Abstract
We previously identified cyclin B1-specific T cells and antibodies in cancer patients with cyclin B1+ tumors and also in some healthy individuals. We also demonstrated that these responses may be important in cancer immunosurveillance by showing that vaccination against cyclin B1 prevents growth of transplantable cyclin B1+ tumors in mice. Constitutive overexpression of cyclin B1 was determined to correlate with the lack of p53 function. This allowed us to use p53−/− mice as a model that better approximates human disease. p53−/− mice spontaneously develop cyclin B1+ tumors. At 5–6 weeks of age, when the mice were still healthy with no evidence of tumor, they received the cyclin B1 vaccine and were then observed for tumor growth. We demonstrate that cyclin B1 vaccination can delay spontaneous cyclin B1+ tumor growth and increases median survival of tumor bearing p53−/− mice.
Keywords: cancer vaccines, immunosurveillance, mouse model
Introduction
Cyclin B1 is both a self molecule and a tumor antigen. During normal cell replication, cyclin B1 is expressed transiently in the nucleus at the transition from G2 to M phase of the cell cycle. In cancers of many tissues, cyclin B1 is overexpressed constitutively in the cytoplasm, and higher levels of expression correlate with worse disease prognosis.1 We previously published that cyclin B1 specific memory T cell responses exist in cancer patients who have cyclin B1-overexpressing tumors. While we initially proposed that these immune responses were elicited by abnormal expression of cyclin B1 by the tumor, our recent studies have demonstrated that some healthy individuals with no evidence of cancer also have humoral and cellular anti-cyclin B1 immune responses (Vella et al, submitted). This led us to explore the importance of pre-existing anti-cyclin B1 immune responses in the immunosurveillance of cancer. Specifically, we wanted to know whether eliciting or boosting these immune responses can slow or prevent growth of spontaneous cyclin B1-positive tumors.
Our previous work demonstrated the inverse relationship between the expression of p53 and the expression of cyclin B1. 2 We showed that tumor cell lines with deletions or mutations of p53 overexpressed cyclin B1. Furthermore, knock down of p53 in tumor cells with normal p53 function resulted in cyclin B1 overexpression while restoration of p53 function led to normalization of cyclin B1. Mice deficient in p53 have been demonstrated to spontaneously develop tumors. All three of the initial p53−/− mouse models demonstrated that mice died most often of lymphoma at approximately 6 months of age.24–26 We thus postulated that tumors arising spontaneously in p53−/− mice would overexpress cyclin B1 and could be a good model to study cyclin B1-based immunosurveillance.3 This mouse model is even more relevant considering that cyclin B1 is highly conserved between species and human and mouse cyclins exhibit over 85% homology.
Here, we demonstrate that, like human p53−/− tumors, tumors arising in p53−/− mice strongly overexpress cyclin B1. We show that vaccinating p53−/− mice early in life can provide protection by delaying spontaneous tumor occurrence, slowing tumor growth and extending survival. These and other data that we have already published suggest that both spontaneous and vaccine-induced anti-cyclin B1 immunity can play a role in tumor immunosurveillance and that boosting or eliciting this immune response could impact survival, especially in individuals at high risk for developing cyclin B1+ tumors such as lung cancer, breast cancer, head and neck cancer and others.
Results
Spontaneous tumors in p53 deficient mice overexpress cyclin B1
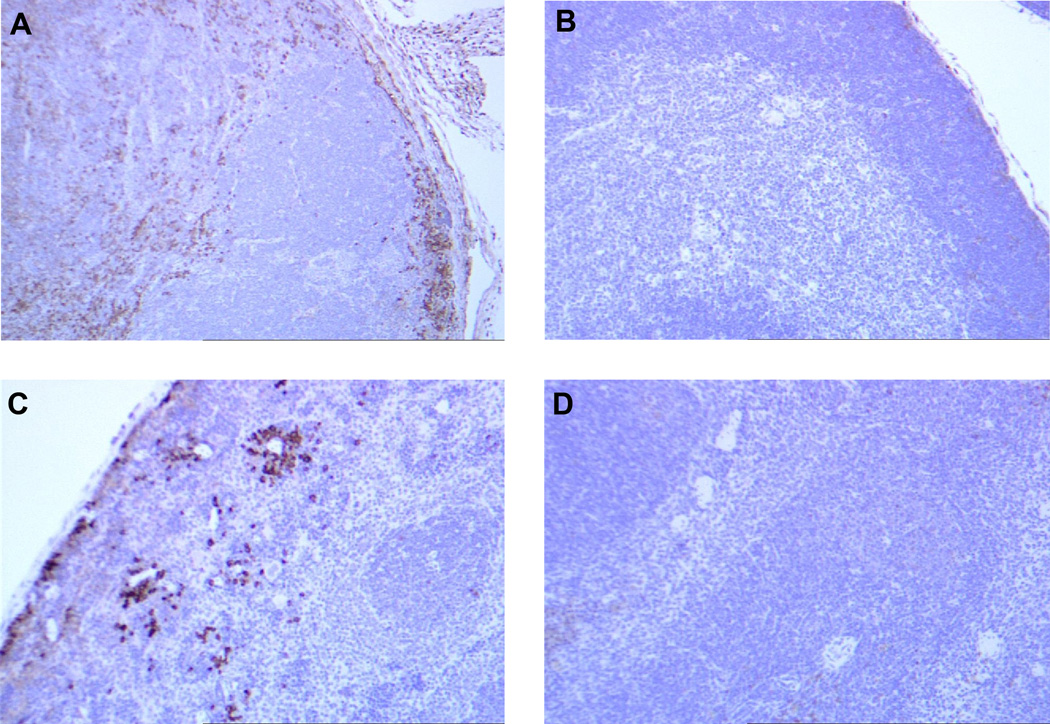
Figure 5 shows immunohistochemical staining of cyclin B1 overexpression in a spontaneous thymoma compared to normal cells in a p53−/− mouse. Tissues from wild type (WT) mice are also used for comparison. Cells in the primary tumor arising in the p53−/− thymus (Fig 1A) show uniformly high expression of cyclin B1 with some cells having very high expression. Tumor cells metastasizing to the spleen (Figure 1C) and other organs (not shown) are the high expressers of cyclin B1, suggesting that the cells in the primary tumor with the highest level of cyclin B1 expression might be those that preferentially metastasize to the periphery. It is important to point out that normal cells in the p53−/− thymus and spleen do not overexpress cyclin B1 (WT thymus (Figure 1B) and WT spleen (Figure 1D) are used for comparison) even though they all lack p53. Thus overexpressed cyclin B1 is a bona fide tumor antigen in mouse tumors as well.
Figure 1. Spontaneous tumors that develop in p53−/− mice overexpress cyclin B1 in the cytoplasm.
Thymus (B) and spleen (D) from a wild type mouse do not overexpress cyclin B1. In a p53−/− mouse, (A) the primary tumor (thymoma) and (C) tumor cells metastasizing to the spleen exhibit cyclin B1 overexpression. Non-tumor cells in both the thymus and the spleen of the p53−/− mouse are negative for cyclin B1 overexpression.
Cyclin B1 DNA prime/protein boost vaccine delays spontaneous tumor growth and prolongs overall survival in p53−/− mice
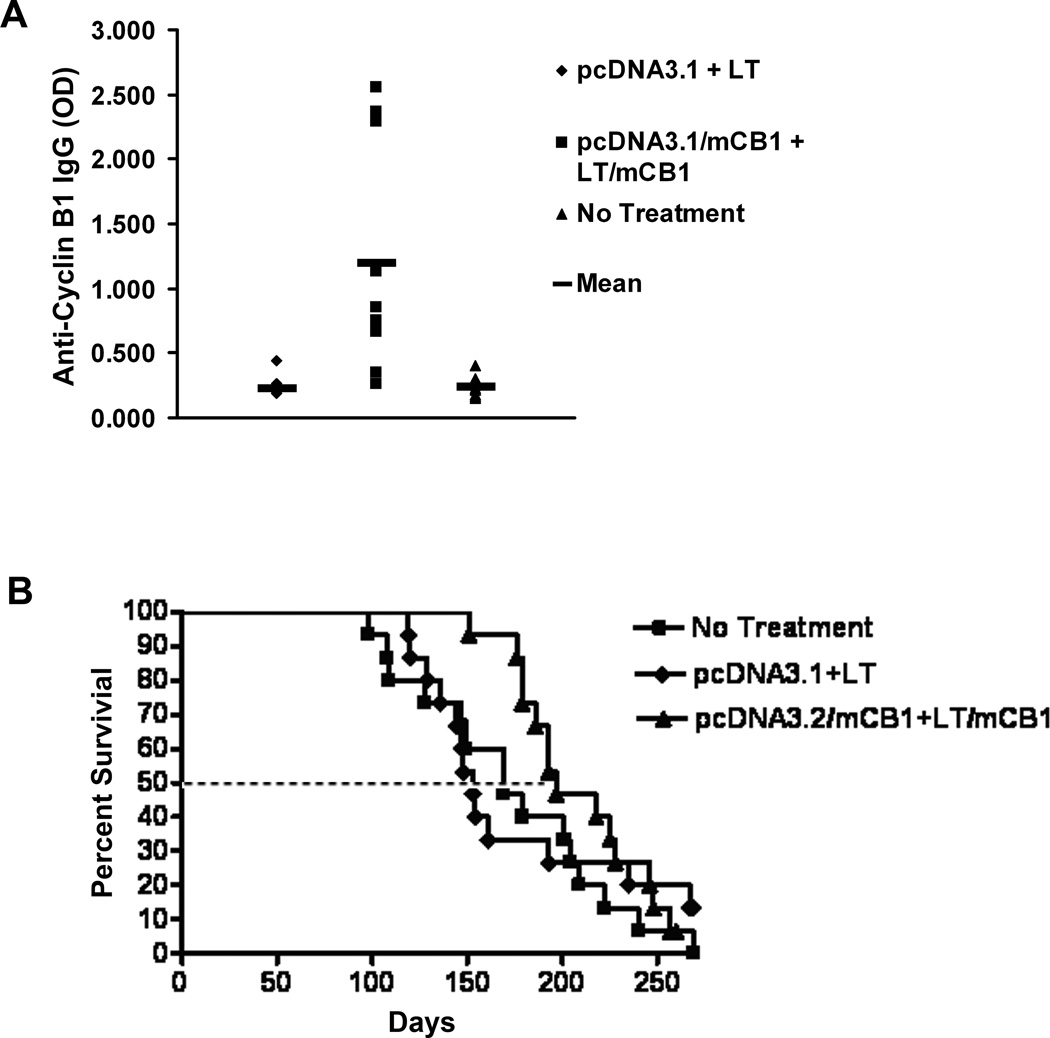
We initiated DNA prime-protein boost vaccination of mice at 6 weeks of age, prior to any evidence of tumor growth. The mice were divided in three groups that received the following treatments: mouse cyclin B1 DNA vaccine with a mouse cyclin B1 protein boost in the presence of an LT/IS adjuvant patch; empty-vector DNA control vaccine followed by the LT/IS patch; and no treatment. While from our preliminary studies we knew that this DNA prime-protein boost vaccination induced antibodies and CD4+ as well as CD8+ T cell responses, we chose in this study to measure humoral responses only in order not to sacrifice the mice that were observed long term for spontaneous tumor development and overall survival. Sera were collected 3 weeks after immunization to test for anti-cyclin B1 IgG. Mice were monitored for survival and/or tumor development and eliminated from the survival curve after death, or after the mice met IACUC standards for euthanasia. Figure 2A shows that vaccinated mice developed a strong IgG response, which indirectly also indicated that specific helper T cell responses were elicited. Figure 2B demonstrates that the immune response induced by the cyclin B1 DNA prime-protein boost vaccine controlled tumor growth and extended overall survival time.
Figure 2. Cyclin B1 DNA prime/protein boost vaccine elicits an anti-cyclin B1 immune response and delays spontaneous tumor growth in p53−/− mice.
p53−/− mice at 4–6 weeks of age were vaccinated with pcDNA3.1 with mouse cyclin B1 (mCB1) cDNA and boosted with the LT/IS patch + mCB1 protein twice at 3 week intervals. Control mice received empty DNA vector and the LT/IS patch or no treatment. (A) DNA prime/protein boost vaccination elicits anti-cyclin B1 IgG. Bars indicate mean OD. (B) Cyclin B1 vaccination retarded spontaneous tumor growth. The dotted line indicates the time point at which 50% of the mice in each group died.
Discussion
Vaccines based on normal self antigens abnormally expressed in tumor cells, such as cyclin B1, have not yet been tested in humans in the setting of early disease or in cancer prevention because of the concern that they might elicit autoimmunity. We would like to propose that the immune response generated against these molecules targets strictly the abnormal expression of these antigens found only on tumor cells and thus the chances of inducing autoimmunity are greatly diminished. The data presented here show that abnormal cyclin B1 expression is restricted to the developing tumor while the tissues throughout the p53−/− mouse remain negative. Cyclin B1 vaccine induced immunity in the p53−/− mouse, an extreme model of an individual at risk for developing cyclin B1+ tumors, showed a protective effect that extended life rather than cause autoimmune damage to normal tissues, which would have been expected to shorten survival.
The use of cyclin B1 as a target for both preventative and therapeutic vaccination would have several advantages. First, given that cyclin B1 overexpression in tumors is not associated with random mutations, cyclin B1 is a shared tumor antigen and cyclin B1 vaccines can be given to more than one individual. Secondly, cyclin B1 overexpression has been documented in melanoma, cervical cancer, non-small cell lung cancer, acute myelogenous leukemia, large B cell lymphoma, non-Hodgkin’s lymphoma, breast cancer, gastric carcinoma, colorectal carcinoma, and several head and neck cancers, including those of the esophagous, tongue, and larynx.4–20. This goes along with our observation that cyclin B1 overexpression is correlated with defects in p53, and many of these tumors are known to have either mutations or deletions of p53 from relatively early in the tumorigenic process. Thus this vaccine would cover a large number and variety of tumors. Third, vaccination against cyclin B1 is not likely to result in tumor immune evasion through antigen loss, since cyclin B1 is required for the transition from G2 into mitosis.21 Finaly, given that cyclin B1 overexpression is associated with poor prognosis and a more malignant phenotype in lung, gastric, breast, tongue, esophageal and laryngeal cancers, 7, 10, 13, 18–20, 22, 23 vaccination against cyclin B1 would target the most aggressive tumor clones and change the course of the disease.
Materials and Methods
Animals
C57Bl/6 p53 deficient female mice, TSG-p53N12-M, 5–6 weeks old, were purchased from Taconic Transgenics. All animals were housed in the University of Pittsburgh Animal Facility.
Immunohistochemical staining of cyclin B1 in tumor sections
Sections from mice were fixed in formalin (Thermo Fisher) for 24 hours and then embedded and sectioned (3–5µm). After drying overnight at 37°C, samples were deparaffinized, dehydrated, and stained with anti-cyclin B1 antibody (BD Biosciences) in the Pathology Laboratory, University of Pittsburgh. Briefly, samples were blocked with 2.5% BSA (Sigma) for 30 min at room temperature and stained with anti-cyclin B1 antibody. The avidin-biotin peroxidase method was then applied according to manufacturer’s protocol using the Vectastain ABC Elite staining kit (Vector laboratories, Inc., Burlingame, CA).
Construction of mouse and human cyclin B1 pcDNA3.1 DNA vectors
Human cyclin B1 cDNA derived from the HeLa cell line was a gift from Dr. Qimin Zhan at the University of Pittsburgh. Mouse cyclin B1 cDNA was an RT-PCR product derived from the mouse p53−/− cell line. Briefly, RT-PCR was performed using primers ATGGCGCTCAGGGTCACTAG (forward) and CAGTCTATTGGAGTTATGCCTTTG (reverse). A band at approximately 1.3kbp migrated on a 1.2% E-Gel Agarose gel (Invitrogen). The mouse cyclin B1 band was eluted using a MiniElute Kit (Qiagen, Valencia, CA) and subcloned into PCR2.1-TOPO vector (Invitrogen) and used to transform One-Shot TOP10 competent cells (Invitrogen) as described by the manufacturer. Colonies were picked for culture, and plasmids were isolated and identified positively by an EcoRI digest. Both cDNAs were then subcloned into the BamHI-XhoI site of the pcDNA3.1 expression vector (Invitrogen). All inserts were verified by DNA sequencing.
Synthesis of mouse cyclin B1 recombinant protein
Mouse cyclin B1 was a generous gift from IOMAI Corporation and was synthesized as described previously (Vella, et al, submitted). Briefly, pDEST-17 (Invitrogen) vector containing mouse or human cyclin B1 cDNA was used to transform Bl21 codon+ BL21 RIPL (Invitrogen) bacteria. Cyclin B1 was purified from inclusion bodies by extraction with guanidine-HCL under reducing conditions and passage over a Q-Sepharose FF (Amersham Biosciences) packed column to be collected in flow through fractions. Fractions were run over Nickel-HP 5 ml HighTrap columns (Amersham Biosciences) eluted in an imidazole gradient, and fractions collected and run on a gel to determine which ones contained the full-length protein. Protein purity was analyzed by coomassie blue, Western blot, HPLC, and Limulus amebocyte lysate assay (LAL, for endotoxin assessment).
DNA prime-protein boost vaccination and tumor measurements
At 6 weeks of age, experimental groups (n=15) were immunized with either pcDNA3.1 vector carrying mouse cyclin B1 cDNA, with the pcDNA3.1 empty vector, or left untreated. Mice were shaved on the abdomen with a No. 40 clipper 24 hours prior to treatments. The cDNA (4µg) was coated on 1–3µm gold particles (Bio-Rad, Hercules, CA) and fired into the shaved abdominal skin using a helium-powered gene gun. Three weeks later, the cyclin B1 immunized mice were boosted with protein in the presence of heat-labile enterotoxin (LT) placed on an immunostimulatory (IS) patch (IOMAI Corporation). Briefly, 25µg/100µl/mouse of mouse cyclin B1 recombinant protein was injected subcutaneously, with PBS injections used as controls in the adjuvant only group. In order to prevent grooming during the immunization procedure, mice were anesthetized intramuscularly with 100mg/kg ketamine (Phoenix Scientific Inc., St. Joseph, MO) mixed with 11mg/kg xylazine (Phoenix Scientific). The shaved skin was hydrated by rubbing with saline-drenched gauze. Hydrated skin was lightly blotted with dry gauze prior to immunization. The skin was abraded with sandpaper using gentle pressure 10 times in one direction. The area was then blotted with saline-saturated gauze and blotted dry. An immunostimulatory (IS) patch containing 20µg of heat-labile enterotoxin (LT) was applied to the treated skin. Mice were monitored for survival, the development visible tumors more than 2cm in diameter, or distress that met IACUC standards for euthanasia.
Measurement of Anti-Cyclin B1 Antibody Responses
Sera were drawn 3 weeks after the final immunization and tested for anti-cyclin B1 IgG by ELISA. Wells of 96-well ELISA plates (Thermo, Milford, MA) were each coated with 0.6µg recombinant mouse cyclin B1 protein in 50µl PBS. Plates were sealed overnight at 4°C and washed with PBS before use. Cyclin B1-coated wells and empty, background control wells were then blocked with 2.5% BSA in PBS (blocking buffer) for 1 hour. Plasma samples were diluted 1:100 in blocking buffer in 96-well polypropylene plates (Nunc, Thermo Fisher, Rockford, IL). 50µl of each diluted sample was then transferred to the ELISA plates. Samples were incubated for 1 hr and were subsequently washed with 1% PBS-Tween. Anti-mouse IgG (Sigma, St. Louis, MO) was diluted in blocking buffer and incubated on the plates for 1 hour. Plates were then washed as before and incubated substrate (TMB, BD Biosciences) for 30 minutes in the dark. The reaction was stopped with 2N H2SO4 and plates were read immediately at 450nm.
Acknowledgements
This work was supported by the NCI SPORE P50 CA90440.
References
- 1.Egloff AM, Vella LA, Finn OJ. Cyclin B1 and other cyclins as tumor antigens in immunosurveillance and immunotherapy of cancer. Cancer Res. 2006;66:6–9. doi: 10.1158/0008-5472.CAN-05-3389. [DOI] [PubMed] [Google Scholar]
- 2.Yu M, Zhan Q, Finn OJ. Immune recognition of cyclin B1 as a tumor antigen is a result of its overexpression in human tumors that is caused by non-functional p53. Mol Immunol. 2002;38:981–987. doi: 10.1016/s0161-5890(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 4.Arinaga M, et al. Clinical implication of cyclin B1 in non-small cell lung cancer. Oncol Rep. 2003;10:1381–1386. [PubMed] [Google Scholar]
- 5.Ersvaer E, et al. Cyclin B1 is commonly expressed in the cytoplasm of primary human acute myelogenous leukemia cells and serves as a leukemia-associated antigen associated with autoantibody response in a subset of patients. Eur J Haematol. 2007;79:210–225. doi: 10.1111/j.1600-0609.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 6.Jin YH, Park CK. Expression of cyclin B1 and cdc2 in nodal non-Hodgkin's lymphoma and its prognostic implications. J Korean Med Sci. 2002;17:322–327. doi: 10.3346/jkms.2002.17.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DH. Prognostic implications of cyclin B1, p34cdc2, p27(Kip1) and p53 expression in gastric cancer. Yonsei Med J. 2007;48:694–700. doi: 10.3349/ymj.2007.48.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obermann EC, et al. Cyclin B1 expression is an independent prognostic marker for poor outcome in diffuse large B-cell lymphoma. Oncol Rep. 2005;14:1461–1467. [PubMed] [Google Scholar]
- 9.Stefanaki C, et al. Cell cycle and apoptosis regulators in Spitz nevi: comparison with melanomas and common nevi. J Am Acad Dermatol. 2007;56:815–824. doi: 10.1016/j.jaad.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, et al. Nuclear cyclin B1 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2007;98:644–651. doi: 10.1111/j.1349-7006.2007.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda M, et al. Overexpression of cyclin B1 in gastric cancer and its clinicopathological significance: an immunohistological study. J Cancer Res Clin Oncol. 2002;128:412–416. doi: 10.1007/s00432-002-0359-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, et al. Expression profiling of cyclin B1 and D1 in cervical carcinoma. Exp Oncol. 2006;28:44–48. [PubMed] [Google Scholar]
- 13.Dong Y, et al. Clinical relevance of cyclin B1 overexpression in laryngeal squamous cell carcinoma. Cancer Lett. 2002;177:13–19. doi: 10.1016/s0304-3835(01)00770-4. [DOI] [PubMed] [Google Scholar]
- 14.Harada H, et al. Cyclin B1 is useful to predict occult cervical lymph node metastases in tongue carcinoma. J Exp Clin Cancer Res. 2006;25:351–356. [PubMed] [Google Scholar]
- 15.Hassan KA, et al. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res. 2002;62:6414–6417. [PubMed] [Google Scholar]
- 16.Korenaga D, et al. The relationship between cyclin B1 overexpression and lymph node metastasis in human colorectal cancer. Surgery. 2002;131:S114–S120. doi: 10.1067/msy.2002.119362. [DOI] [PubMed] [Google Scholar]
- 17.Li JQ, et al. Cyclin B1, unlike cyclin G1, increases significantly during colorectal carcinogenesis and during later metastasis to lymph nodes. Int J Oncol. 2003;22:1101–1110. [PubMed] [Google Scholar]
- 18.Nozoe T, et al. Significance of cyclin B1 expression as an independent prognostic indicator of patients with squamous cell carcinoma of the esophagus. Clin Cancer Res. 2002;8:817–822. [PubMed] [Google Scholar]
- 19.Song Y, et al. Overexpression of cyclin B1 in human esophageal squamous cell carcinoma cells induces tumor cell invasive growth and metastasis. Carcinogenesis. 2008;29:307–315. doi: 10.1093/carcin/bgm269. [DOI] [PubMed] [Google Scholar]
- 20.Takeno S, et al. Prognostic value of cyclin B1 in patients with esophageal squamous cell carcinoma. Cancer. 2002;94:2874–2881. doi: 10.1002/cncr.10542. [DOI] [PubMed] [Google Scholar]
- 21.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 22.Soria JC, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4004. [PubMed] [Google Scholar]
- 23.Hassan KA, et al. Clinical significance of cyclin B1 protein expression in squamous cell carcinoma of the tongue. Clin Cancer Res. 2001;7:2458–2462. [PubMed] [Google Scholar]