased BOLD responses compared to first-degree relatives in Brodmann areas 9, 8 and 6
3.3.3.3. First-degree relatives compared to Healthy Controls
First-degree relatives had increased BOLD response compared to healthy controls in left inferior parietal lobule (BA 40) and left cuneus (BA 19). There was a trend for first-degree relatives to show increased BOLD responses in the prefrontal region (p=0.057). First-degree relatives did not show BOLD response differences in the medial temporal regions nor were there any regions with decreased BOLD signals.
4. DISCUSSION
We examined the neural correlates of visual episodic memory encoding and retrieval in a well-characterized cohort of early course schizophrenia subjects, first-degree relatives and healthy subjects. Our main findings were increased prefrontal (BA 8 and 9), thalamic and insular (BA 13) BOLD responses to visual episodic memory retrieval in schizophrenia subjects compared to healthy subjects and first-degree relatives. Increased BOLD responses in schizophrenia subjects compared to healthy controls were in conjunction with increased response time for retrieval. However, increased BOLD responses in schizophrenia subjects compared to first-degree relatives were associated with non-significantly prolonged response time for retrieval. First degree-relatives showed increased BOLD response in the inferior parietal lobule and a trend toward increased BOLD signals in the prefrontal region compared to healthy controls during retrieval with no differences in response time. During encoding, we did not observe group differences in BOLD responses although there was a progressive increase in response time across groups (Healthy Controls<First-degree relatives<Schizophrenia) and main effect of encoding in BA 10, 6 and 19. These observations are consistent with some of the previous studies and reveal the complex nature of visual episodic memory and its relationship with familial risk for schizophrenia.
Contrary to our hypothesis, increased activation was observed during episodic memory retrieval in schizophrenia subjects compared to healthy controls and first-degree relatives in the prefrontal regions (BA 9 and 8) as opposed to hypothesized decrease in these regions. Majority of studies have reported decreased BOLD responses in the prefrontal regions although increased BOLD response has been noted in six studies included in a recent meta-analysis (Ragland et al., 2009). Increased BOLD response in the presence of increased response time with no change in accuracy suggests an increased effort in the prefrontal regions engaged in processing the cognitive challenge. For example, BA 9 may be involved in the retrieval as well as post-retrieval monitoring as it allows one to consider retrieved information in conjunction with task instructions to determine if the recovered material is pertinent to the task at hand (Achim and Lepage, 2005b). Thus, in addition to retrieval, BA 9 may be involved more intricately with the organization of task instructions juxtaposed with recovered material, which assists in achieving the correct behavioral response (Henson et al., 2005; Henson et al., 2000; Maguire et al., 2001; Rugg et al., 2003; Shallice and Burgess, 1996). Hence, increased BOLD response may be secondary to enhanced post-retrieval monitoring.
In addition, increased dorsolateral PFC (DLPFC) activation is linked to more effortful post-retrieval monitoring where subjects are less confident in their recalled memories (Henson et al., 1999a) as task “difficulty” increases (Buckner et al., 1999; Buckner et al., 1998). Lower confidence in a response increases post-retrieval monitoring and activation (Henson et al., 1999a). Using a similar paradigm to the present study, elevated DLPFC activation was associated with lower level of confidence, perhaps requiring greater level of post-retrieval monitoring in order to meet the requirements of the instructions (Buckner et al., 1998; Henson et al., 1999a). Similarly, in the present study, increased BA 9 activity among schizophrenia subjects compared to healthy controls and first-degree relatives may suggest that schizophrenia subjects required a greater level of post-retrieval monitoring leading to recruitment of more resources to achieve similar degree of accuracy on retrieval. This may be a result of schizophrenia subjects being less certain of their “old” image judgments, which would require schizophrenia subjects to consider retrieved images more rigorously than the first-degree relatives and healthy subjects.
The disparate mix of hyper-(Hofer et al., 2003) and hypo-(Ragland et al., 2009) frontality found in schizophrenia during episodic memory tasks may be elucidated through the Monoach (2003) cortical inefficiency model for working memory (WM) deficits in schizophrenia. Studies on WM in schizophrenia have predominantly observed prefrontal hypoactivation in schizophrenia subjects (Weinberger et al., 1992) compared to healthy controls while a few have shown hyperactivation (Callicott et al., 2003; Manoach et al., 2000; Manoach et al., 1999). Manoach (2003) posits that low task demand is associated with increased prefrontal activation in schizophrenia subjects compared to healthy controls because the task is within the ability of schizophrenia subjects but is more resource-intensive. Hypofrontality may occur in schizophrenia groups with high task demand possibly secondary to reduced availability of resources leading to schizophrenia subjects not participating in the task marking the decline in performance and DLPFC hypoactivation. Healthy subjects may manage high task demand by increasing the DLPFC activity. This pattern may reflect reduced cortical efficiency in schizophrenia (Manoach, 2003). In the present study, non-significant difference in accuracy with slower response times of patients and first-degree relatives compared to controls during encoding supports such a mechanism. Similarly, during retrieval the schizophrenia subjects had slower response time than healthy controls with similar accuracies. Observed increased effort and elevation in PFC BOLD response in schizophrenia subjects compared to other groups suggests a cortical inefficiency. Similar phenomenon of capacity-constrained reduction of DLPFC activation is noted in healthy subjects, too (Callicott et al., 1999).
Increased activation was noted in the insula and the thalamus among schizophrenia subjects compared to healthy controls and first-degree relatives. Both the insula and thalamus showed increased activation during accurate retrieval compared to correct rejection of the new stimuli in an event-related fMRI study (Konishi et al., 2000). It was not feasible to make such distinctions in our block design. The thalamus plays an integral role in transmitting and processing a majority of sensorimotor information (Cabeza et al., 2002). The DLPFC receives projection fibers from the medial dorsal nucleus of thalamus through the anterior thalamic radiation (Pandya and Yeterian, 1996). Our study showed differences in activation in the pulvinar and the ventral posterior regions of thalamus. Both schizophrenia group and the first-degree relatives were activating the prefrontal and thalamic neural circuitry. Increased thalamic activation may represent an increased effort in processing of this sensory information (Ragland et al., 2009). Elevated fronto-thalamic BOLD response suggesting increased fronto-thalamic connectivity observed in schizophrenia subjects compared to other groups may interfere with cognitive processing (Schlösser et al., 2003). Fronto-thalamic regions take part in the creation and regulation of γ-oscillations (Buzsaki, 2006) that are thought to underlie higher order cognition (Herrmann et al., 2004). Increased fronto-thalamic connectivity has been associated with altered synchrony and amplitude of γ-band oscillations. Schizophrenia subjects (Uhlhaas and Singer, 2010) and first-degree relatives (Hong et al., 2004) have displayed altered synchronization and amplitude of γ-band oscillations and is hypothesized to underlie cognitive dysfunction in schizophrenia (Uhlhaas and Singer, 2010). In our study, increased fronto-thalamic activation in schizophrenia subjects may represent enhanced fronto-thalamic connectivity and dysfunctional γ-band oscillations that may underlie the observed episodic memory deficit. Insular activation has been noted in earlier fMRI studies that examined verbal episodic memory retrieval (Daselaar et al., 2001; McDermott et al., 1999) although such activation was noted to be more specific for semantic processing (Dalla Barba et al., 1998). In a recent meta-analysis, insular activation was associated with increasing familiarity with the stimulus during retrieval (Kim, 2010).
Visual episodic memory has been less well investigated among the first-degree relatives (Lefebvre et al., 2010; Seidman et al., 2003). In our sample, the first-degree relatives exhibited an intermediate BOLD response in the prefrontal regions as hypothesized suggesting that the degree of impairment in episodic memory processing was milder compared to schizophrenia subjects. However, previous studies suggest that adult unaffected relatives of individuals with schizophrenia do not have pronounced visual episodic memory deficits while subjects with schizophrenia do (Maziade et al., 2011; Skelley et al., 2008). A recent study noted that schizophrenia subjects and their healthy relatives had impaired verbal episodic memory; however, visual episodic memory impairments were noted in young offspring of schizophrenia subjects and patients but not in adult unaffected relatives suggesting different developmental trajectories in those who convert to psychosis compared to those who do not (Maziade et al., 2011). Our study suggests that the BOLD responses to visual episodic memory retrieval in adult first-degree relatives were intermediate between that observed among schizophrenia subjects and healthy controls although no deficits in accuracy of performance were noted. Interestingly, non-significant correlation of encoding and retrieval times is perhaps suggestive of greater heterogeneity in first-degree relatives compared to schizophrenia and healthy subjects in this sample.
We did not notice differences in accuracy of either encoding or retrieval among the groups consistent with some studies (Henson et al., 1999b). However, subjects took more time to process the task to provide accurate responses. More effortful processing possibly contributed by increased post-retrieval monitoring may have resulted in similar task accuracy across the groups along with increased BOLD responses for retrieval. We did not notice BOLD response differences for encoding although there was an incremental increase in response time. One plausible explanation for our observations is that the encoding task might have been easy, and did not elicit BOLD response differences that reached our stringent statistical threshold. Further, the ease of encoding may not have elicited neural responses of sufficient amplitude to evoke cerebral blood flow sufficient to evoke strong BOLD signals (Sheth et al., 2004). In addition, the tasks might have been relatively easier reflecting floor effect for this cohort.
We did not observe activations in the MTL structures during encoding although other studies have reported such observations (Achim and Lepage, 2005b; Ragland et al., 2009). The hippocampus may be involved in many aspects of memory formation including the role of binding multiple aspects of a learning opportunity into a cohesive memory (Chalfonte and Johnson, 1996; Cohen and Eichenbaum, 1991). This may be due to the single-item recognition paradigm that requires less relational-binding, and less hippocampal activity compared to associative memory tasks in studies of healthy subjects (Davachi and Wagner, 2002). Since the task may not have required subjects to use relational binding, there was no opportunity for a difference in the hippocampal area. In addition, it is likely that the differences in the amplitude of neural responses while encoding in this cohort may not have been sufficient to evoke detectable differences in hemodynamic response (Sheth et al., 2004).
Our study was limited by small sample size. To improve power, we used block design. Arguably, an event-related design could have been more informative regarding the cognitive processes that may underlie our observations. We designed the paradigm by incorporating a minimum time interval between the encoding stimulus and the retrieval stimulus to be longer than 70 seconds. Since it is known that working memory becomes less reliable after 10 seconds (Peterson and Peterson, 1959), we believe that the BOLD responses may not have been significantly contaminated by the working memory. Future studies need to address these issues and also examine the genetic variations that may contribute to the observed functional differences associated with visual episodic memory.
Supplementary Material
Acknowledgments
This study was funded through grants from the National Institute of Mental Health (MH072995) to KMP. We thank our research staff Stephen Goodnow, Debra Montrose, Kevin Eklund, Diana Dworakowski, Alicia Thomas and Jean Miewald for clinical characterization of subjects and managing research data.
Footnotes
This paper was presented at the 65th Annual Meeting of the Society of Biological Psychiatry, May 20–22, New Orleans, Louisiana.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Dorsolateral prefrontal cortex involvement in memory post-retrieval monitoring revealed in both item and associative recognition tests. Neuroimage. 2005a;24:1113–1121. doi: 10.1016/j.neuroimage.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. The British journal of psychiatry: the journal of mental science. 2005b;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EHF, Kahn RS. Memory Impairment in Schizophrenia: A Meta-Analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Braff D, Schork NJ, Gottesman II. Endophenotyping Schizophrenia. Am J Psychiatry. 2007;164:705–707. doi: 10.1176/ajp.2007.164.5.705. [DOI] [PubMed] [Google Scholar]
- Brebion G, Amador X, Smith MJ, Gorman JM. Mechanisms underlying memory impairment in schizophrenia. Psychol Med. 1997;27:383–393. doi: 10.1017/s0033291796004448. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. Neuroimage. 1998;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. The brain’s default state: self-organized oscillations in rest and sleep. In: Buzsaki G, editor. Rhythms of the Brain. Oxford University Press; New York: 2006. pp. 175–205. [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ, Moberg P, Price RA. Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry. 1994;51:651–661. doi: 10.1001/archpsyc.1994.03950080063009. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. The theory that wouldn’t die: A critical look at the spatial mapping theory of hippocampal function. Hippocampus. 1991;1:265–268. doi: 10.1002/hipo.450010312. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G, Parlato V, Jobert A, Samson Y, Pappata S. Cortical networks implicated in semantic and episodic memory: common or unique? Cortex. 1998;34:547–561. doi: 10.1016/s0010-9452(08)70513-6. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Lazeron RH, Jonker C. Parahippocampal activation during successful recognition of words: a self-paced event-related fMRI study. Neuroimage. 2001;13:1113–1120. doi: 10.1006/nimg.2001.0758. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- First MB. The Structured Clinical Interview for DSM-IV for Axis I disorders: Clinical Version, Administration Booklet. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. Journal of abnormal psychology. 1992;101:487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Ragland JD, Torrey EF, Gold JM, Bigelow LB, Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Archives of General Psychiatry. 1990;47:1066–1072. doi: 10.1001/archpsyc.1990.01810230082013. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, Coppola R, Egan MF, Weinberger DR. The G72/G30 gene complex and cognitive abnormalities in schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:2022–2032. doi: 10.1038/sj.npp.1301049. [DOI] [PubMed] [Google Scholar]
- Goldstein G. Neuropsychological heterogeneity in schizophrenia: a consideration of abstraction and problem-solving abilities. Arch Clin Neuropsychol. 1990;5:251–264. [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Contributions of twin studies to perspectives on schizophrenia. Prog Exp Pers Res. 1966;3:1–84. [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Henson RN, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: an FMRI study. J Cogn Neurosci. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J Cogn Neurosci. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999a;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999b;122 ( Pt 7):1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW. Neural Correlates of Episodic Encoding and Recognition of Words in Unmedicated Patients During an Acute Episode of Schizophrenia: A Functional MRI Study. Am J Psychiatry. 2003;160:1802–1808. doi: 10.1176/appi.ajp.160.10.1802. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Holthausen EA, Wiersma D, Sitskoorn MM, Hijman R, Dingemans PM, Schene AH, van den Bosch RJ. Schizophrenic patients without neuropsychological deficits: subgroup, disease severity or cognitive compensation? Psychiatry Res. 2002;112:1–11. doi: 10.1016/s0165-1781(02)00184-1. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK. Evoked gamma band synchronization and the liability for schizophrenia. Schizophrenia Research. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn KU, Maier W, Schild HH, Heun R. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. The American journal of psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Roiser JP. Cognitive heterogeneity in schizophrenia. Curr Opin Psychiatry. 2007;20:268–272. doi: 10.1097/YCO.0b013e3280ba4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Silverman JM, Roitman SE, Harvey PD, Duncan MA, Alroy D, Siever LJ, Davis KL, Mohs RC. Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Research. 1994;53:1–12. doi: 10.1016/0165-1781(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50:1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Lang P, Ohman DV. Technical Report. The Center for Research in Psychophysiology, University of Florida; Gainsville, FL: 1988. The international affective picture system [photographic slides] [Google Scholar]
- Lefebvre AA, Cellard C, Tremblay S, Achim A, Rouleau N, Maziade M, Roy MA. Familiarity and recollection processes in patients with recent-onset schizophrenia and their unaffected parents. Psychiatry Res. 2010;175:15–21. doi: 10.1016/j.psychres.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Henson RN, Mummery CJ, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12:441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biological Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Maziade M, Rouleau N, Merette C, Cellard C, Battaglia M, Marino C, Jomphe V, Gilbert E, Achim A, Bouchard RH, Paccalet T, Paradis ME, Roy MA. Verbal and visual memory impairments among young offspring and healthy adult relatives of patients with schizophrenia and bipolar disorder: selective generational patterns indicate different developmental trajectories. Schizophr Bull. 2011;37:1218–1228. doi: 10.1093/schbul/sbq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Ojemann JG, Petersen SE, Ollinger JM, Snyder AZ, Akbudak E, Conturo TE, Raichle ME. Direct comparison of episodic encoding and retrieval of words: an event-related fMRI study. Memory. 1999;7:661–678. doi: 10.1080/096582199387797. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Comparison of prefrontal architecture and connections. Philos Trans R Soc Lond B Biol Sci. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, Kuck J, Zisook S, Jeste DV. The nature of learning and memory impairments in schizophrenia. Journal of the International Neuropsychological Society: JINS. 1995;1:88–99. doi: 10.1017/s135561770000014x. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. J Exp Psychol. 1959;58:193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Rabinowitz J, Weiser M, Mark M, Kaplan Z, Davidson M. Premorbid functioning in a national population of male twins discordant for psychoses. The American journal of psychiatry. 2000;157:1514–1516. doi: 10.1176/appi.ajp.157.9.1514. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Lanca M, Kremen WS, Faraone SV, Tsuang MT. Organizational and visual memory deficits in schizophrenia and bipolar psychoses using the Rey-Osterrieth complex figure: effects of duration of illness. J Clin Exp Neuropsychol. 2003;25:949–964. doi: 10.1076/jcen.25.7.949.16482. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philos Trans R Soc Lond B Biol Sci. 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. discussion 1411-1402. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Skelley SL, Goldberg TE, Egan MF, Weinberger DR, Gold JM. Verbal and visual memory: Characterizing the clinical and intermediate phenotype in schizophrenia. Schizophrenia Research. 2008;105:78–85. doi: 10.1016/j.schres.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, III, Carter CS. Cognitive Deficits in Unaffected First-Degree Relatives of Schizophrenia Patients: A Meta-analytic Review of Putative Endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for fMRI data. Author; Milwaukee, WI: 2000. [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scandinavian journal of psychology. 2001;42:239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, Goff CD, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biological Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Levy BJ. Dissociating familiarity from recollection in human recognition memory: different rates of forgetting over short retention intervals. Psychonomic bulletin & review. 2002;9:575–582. doi: 10.3758/bf03196315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.