Abstract
Previously, we demonstrated that sphingosine 1-phosphate (S1P) increased the excitability of small-diameter sensory neurons, in part, through activation of S1P receptor 1 (S1PR1), suggesting that other S1PRs can modulate neuronal excitability. Therefore, studies were undertaken to establish the expression profiles of S1PRs in the intact dorsal root ganglion (DRG) and in defined single isolated sensory neurons. To determine mRNA expression of S1PRs in the DRG, SYBR green quantitative PCR (qPCR) was used. To determine the expression of S1PR mRNAs in single neurons of defined diameters, a preamplification protocol utilizing Taqman primer and probes was used to enhance the sensitivity of detection. The preamplification protocol also permitted detection of mRNA for two hallmark neuronal receptor/ion channels, TRPV1 and P2X3. Expression profiles of S1PR mRNA isolated from lung and brain were used as positive control tissues. In the intact DRG, the order of expression of S1PRs was S1PR3>>R1≈R2>R5≈R4. In the single neurons, the expression of S1PRs was quite variable with some neurons expressing all five subtypes, whereas some expressing only one subtype. In contrast to the DRG, S1PR1 was the highest expressing subtype in 10 of the 18 small-, medium-, and large-diameter sensory neurons. S1PR1 was the second highest expressor in ∼50% of those remaining neurons. Overall, in the single neurons, the order of expression was S1PR1>>R3≈R5>R4>R2. The results obtained from the single defined neurons are consistent with our previous findings wherein S1PR1 plays a prominent but not exclusive role in the enhancement of neuronal excitability.
Keywords: single cell qPCR, neuronal sensitization, Taqman preamplification
sphingosine 1-phosphate (S1P) has proven to be an important signaling molecule between cells, but this lysophospholipid also plays an important role as an intracellular second messenger (15, 18, 20, 49, 51, 52). S1P interacts with a family of five G protein-coupled receptors (S1PR1–5) that have been demonstrated to significantly influence the development and activation of many cell types (3, 31, 43, 46). An array of immunocompetent cells, such as platelets and mast cells, release S1P upon activation where it may function in an autocrine and/or paracrine signaling capacity (13, 35, 41, 55). The interaction between S1P and the S1P receptor subtype S1PR1 plays a significant role in controlling many facets of the inflammatory response. For example, the relationship between circulating levels of S1P and S1PR1 has a critical impact on the trafficking of T lymphocytes in and out of lymph nodes (reviewed Refs. 41, 42). Depletion of S1P levels or agonist-induced internalization of S1PR1 prevents T lymphocytes from leaving the node, consequently removing these cells from circulation (1, 5, 9, 29, 30, 37, 47). These seminal observations gave rise to the idea that removal of S1PR1 (e.g., with the S1PR agonist FTY720) can be a potent immunosuppressant and provides a mechanistic model for the development of selective S1PR1 agonists that produce immunosuppression.
Significantly, inflammation and nerve injury are two pathological conditions that enhance the sensitivity of nociceptive sensory neurons to a variety of stimuli. The role of S1P as a critical primary messenger communicating between activated immune cells and neuronal sensitivity is unexplored. Emerging results demonstrate, however, that S1P/S1PRs are instrumental in regulating nociceptive events. Recent studies have shown that S1P via S1PRs directly enhances excitability of small-diameter sensory neurons (8, 59) and that this sphingolipid augments heat- and transient receptor potential vanilloid type 1 (TRPV1)-activated currents (28). In the skin-nerve preparation S1P significantly increased the discharge rate of polymodal C-fibers in response to thermal stimulation, indicating that the heightened response was not dependent on invasion of immune cells (28). In vivo studies show that injection of S1P into the rat's hind paw produces symptoms of inflammation (e.g., edema) (44, 45), as well as significant thermal and mechanical hyperalgesia (12, 28). Consistent with these observations, S1P perfusion of the L5 dorsal root ganglion (DRG) enhances neuronal excitability in the intact DRG and gives rise to a significant mechanical hypersensitivity (56). In contrast to these peripheral actions, intrathecal delivery of S1P decreased nociceptive behaviors in an animal model of inflammatory pain (formalin treatment) (11). The basis for these differences remains to be defined. Taken together, these results show that S1P has multiple tasks in orchestrating the inflammatory response, including the chemotaxis of immune cells and direct sensitizing actions on sensory neurons. Very little is known about the role that S1P plays in communicating the status of the immunological environment to sensory neurons and raises important questions regarding the chemical interaction between immune cells and neurons responsible for perception of local environments.
Our previous work showed that S1P sensitized sensory neurons through activation of S1PRs, a family of five G protein-coupled receptors (GPCRs) (8, 59). These studies demonstrated that this novel sphingolipid regulates neuronal excitability and that S1PR1 plays a prominent but not exclusive role in enhancing excitability. Although the S1P/S1PR1 axis is a key regulatory mediator of the inflammatory response, to date, the roles of S1PRs in neuronal function are largely unexplored. Therefore, to establish the expression profiles of S1PRs in the intact DRG as well as in defined populations of isolated sensory neurons, we used a unique approach that involves the preamplification of mRNA isolated from single neurons to identify in a quantitative manner the expression of S1PRs.
METHODS
cDNA from rat tissues: whole organ studies.
The DRG, whole brain, and peripheral lung tissue from three rats were harvested. Male Sprague-Dawley rats (100–150 g) were killed by placing them in a chamber that was then filled with CO2, and the brain, lungs, and spinal column were removed. The spinal column was hemisected; lumbar, cervical, and thoracic DRG were collected and trimmed in cold, sterilized Puck's solution composed of (in mM): 171 NaCl, 6.7 KCl, 1.6 Na2PO4, 0.5 KH2PO4, 6 d-glucose, and 0.01% phenol red, pH 7.3. The ganglia were then transferred to a conical tube and washed with sterilized PBS. All procedures have been approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
Total RNA was extracted using the RNeasy Plus Mini Kit (cat. #74134; Qiagen, Valencia, CA). Each individual RNA was assessed on a NanoDrop ND-1000 Spectrophotometer (Thermoscientific, Franklin, MA) for concentration (A260) and purity by optical density (OD) ratios (A260/A280, ranging between 2.0 and 2.2). RNA integrity was also assessed using the Experion RNA StdSens Analysis Kit and Experion automated electrophoresis system (Bio-Rad, Hercules, CA). All individual rat organ RNA samples had a RNA quality indicator (RQI) value at least 8.0. To ensure removal of any residual genomic DNA, 4 μg of RNA was treated with 4 units of DNase in a 40 μl reaction for 15 min at room temperature. DNase activity was stopped by adding 4 μl of 25 mM EDTA to each reaction and incubating the reactions for 10 min at 65°C. To convert the RNA to cDNA, each reaction received 36 μl of a Master Mix containing 16 μl 5× iScript Reaction Buffer, 4 μl iScript reverse transcriptase, and 16 μl nuclease-free water. The reactions were incubated as follows: 25°C for 5 min, 42°C for 30 min, 85°C for 5 min and then stored at −20°C. To assess the specificity of these reactions, no reverse transcriptase (RT), and no template controls were also generated.
Real-time SYBR-green quantitative PCR: whole organ studies.
Quantitative PCR (qPCR) reactions were run in triplicate on an Applied Biosystems 7500 Fast Real-Time PCR System using MicroAmp Fast 96-well reaction plates sealed with MicroAmp optical adhesive film. Amplification reactions were run with Power SYBR-Green Master Mix and 500 nM primers in a 25 μl volume. All reactions began with an initial cycle of 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Validation of each primer pair was determined in rat lung, since rat lung expresses all five S1PR subtypes to a measurable level. Efficiencies for each primer pair were determined from the fitted slope of a seven-point standard curve and calculated from the relation where Efficiency = 10(−1/slope) (see Refs. 39, 40). The regression fit was considered to be acceptable when the r2 > 0.99. Sequences and calculated efficiencies for all validated primers for S1PRs and the two reference genes, hypoxanthine-guanine phosphoribosyl transferase (HPRT) and acidic ribosomal protein P0 (Arbp) are listed in Table 1. Except for rat S1PR1, all S1PRs have only one exon; S1PR1 has two exons with the first exon being only 61 nts. Thus all S1PR primers, either SYBR-Green or TaqMan (see below), only spanned 1 exon. The Taqman primers for TRPV1, P2X3, Arbp, and HPRT used in the single cell qPCR all spanned exons. The rat HPRT SYBR-Green primers span exons 6–9 of the nine total exons, whereas the rat Arbp primer is located within exon 6 of the six total exons. The specificity of these amplifications was verified by melt curve analysis with detection of only a single peak and electrophoresis on a 3% agarose gel with detection of only a single band at the appropriate size. The quantification cycle (Cq) was chosen to be number of cycles when the value of normalized fluorescence generated by SYBR green emission (ΔRn) attained 0.3. To determine the expression levels of S1PR subtypes, five-point (triplicate values) standard curves of each S1PR and the two reference genes, HPRT and Arbp, were constructed in each tissue (rat DRG, lung, and brain). The primer efficiency from the slope of the standard curve was used to convert Cq values at threshold (ΔRn = 0.3) to the number of copies of each gene where Number of Copies = Efficiency−Cq. The triplicate values for number of copies at each dilution on the standard curve were averaged. The relative number of copies for each S1PR subtype was determined by dividing the average copy number for each S1PR by the average copy number for the reference gene at each respective dilution point on the standard curves. The relative copy number for each S1PR over the five dilution points were then averaged to obtain the grand mean for the relative number of copies for each S1PR.
Table 1.
Sequences and calculated efficiencies for SYBR green S1PR and reference gene primers
| Gene | Acc. No. | Prod., bp | Pos. | Seq. (5′ to 3′) | Efficiency |
|---|---|---|---|---|---|
| S1PR1 | NM_017301.2 | 125 | 626 . . . 750 | f-TTCAGCCTCCTTGCTATCGC | 1.976 |
| r-AGGATGAGGGAGATGACCCAG | |||||
| S1PR2 | NM_0017192.1 | 138 | 216 . . . 353 | GACGCTGGACATGCAGGAG | 1.997 |
| TACATGGCTGAGTGGAACTTGC | |||||
| S1PR3 | XM_225216.4 | 64 | 411 . . . 474 | GCCACCCGCCAGTCTTG | 1.944 |
| GCCAGCTTCCCCACGTAAT | |||||
| S1PR4 | NM_001108075.1 | 86 | 1531 . . . 1616 | CGTTTCCAGCATCCGCAG | 1.960 |
| CCAGTCCCTTCTCACCTCTCCT | |||||
| S1PR5 | NM_021775.2 | 85 | 832 . . . 916 | CCTATGTGCTCTTCTGCGTGCTG | 1.976 |
| CGCACCTGACAGTAAATCCTTG | |||||
| HPRT | NM_012583 | 278 | 495 . . . 772 | GCAGACTTTGCTTTCCTTGG | 1.967 |
| TACTGGCCACATCAACAGGA | |||||
| Arbp | NM_022402.1 | 57 | 913 . . . 970 | CAGCCAAGGTCGAAGCAAA | 1.978 |
| CCGAATCCCATGTCCTCATC |
Acc. No., accession number; Prod, amplicon size in base pairs; Pos., amplicon start and finish positions in NCBI Reference Sequence; Seq., targeted sequence, listed first is the forward primer (f), second is the reverse primer (r); Efficiencies were determined in lung.
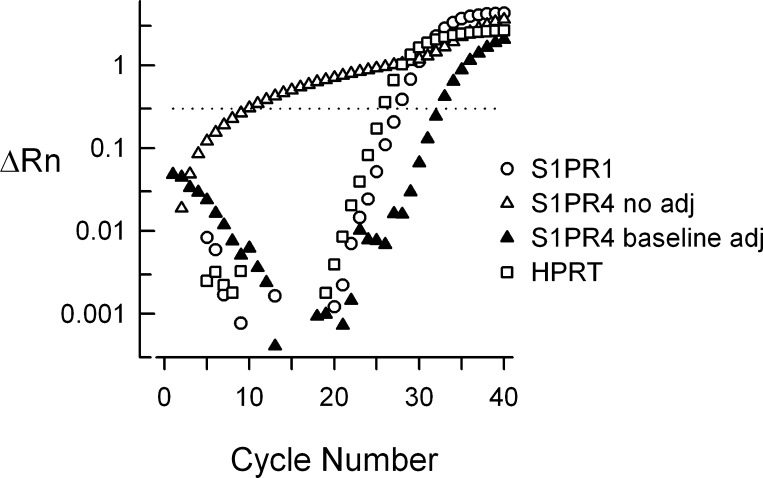
Baseline adjustment for S1PR4.
In our initial studies, we found that the ΔRn vs. cycle number relation for S1PR4 was quite different than our observations with S1PR1 and HPRT (see Fig. 1). To extract a meaningful result above background signal, the Cq values for the start and end were manually adjusted from the automatic settings selected by the ABI 7500 software. For experiments measuring expression of S1PR4, the start Cq and end Cq for the baseline were adjusted to 12 and 22, respectively, to establish the baseline from which S1PR4 expression would be measured. Representative traces shown for S1PR4 expression before and after baseline adjustments are shown in Fig. 1. For all other genes of interest (GOI), the software-determined start and end Cqs were used.
Fig. 1.
Baseline correction for sphingosine 1-phosphate receptor 4 (S1PR4) qPCR. These representative traces illustrate the qPCR runs obtained for S1PR4 with no adjustment [quantification cycle (Cq) 9.8] and after adjustment (Cq 32.4) to the baseline as described in methods. The qPCR runs for S1PR1 (Cq 27.5) and hypoxanthine-guanine phosphoribosyl transferase (HPRT) (Cq 25.7) are shown as a reference. These qPCR measurements were obtained with tissue isolated from rat lung.
Isolation and maintenance of adult rat sensory neurons: single cell studies.
The procedures for primary culture of rat sensory neurons have been described previously (27) with slight modification (21). In brief, male Sprague-Dawley rats (100–150 g) were killed by placing them in a chamber that was then filled with CO2. DRGs were removed and collected in a culture dish filled with sterilized Puck's solution. The tube was centrifuged for 50 s at low speed (∼2,000 g) and the pellet was resuspended in Puck's solution containing collagenase (1 mg/ml, type 1A) and dispase II (2.5 mg/ml). After a 1 h incubation at 37°C, the tube was centrifuged for 50 s, after which the enzyme-containing supernatant was removed. The pellet was resuspended in F-12 medium supplemented with 30 ng/ml 7S nerve growth factor (Harlan Bioproducts, Indianapolis, IN) and mechanically dissociated with fire-polished pipettes until all obvious chunks of tissues were gone. Isolated cells were plated onto plastic coverslips that had been previously coated with poly d-lysine and laminin. The cells were maintained in F-12 medium containing nerve growth factor at 37°C and 3% CO2 for 18–24 h prior to aspirating the individual cells for isolation of mRNA. This was the same procedure and culture time as previously used in our electrophysiological studies (8). All procedures have been approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
To collect single cells, a coverslip with the sensory neurons was placed in a recording chamber where the neurons were bathed in normal Ringer solution of the following composition (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH. Pipettes were pulled from borosilicate glass tubing and fire-polished; the glass tubes were previously baked at 250° C for 2 h. The tip of the pipette (20–25 μm diameter) was dipped into DEPC Millipore-filtered distilled water to draw up ∼5 μl. The pipette was then positioned against a cell's membrane, and negative pressure was applied to draw the cell into the pipette tip. Cell diameters were measured by a calibrated eye-piece micrometer. The lysed cell was transferred from the pipette tip into an RNase-, DNase-free 200 μl PCR tube. Final volumes of cell lysate generally ranged from 5 to 8 μl. Cell lysates were immediately placed on ice and then processed to isolate RNA.
cDNA from single cells.
All single cell lysates were first incubated with DNase (deoxyribonuclease I; amplification grade kit, catalog #18068-015, Invitrogen) for 15 min at room temperature in a final volume of 10 μl. To stop the DNase reaction, 1 μl of 25 mM EDTA was added to each tube and the tube was incubated at 65°C for 10 min. Reverse transcription was achieved with 0.5 μl iScript reverse transcriptase (iScript cDNA Synthesis Kit Bio-Rad catalog #170-8891) in a cumulative volume of 15 μl. The reactions were incubated as follows: 25°C for 5 min, 42°C for 30 min, 85°C for 5 min, and then stored at −20°C. To test that genomic DNA was not responsible for any amplification in qPCR, a limited number of cell lysates were split after the DNase reaction, omitting the reverse transcription reaction in one half, but otherwise completing all other treatments as described below.
Preamplification of single cell cDNA.
A pooled assay mix was prepared by adding 2 μl of 20× TaqMan Gene Expression Assay for each GOI (see Table 2) to Tris-EDTA buffer pH 8.0, final volume 80 μl. All Gene Expression Assays are labeled with the reporter dye FAM, except for HPRT, which was labeled with reporter dye VIC. To each 15 μl single cell cDNA, 25 μl of a Complete Pre-amp Master Mix was added. Each 25 μl of Complete Pre-amp Master Mix contained 20 μl of 2× TaqMan Pre-Amp Master Mix (ABI catalog #4391128), 4 μl of 0.5× pooled assay mix, and 1 μl nuclease-free H2O (Ambion catalog #9932). After a 10 min incubation at 95°C, 14 cycles of 95°C/15 s and 60°C/4 min were run, followed by −20°C storage.
Table 2.
Genes of Interest and primer efficiencies for TaqMan Gene Expression Assay
| Gene of Interest | ABI Catalog Number | Efficiency |
|---|---|---|
| S1PR1 | Rn02758712_s1 | 1.968 |
| S1PR2 | Rn02130568_s1 | 1.957 |
| S1PR3 | Rn02758880_s1 | 1.953 |
| S1PR4 | Rn01408085_s1 | 1.896 |
| S1PR5 | Rn00572952_s1 | 1.966 |
| TRPV1 | Rn00583117_m1 | 2.020 |
| P2X3 | Rn00579301_m1 | 2.011 |
| Arbp | Rn00821065_g1 | 1.950 |
| HPRT | Rn01527840_m1 | 1.950 |
Efficiency was calculated from the average slope obtained from 3 separate standard curve experiments.
Preamplification uniformity analysis.
To test that the preamplification reaction does not change the relative levels of expression of the S1PR isoforms and reference genes, preamplification was performed in a 20 μl reaction volume using 250 ng or 2.5 ng of cDNA isolated from rat lung. A negative control reaction used nuclease-free water as the template. qPCR was run using a 10-fold dilution of the preamplified cDNA and 10-fold dilutions of the original 250 ng and 2.5 ng rat lung cDNA as templates. Cq values of the preamplified and original rat lung cDNAs were analyzed as described in the TaqMan Preamp Master Mix Kit Protocol Appendix A (http://tools.invitrogen.com/content/sfs/manuals/cms_039316.pdf). Briefly, this involves determination of Cq values for nonamplified and preamplified GOI and reference genes and respective calculations of ΔCq and ΔΔCq for preamplified vs. nonamplified samples. According to the guidelines in TaqMan Preamp Appendix A, values of ΔΔCq between ± 1 indicate uniform preamplification. The results for the preamplification uniformity analysis for S1PR2 and S1PR5 are summarized in Table 3; these two receptors were chosen because of their range in relative expression, S1PR2 was the second highest expressing receptor, whereas S1PR5 was the smallest in lung. These results demonstrate that values of ΔΔCq for both S1PR2 and S1PR5 were within the ± 1 criteria values, thus demonstrating uniform preamplification. In addition, the ratios for ΔCq 250 ng/ΔCq 2.5 ng were very close to 1, indicating that the extent of amplification was not dependent on the amount of starting template.
Table 3.
Uniformity analysis for preamplification
| Target | Pre | Cq | ΔΔCq | ΔCq | Ampl | ΔCq 250 ng/ΔCq 2.5 ng |
|---|---|---|---|---|---|---|
|
Lung 250 ng cDNA | ||||||
| S1PR2 | + | 13.518 | 0.638 | 11.712 | 0.948 | 1.013 |
| S1PR2 | − | 25.230 | ||||
| S1PR5 | + | 16.280 | 0.224 | 12.126 | 0.982 | 0.911 |
| S1PR5 | − | 28.406 | ||||
| Arbp | + | 9.098 | −0.388 | 12.738 | 1.031 | 1.038 |
| Arbp | − | 21.837 | ||||
| HPRT | + | 12.833 | 0 | 12.350 | 1.0 | 1.0 |
| HPRT | − | 25.183 | ||||
|
Lung 2.5 ng cDNA | |||||
|---|---|---|---|---|---|
| Target | Pre | Cq | ΔΔCq | ΔCq | Ampl |
| S1PR2 | + | 21.468 | 0.770 | 11.564 | 0.938 |
| S1PR2 | − | 33.032 | |||
| S1PR5 | + | 23.348 | −0.974 | 13.309 | 1.079 |
| S1PR5 | − | 36.656 | |||
| Arbp | + | 16.635 | 0.060 | 12.274 | 0.995 |
| Arbp | − | 28.909 | |||
| HPRT | + | 20.244 | 0 | 12.335 | 1.0 |
| HPRT | − | 32.579 | |||
Pre, preamplification; Cq, quantitation cycle; GOI, gene of interest; ΔΔCq, ΔCqpreamp− ΔCqnoampwithin a gene target where ΔCqpreamp= CqGOIpreamp− CqHPRTpreampand ΔCqnoamp= CqGOInoamp− CqHPRTnoamp; ΔCq = Cqnoamp− Cqpreampwithin a gene target; Ampl: amplification within a gene target = ΔCqGOI− ΔCqHPRT.
cDNA from rat lung: single cell studies.
To create a large pool of rat lung cDNA to use as a positive control throughout the entire study, peripheral lung tissue from four different rats was harvested, and total RNA was extracted using the RNeasy Plus Mini Kit. Each individual RNA was assessed on a NanoDrop ND-1000 Spectrophotometer (Thermoscientific) for concentration (A260) and purity by OD ratios (A260/A280, ranging between 2.0 and 2.2). RNA integrity was also assessed using the Experion RNA StdSens Analysis Kit and Experion automated electrophoresis system (Bio-Rad). All individual rat lung RNA samples had an RQI value of at least 8.0. These RNAs were pooled, a new NanoDrop assessment was made, and 30 μg of pooled RNA was treated with 5 units of DNase in 6 × 5 μg reactions (50 μl per reaction) for 15 min at room temperature. DNase activity was stopped by adding 5 μl of 25 mM EDTA to each reaction and incubating the reactions for 10 min at 65°C. To convert the RNA to cDNA, each reaction received 45 μl of a Master Mix containing 20 μl 5× iScript Reaction Buffer, 5 μl iScript reverse transcriptase, and 20 μl nuclease-free water. The reactions were incubated as follows: 25°C for 5 min, 42°C for 30 min, 85°C for 5 min, hold at 4°C, or store at −20°C. To assess the specificity of these reactions, no RT and no template controls were also generated.
Real-time TaqMan qPCR: single cell studies.
qPCR reactions were run in triplicate on an Applied Biosystems 7500 Fast Real-Time PCR System using MicroAmp Fast 96-well reaction plates sealed with MicroAmp optical adhesive film. Amplification reactions were run with TaqMan Gene Expression Master Mix and 900 nM primers in a 10 μl volume. All reactions began with an initial cycle of 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Validation of each S1PR, HPRT, and Arbp primer pair were determined in rat lung, since rat lung expresses all five S1PR subtypes to a measurable level. Primer pairs for TRPV1 and P2X3 were validated in rat DRG. Efficiencies for each primer pair were determined from the fitted slope of a seven-point standard curve. The regression fit was considered to be acceptable when the r2 > 0.99. Calculated efficiencies for all validated primers are listed in Table 2. The Cq was chosen to be number of cycles when the value of normalized fluorescence generated by FAM or VIC emission (ΔRn) attained 0.3. The template for single cell qPCR was a fivefold dilution of the preamplified cDNA stock solution. Positive control template was a 15-fold dilution of the pooled rat lung cDNA. For determination of the distribution of S1PR isoforms in each single cell (and rat lung positive control), the slope from the standard curve of the appropriate GOI and the Cq at ΔRn = 0.3 were used to estimate the number of copies of each gene as described above. Using the results obtained in the validation of TRPV1 and P2X3 primers, we determined that the limit of reliable detection for assessing the absence or presence of mRNA for TRPV1 was a Cq value of ≤35, whereas for P2X3 the Cq was 36.
Data analysis.
Data are presented as the means ± SE. To determine the possible correlation between expression levels of the different S1PRs normalized to Arbp, the correlation function in Excel was used to determine Pearson's correlation coefficient. Statistical differences between expression levels were determined by either a Student's t -test or an ANOVA. When a significant difference was obtained with an ANOVA, post hoc analysis was performed by a Tukey all-pairwise test. If the data set failed the normality test, a Kruskal-Wallis ANOVA on ranks was performed with a Tukey all-pairwise test. Values of P < 0.05 were judged to be statistically significant.
Chemicals.
Tissue culture supplies were purchased from Invitrogen (Carlsbad, CA). Unless specifically designated, all other chemicals were obtained from Sigma Chemical (St. Louis, MO).
RESULTS
Expression of S1PR mRNA in lung, brain, and DRG.
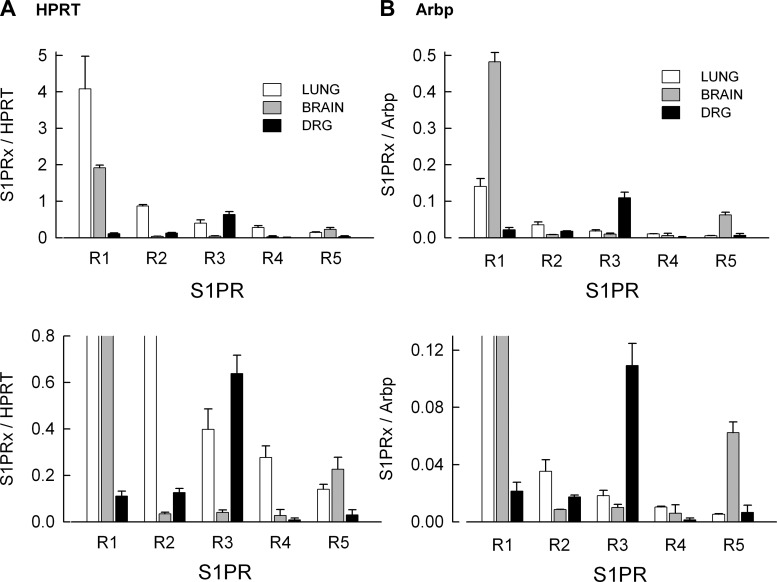
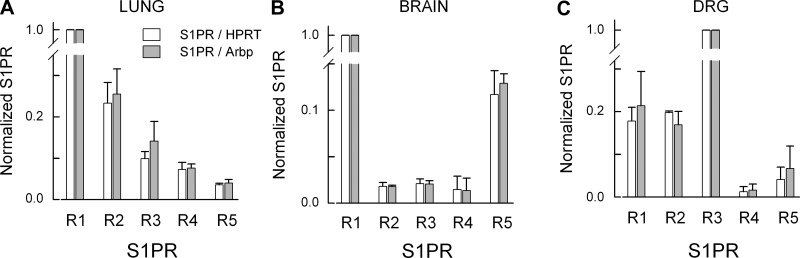
The expression levels of mRNA for each S1PR measured in rat lung, brain, and DRG is summarized in Fig. 2. Figure 2A (top) represents expression levels of S1PRs after normalization to the reference gene HPRT; Fig. 2B (top) shows normalization to Arbp. In both lung and brain the highest expressing receptor was S1PR1. Although the Cq values for S1PR1 in lung and brain are similar (see Table 4), in lung the Cq for the reference gene, HPRT, is higher than in brain, while in lung the Cq for Arbp is lower than in brain. Thus, when normalized to HPRT, S1PR1 levels were greater in lung than brain, whereas when normalized to Arbp, this was reversed. In contrast to lung and brain, the most highly expressed receptor in the DRG was S1PR3 after normalization to either HPRT or Arbp. Based on the values of Cq, the expression levels of HPRT are quite similar in brain and DRG; similar results were obtained with Arbp and suggest that these are stable reference genes for the normalization of S1PR expression in these neural tissues. The bottom panels of Fig. 2, A and B, demonstrate the expression levels of S1PRs at an expanded scale to better show the lower levels for S1PR2 -S1PR5 in these tissues. To further illustrate the levels of each S1PR relative to the other receptors, the expression for each receptor (relative to the reference genes) was then normalized to the most highly expressed receptor in each respective tissue (lung and brain S1PR1, DRG S1PR3). As shown in Fig. 3A, normalization to S1PR1 demonstrated that the rank order for expression in lung was R1 >>R2 >R3 ≈R4 >R5. In brain, normalization to S1PR1 indicated that R1 >>R5 >>R2 ≈R3 ≈R4 (see Fig. 3B); however, in the DRG normalization to S1PR3 showed that R3 >>R1 ≈R2 >R4 ≈R5. In the three tissues, the fractional expression for any given S1PR relative to either HPRT or Arbp is quite consistent indicating the stability of HPRT and Arbp as reference genes for quantification of S1PR expression levels.
Fig. 2.
Expression of S1PRs in tissues isolated from lung, brain, and dorsal root ganglion (DRG). A, top: the expression levels of the 5 S1PRs relative to the reference gene HPRT. Bottom: the results shown at top at an expanded scale to better demonstrate the differences at low levels of expression for S1PR2-R5. B, top and bottom: expression of S1PRs relative to the reference gene acidic ribosomal protein P0 (Arbp). The results represent means ± SE for 3 separate tissue harvests.
Table 4.
Cq values for S1PRs in lung, brain, and DRG
| Lung | Brain | DRG | |
|---|---|---|---|
| S1PR1 | 19.262 ± 0.096 | 19.228 ± 0.125 | 24.046 ± 0.116 |
| S1PR2 | 21.873 ± 0.054 | 25.566 ± 0.256 | 23.811 ± 0.044 |
| S1PR3 | 22.869 ± 0.546 | 26.406 ± 0.449 | 21.922 ± 0.289 |
| S1PR4 | 23.727 ± 0.073 | 29.818 ± 0.195 | 30.420 ± 0.138 |
| S1PR5 | 24.872 ± 0.224 | 22.338 ± 0.417 | 28.758 ± 0.170 |
| HPRT | 21.333 ± 0.188 | 20.387 ± 0.209 | 20.615 ± 0.145 |
| Arbp | 16.227 ± 0.088 | 17.785 ± 0.088 | 17.626 ± 0.042 |
Values are means ± SE obtained from 3 separate tissue harvests.
Fig. 3.
S1PR expression normalized to the most highly expressed receptor subtype in each tissue. In lung and brain, S1PR1 was the highest expressor, whereas for the DRG it was S1PR3. Note the similar levels of S1PR expression for HPRT and Arbp.
Expression levels of S1PR mRNA in single defined isolated sensory neurons.
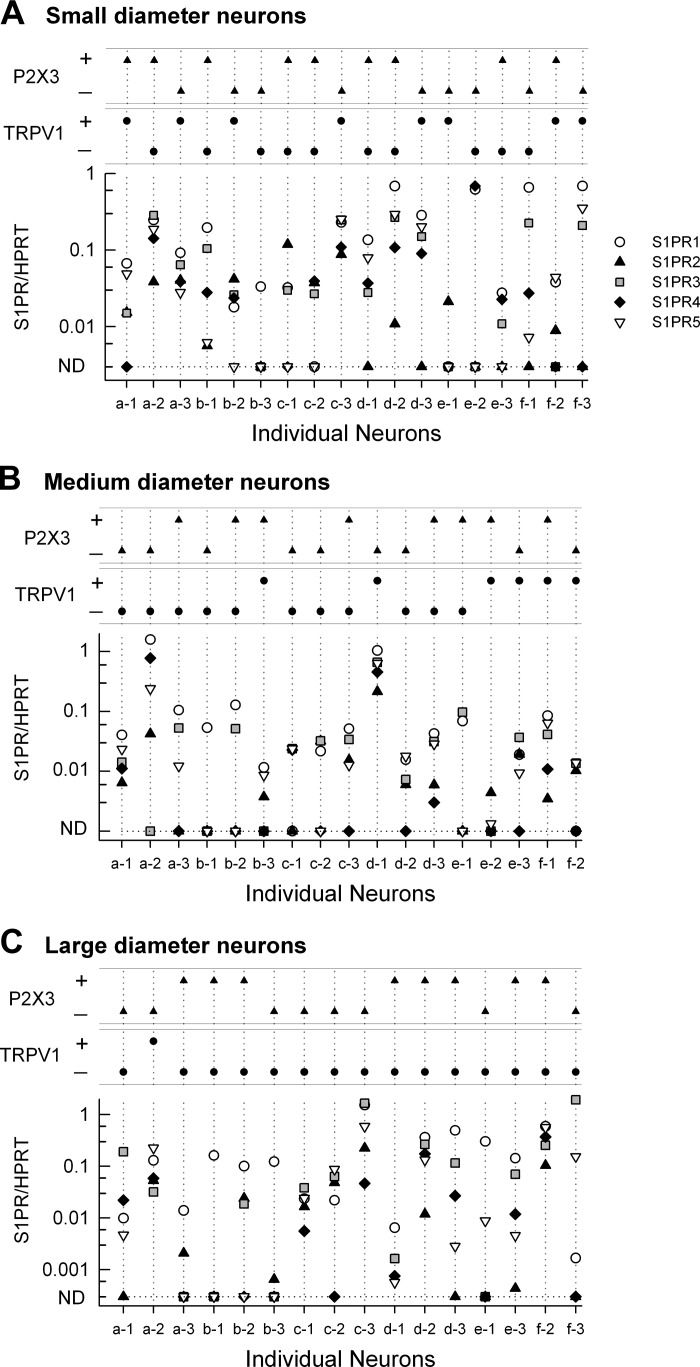
Our finding that expression of message for S1PR3 was by far the highest in the DRG was unexpected. In our previous work, recordings from small-diameter sensory neurons that had been treated with siRNA targeted to S1PR1 demonstrated that the sensitization produced by the S1PR1 selective agonist SEW2871 was completely blocked, whereas one-third of these SEW2871-insensitive neurons were sensitized by the general agonist, S1P (8). These results suggested that S1PR1 plays a prominent, although not exclusive, role in augmenting excitability and raise the question as to what are the expression levels of S1PRs in single defined isolated sensory neurons. Initial experiments indicated that using our previous single-cell PCR protocol (8) would be ineffective in the analysis of expression for all five S1PRs; therefore, we adopted the preamplification approach described in methods to measure mRNA levels for multiple genes (see Table 2). Sensory neurons were isolated from the DRG and maintained in culture for <24 h using our standard protocol for electrophysiological studies. Single neurons were visually identified, and cell body diameters were recorded and then aspirated into a large-diameter glass pipette. These single neurons then underwent the preamplification PCR protocols described in methods.
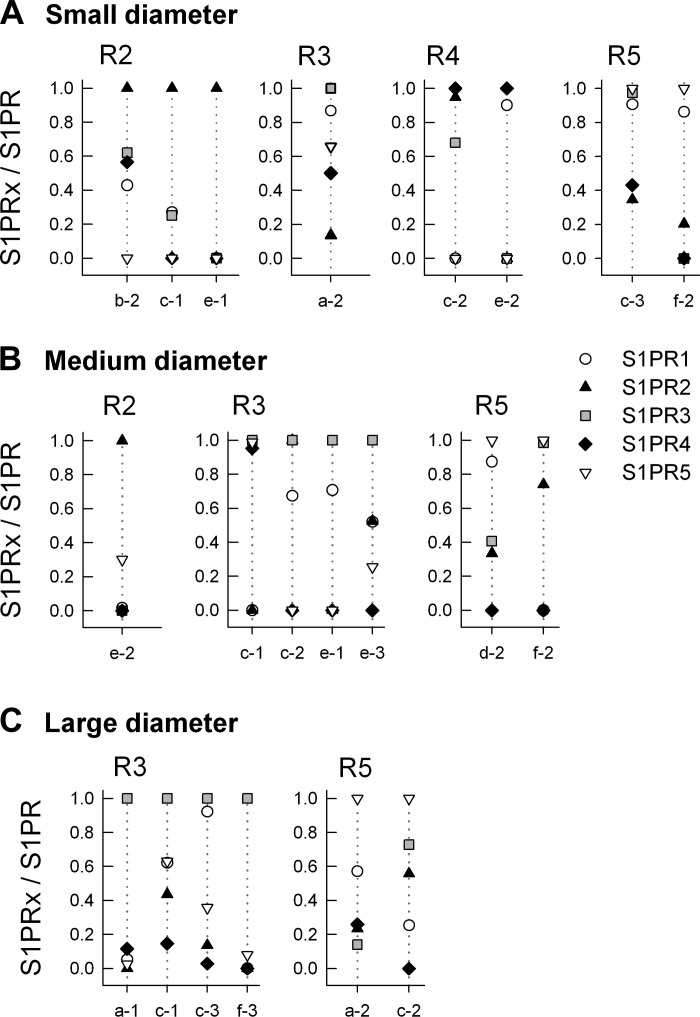
Previous studies by Lawson (16, 17, 26) demonstrated that sensory neurons of the DRG could be phenotypically classified based on the diameter of the neuronal cell body. Using their established classifications, we sorted sensory neurons into three groups based on cell body diameter: small-diameter neurons were <25 μm, medium-diameter neurons were 25–40 μm, and large-diameter neurons were >50 μm. The expression profiles for S1PRs in the three classes of sensory neurons are shown in Fig. 4. An advantage of the preamplification protocol was that in addition to S1PRs, single cell mRNA could also be probed for other phenotypic markers of sensory neurons, namely the capsaicin-sensitive channel TRPV1 and the purinergic channel P2X3. The S1PR, TRPV1, and P2X3 profiles for 18 small-diameter sensory neurons are shown in Fig. 4A. The levels of expression were surprisingly variable wherein some neurons expressed all five receptors (e.g., neurons a-2 and c-3), whereas some neurons expressed only one receptor subtype (e.g., b-3 and e-1). Of the 18 small-diameter neurons, 16 expressed S1PR1, 11 expressed S1PR2, 14 expressed S1PR3, 12 expressed S1PR4, and 11 expressed S1PR5. Similar results were obtained for medium- and large-diameter neurons (see Fig. 4, B and C). Of the 17 medium-diameter neurons, 14 expressed S1PR1, 12 expressed S1PR2, 13 expressed S1PR3, 6 expressed S1PR4, and 13 expressed S1PR5. Of the 16 large-diameter neurons, 16 expressed S1PR1, 10 expressed S1PR2, 12 expressed S1PR3, 9 expressed S1PR4, and 12 expressed S1PR5. The expression of the phenotypic markers, TRPV1 and P2X3, for these three neuronal diameters is summarized in Table 5. Consistent with the existing literature (6, 10, 14, 32, 53), ∼50% of the small-diameter neurons were TRPV1 positive and 50% were P2X3 positive, whereas for the large-diameter neurons, 15 of the 16 were TRPV1 negative.
Fig. 4.
Expression profiles of S1PRs in single identified sensory neurons of small, medium, and large diameters. A: expression of S1PR mRNAs in a total of 18 small-diameter sensory neurons (3 isolated neurons from 6 rats). The 2 small panels at the top indicate whether these individual neurons expressed detectable levels of P2X3 and/or TRPV1. B: expression of S1PR mRNAs in a total of 17 medium-diameter sensory neurons (from the same 6 rats as in A). C: expression of S1PR mRNAs in a total of 16 large-diameter sensory neurons (from the same 6 rats as in A). The distributions of P2X3 and TRPV1 expression are summarized in Table 5. ND, not detected.
Table 5.
Expression of phenotypic markers for sensory neurons
| Small | Medium | Large | |
|---|---|---|---|
| TRPV1− P2X3− | 3 | 4 | 7 |
| TRPV1− P2X3+ | 7 | 7 | 8 |
| TRPV1+ P2X3− | 6 | 0 | 1 |
| TRPV1+ P2X3+ | 2 | 6 | 0 |
| Neurons, n | 18 | 17 | 16 |
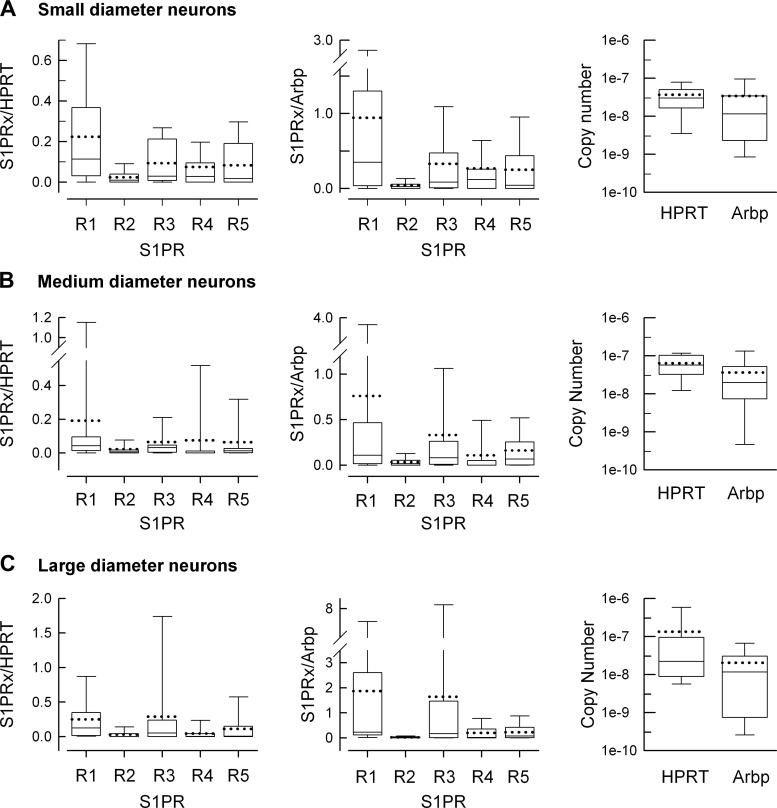
Expression of the five S1PRs in small-, medium-, and large-diameter neurons is summarized in the box plots shown in Fig. 5. The expression levels relative to HPRT and Arbp are illustrated in the left and middle panels, respectively, while the right panels indicate the copy number values of the two reference genes for each diameter group. The copy number calculated at ΔRn = 0.3 for HPRT was significantly greater than for Arbp in both the small (3.67 ± 0.81 × 10−8 vs. 3.40 ± 1.79 × 10−8, respectively, P = 0.04 Mann-Whitney rank sum test) and medium-diameter neurons (6.33 ± 0.95 × 10−8 vs. 3.61 ± 1.15 × 10−8, respectively, P = 0.02 Mann-Whitney rank sum test), whereas there was no difference for the large-diameter neurons (1.33 ± 0.90 × 10−7 vs. 2.02 ± 0.71 × 10−8, respectively, P = 0.052 Mann-Whitney rank sum test). In addition, there was no difference in the levels of either HPRT (P = 0.09 Kruskal-Wallis one-way ANOVA on ranks) or Arbp (P = 0.45 Kruskal-Wallis one-way ANOVA on ranks) for the three different diameters of neurons. For all three diameter groups, there is ∼10- to 20-fold higher levels of S1PR1 than S1PR2, although this difference was only significant for small diameter neurons (P = 0.02 Kruskal-Wallis one-way ANOVA on ranks, Tukey all-pairs post hoc test). The failure to achieve a significant difference for medium- and large-diameter neurons likely results from the large amounts of variance in these measurements. In the large-diameter neurons, there are about equal levels of S1PR1 and S1PR3 along with 5- to 10-fold more S1PR1 than S1PR4 and R5. In medium-diameter neurons, S1PR1 was significantly higher than S1PR4 (P = 0.005 Kruskal-Wallis one-way ANOVA on ranks, Tukey all-pairs post hoc test), and in large-diameter neurons S1PR1 was significantly higher than S1PR2 and S1PR4 (P = 0.008 Kruskal-Wallis one-way ANOVA on ranks, Tukey all-pairs post hoc test). There were no significant differences in S1PR expression levels between TRPV1 positive and negative small- and medium-diameter neurons. For example, the mean value for S1PR1 expression relative to HPRT in TRPV1 positive small-diameter neurons was 0.176 ± 0.081 (n = 8) compared with 0.262 ± 0.089 (n = 10) in TRPV1 negative neurons (P = 0.79 Student's t-test, data not shown). Similarly, there was no difference in S1PR1 expression levels in large-diameter neurons that were P2X3 positive (0.235 ± 0.079, n = 8) compared with P2X3 negative neurons (0.287 ± 0.210, n = 7, P = 0.46 Student's t-test, data not shown). These results indicate that S1PR1 is the most highly expressed S1PR in sensory neurons regardless of cell body diameter. This is in contrast to the results obtained from the whole DRG where S1PR3 was the most highly expressed receptor, suggesting that other cell types in the DRG, such as satellite cells, express significant levels of S1PR3. Future studies clearly are warranted to address this difference in S1PR3 levels.
Fig. 5.
Summary of S1PR expression in small-, medium-, and large-diameter sensory neurons. A, left: a box plot summary of S1PR expression relative to HPRT in the 18 small-diameter neurons represented in Fig. 4A. Middle: summary of S1PR expression relative to Arbp. Right: the copy numbers for HPRT and Arbp calculated at ΔRn = 0.3. B, left: a box plot summary of S1PR expression relative to HPRT in the 17 medium-diameter neurons represented in Fig. 4B. Middle: summary of S1PR expression relative to Arbp. Right: the copy numbers for HPRT and Arbp. C, left: a box plot summary of S1PR expression relative to HPRT in the 16 large-diameter neurons represented in Fig. 4C. Middle: summary of S1PR expression relative to Arbp. Right: the copy numbers for HPRT and Arbp. For the box plot the top and bottom error bars represent the 90 and 10% range of the data, respectively; the top and bottom tiles of the box represent the 75 and 25% range, respectively; the solid line through the box represents the median value, and the dotted line through the box represents the mean value.
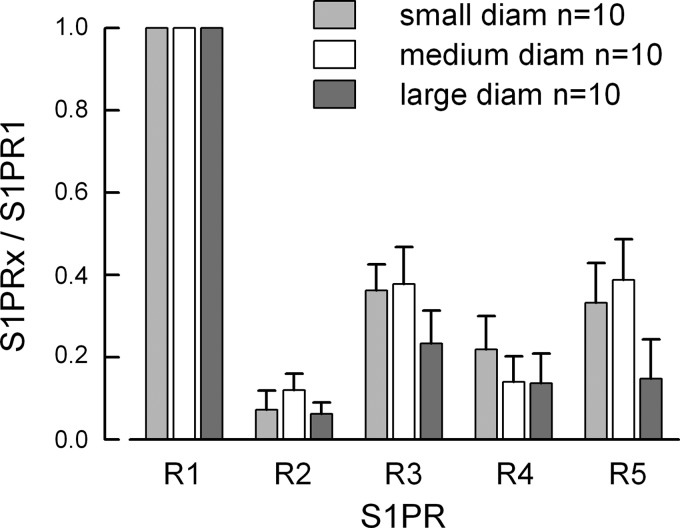
Further analysis indicated that S1PR1 was the most highly expressed receptor subtype in 10 of the total number of neurons for each group of neuronal diameters. In an attempt to reduce the variance, the expression of the other four S1PRs were normalized to the level of S1PR1 (relative to HPRT) in each of those 10 neurons wherein S1PR1 was the highest expressor; results for small, medium, and large neurons are summarized in Fig. 6. The expression of S1PR1 was significantly greater than R2, R3, R4, and R5 in each group (P < 0.001 Kruskal-Wallis one-way ANOVA on ranks, Tukey all-pairs post hoc test), although there were no differences in expression of individual receptor subtypes between the three diameter groups (P = 0.37, 0.37, 0.59, and 0.17 for R2, R3, R4, and R5 respectively, Kruskal-Wallis one-way ANOVA on ranks). In addition, S1PR1 was the second most highly expressed receptor subtype in four of the remaining eight small-diameter, three of seven medium-diameter, and three of six large-diameter neurons, demonstrating that S1PR1 exhibited the most prominent expression profile of the five S1PRs in sensory neurons. In the eight small-diameter neurons where S1PR1 was not the highest expressor there was a mix of the other four receptors; R2, R3, R4, and R5 were the highest expressor in three, one, two, and two neurons, respectively (see Fig. 7A). In the seven medium-diameter neurons, R2, R3, and R5 were the highest in one, four, and two neurons, respectively (Fig. 7B). Lastly, in the six large-diameter neurons, only R3 and R5 were detected as the highest expressors in four and two neurons, respectively (Fig. 7C). These results are consistent with our previous results wherein S1PR1 played a prominent although not exclusive role in the S1P-mediated enhancement of excitability in small-diameter sensory neurons (8).
Fig. 6.
S1PR expression normalized to S1PR1 levels in neurons in which S1PR1 was the most highly expressed subtype. S1PR1 was the highest expressor in 10 of 18, 10 of 17, and 10 of 16 the small-, medium-, and large-diameter neurons, respectively.
Fig. 7.
S1PR expression levels normalized to the most highly expressed subtype in neurons wherein S1PR1 was not the highest expressor. A, B, and C: summaries of the remaining small-, medium-, and large-diameter sensory neurons, respectively.
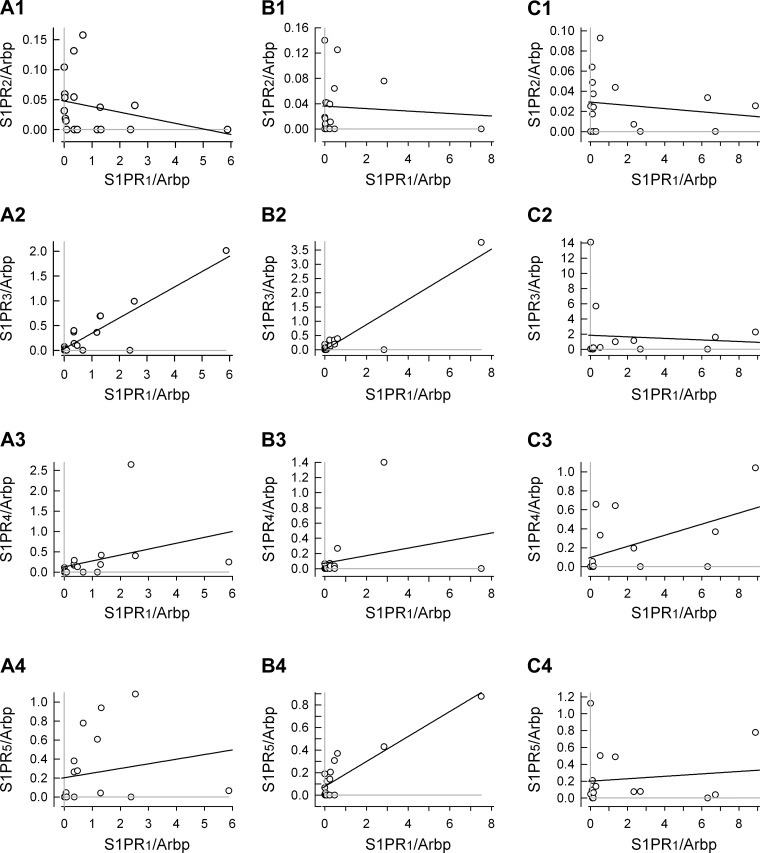
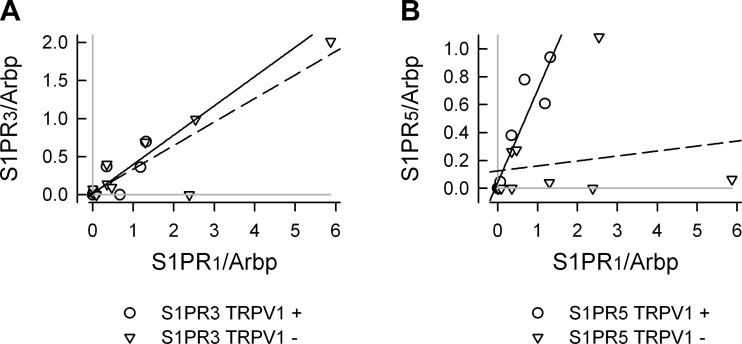
To establish whether there was an association between the expression levels for the different S1PRs a correlation analysis was performed. These results are summarized in Fig. 8 wherein the expression levels for S1PR2–5 /Arbp were correlated with the expression of S1PR1 /Arbp for small (Fig. 8A, 1–4)-, medium (Fig. 8B, 1–4)-, and large (Fig. 8C, 1–4)-diameter neurons. In addition, Table 6 summarizes the values of Pearson's correlation coefficients for the relations between S1PRs. In small-diameter neurons (n = 18), there appeared to be a correlation between the expression of S1PR1 and S1PR3 having a Pearson's coefficient of 0.885. This analysis indicated no other strong correlations between S1PR expression. When the small-diameter neurons were divided into TRPV1-positive (n = 8/18) and negative (n = 10/18)-populations (see Fig. 9), the correlations between S1PR1 and S1PR3 were maintained (correlation coefficients of 0.803 and 0.888 for TRPV1-positive and -negative neurons, respectively; data not shown). However, in TRPV1-positive neurons, there was a strong correlation between S1PR1 and S1PR5 (coefficient of 0.924), whereas in TRPV1-negative neurons this correlation was absent (coefficient of 0.195). In large-diameter neurons, there appeared to be a good correlation between S1PR3 and S1PR5 expression (coefficient of 0.735). Interestingly, the medium-diameter neurons exhibited characteristics of small- and large-diameter neurons. There was a good correlation between S1PR1 expression and S1PR3 as well as S1PR1 and S1PR5 expression (coefficients of 0.922 and 0.895, respectively) but also between S1PR3 and S1PR5 (0.8221). Thus, these results suggest that there may be characteristic expression profiles for S1PRs in these different groups of sensory neurons; however, caution should be taken in making such conclusions because of the limited sample size. Future studies incorporating larger numbers of neurons as well as additional mRNA targets are warranted to corroborate these preliminary indicators.
Fig. 8.
Correlation analysis for the levels of S1PR2–5/Arbp as a function of S1PR1/Arbp in small (A, 1–4)-, medium (B, 1–4)-, and large (C, 1–4)-diameter sensory neurons. The solid black line in all panels represents the linear regression as determined by SigmaPlot 9.01. Pearson's correlation coefficient for each panel was determined by using the correlation function in Excel (Office 12) and is listed in Table 6. The gray lines in each panel indicate the levels for undetected receptor expression; data points that lie on the gray line indicate that a particular receptor was not detected in that neuron.
Table 6.
Correlation analysis of S1PR subtypes in small-, medium-, and large-diameter neurons
| S1PR1 | S1PR2 | S1PR3 | S1PR4 | S1PR5 | |
|---|---|---|---|---|---|
| Small | |||||
| S1PR1 | 1.0 | −0.283 | 0.885 | 0.348 | 0.198 |
| S1PR2 | 1.0 | −0.199 | −0.240 | 0.196 | |
| S1PR3 | 1.0 | −0.015 | 0.286 | ||
| S1PR4 | 1.0 | −0.055 | |||
| S1PR5 | 1.0 | ||||
| Medium | |||||
| S1PR1 | 1.0 | −0.083 | 0.922 | 0.274 | 0.895 |
| S1PR2 | 1.0 | −0.142 | 0.336 | 0.270 | |
| S1PR3 | 1.0 | −0.101 | 0.821 | ||
| S1PR4 | 1.0 | 0.343 | |||
| S1PR5 | 1.0 | ||||
| Large | |||||
| S1PR1 | 1.0 | −0.172 | −0.081 | 0.526 | 0.126 |
| S1PR2 | 1.0 | −0.374 | 0.032 | 0.091 | |
| S1PR3 | 1.0 | 0.123 | 0.735 | ||
| S1PR4 | 1.0 | 0.426 | |||
| S1PR5 | 1.0 | ||||
Numbers represent the Pearson correlation coefficient.
Fig. 9.
Correlation analysis for S1PR1, R3, and R5 in TRPV1-positive and -negative small-diameter neurons. A: the correlation analysis for S1PR3/Arbp vs. S1PR1/Arbp. B: the correlation analysis for S1PR5/Arbp vs. S1PR1/Arbp. The solid lines represents the linear regression for the TRPV1-positive neurons, whereas the dashed lines represent the linear regression for TRPV1-negative neurons. The correlation coefficients are listed in results. The gray lines in each panel indicate the levels for undetected receptor expression; data points that lie on the gray line indicate that a particular receptor was not detected in that neuron.
DISCUSSION
Our earlier work demonstrated that treatment with S1P enhanced the excitability of small-diameter capsaicin-sensitive sensory neurons largely through activation of S1PR1, although this increased excitability could be attributed to other S1PRs (8, 59). In addition, we found that exposure to S1P enhanced the frequency of medium- and large-diameter neurons to fire multiple action potentials in recordings from the isolated but intact DRG (56). Consistent with our electrophysiological results, we report here that S1PR1 was the most highly expressed receptor subtype in over half of small-, medium-, and large-diameter sensory neurons. In addition, S1PR1 was the second highest expressing subtype in about half of those remaining neurons in which other S1PR subtypes were the highest expressors. Compared with the single cell results, S1PR3 was surprisingly the most highly expressed receptor subtype in the intact DRG exceeding S1PR1 by about fivefold. This observation is similar to that reported where qPCR measurements from DRG explants indicated that S1PR3 levels were greater than S1PR1 (28), although not to the extent that we observed in this study. In contrast to our single cell results, these authors measured approximately similar levels of S1PR1, R2, and R3 in either acutely isolated DRG neurons or after 1 day in culture, whereas we detected very low levels of S1PR2 and only moderate levels of S1PR3 in all diameter sizes compared with S1PR1 levels. In addition, they failed to detect S1PR4 or R5 in isolated neurons. Possible differences between our studies may result because our single cell qPCR utilized preamplification of the isolated mRNA, thereby enhancing the sensitivity of our measurements. Consistent with this idea, detection of some S1PRs (e.g., S1PR2) was very near the Cq limit using conventional qPCR approaches. Furthermore, it seems unlikely that these expression levels were not elevated artifactually as the uniformity analysis indicated successful preamplification for even small amounts of cDNA and that many of the neurons exhibited expression profiles wherein receptor subtypes were not detected. These results clearly indicate that we are only beginning to understand the expression levels of S1PRs in the DRG and different sensory neuron populations.
Our results indicate there was no differential distribution of S1PRs amongst the three neuronal groups based on cell body diameter. Small-diameter neurons (<25 μm) give rise to unmyelinated C-fibers, which have slow conduction velocities (<1.4 m/s); medium-diameter neurons (25–40 μm) give rise to lightly myelinated Aδ fibers having faster conduction velocities (2–8 m/s), and large-diameter (>40 μm) give rise to more heavily myelinated Aα/β fibers that have conduction velocities >14 m/s (16, 17, 26). Similarly, there appeared to be no correlation between S1PR expression and expression of TRPV1 and/or P2X3. Thus, it appears that S1PR expression is not linked with any particular modality, i.e., thermal or mechanical sensitivity, for these defined neuronal populations. However, S1PR1 was the highest or second most highly expressed receptor subtype in all three populations, suggesting that it may play an important role in regulating the sensitivity of all three populations. In fact our recent results indicate that treatment of the intact DRG with S1P augments the capacity of medium- and large-diameter neurons to fire multiple action potentials in response to a depolarizing current pulse (56). Furthermore, siRNA knock-down of S1PR1 in L4/L5 DRG significantly reduced the mechanical hypersensitivity produced by a localized injection of zymosan, indicating that inflammation-induced activation of S1PR1 may play an important role in regulating the sensitivity of cells in the DRG to peripheral stimulation (56). Future studies will determine which S1PRs are necessary to augment the excitability of sensory neurons.
Few investigations have examined the neurophysiological actions of S1P and the role of S1PRs in the nervous system (both peripheral and central) (reviewed by Ref. 34). In studies using S1P-induced 35S-GTPγS labeling to assess receptor localization, different brain regions have been shown to have quite variable levels of expression where the thalamus is relatively low compared with high levels in the cerebellum (48, 54). The presence of S1PRs in the central nervous system raises interesting questions as to what role sphingolipids may play in neuronal function. An early study showed that potassium-evoked depolarization released S1P from rat cerebellar granule cells and that S1PR 1–3mRNAs were detected in these neurons (2). Exposure of isolated rat hippocampal neurons to high potassium or S1P evoked the release of glutamate (22). Interestingly, the high potassium effect was blocked by pretreatment with the sphingosine kinase inhibitor, n,n-dimethylsphingosine, whereas the S1P effect was unaltered. Treatment of these hippocampal cells with siRNA targeted to S1PR1 or S1PR3 partially suppressed the S1P-induced effects on glutamate release, while both siRNAs together completely blocked release. In later reports by these authors, exposure to S1P, likely via S1PR3, augmented the frequency of AMPA-mediated excitatory postsynaptic currents (EPSCs) obtained in recordings from CA3 hippocampal neurons in brain slices, whereas S1P had no effect on CA1 neurons (23, 24). These results suggest that depolarization, by an undefined mechanism, caused the production of S1P via activation of sphingosine kinase and somehow release glutamate. Subsequently, this S1P could be released from the neuron wherein autocrine and/or paracrine activation of S1PR1/S1PR3 can also release glutamate from hippocampal neurons. In contrast to these findings, exposure of isolated and cultured cortical pyramidal neurons to S1P or SEW2871 (a selective S1PR1 agonist) decreased both the frequency and amplitude of spontaneous glutamate-mediated EPSCs (48). The EPSC suppression was partially reversed by pretreatment with VPC44116 (S1PR1/S1PR3 antagonist and a partial agonist at S1PR4/S1PR5), indicating that S1PR1 and R3 may be involved. The fact that S1P promotes glutamate release from hippocampal neurons, whereas S1P appears to suppress glutamate release from cortical pyramidal neurons may reflect differences in the downstream signaling effects initiated by these receptors. The physiological impact of these S1P-mediated actions in relation to neuronal function is yet to be fully understood.
Our previous observations in isolated small-diameter sensory neurons suggest that increased S1P levels associated with peripheral inflammatory conditions can enhance neuronal sensitivity through modulation of G protein-coupled signaling cascades. A proinflammatory role for peripheral S1P is supported by the finding that S1P injection into a rat's paw produced a dose-dependent edema that was associated with infiltration of eosinophils (45). Recently, these authors demonstrated that carrageenan-induced inflammation of the mouse paw produced a long-lasting elevation (>6 h) in S1P levels in the paw as well as increased expression of S1PR2 and R3, whereas there was no change in S1PR1 or R4 (44). In addition, synovial tissue from rheumatoid arthritis patients had increased expression of S1PR1 compared with those with osteoarthritis (25). Proliferation of synoviocytes isolated from patients with rheumatoid arthritis was significantly increased after treatment with S1P (25). In contrast to the peripheral actions of S1P, the central actions of S1P on nociceptive behavioral responses suggest that S1P may reduce neuronal sensitivity. Injection of zymosan or formalin into a rat's hind paw produces well-established animal models of inflammatory pain; yet under these conditions the levels of cerebrospinal S1P were reduced with no changes in ceramides (e.g., d16, whereas d18:1 was undetected) or sphingosine levels (sphinganine was undetected) (11). Indicative of a suppressive action, these authors found that intrathecal injection of S1P transiently reduced the intensity of the secondary phase of nociceptive behaviors produced by formalin. It is difficult to know which S1PR(s) mediated these antinociceptive effects as their conventional RT-PCR measurements indicated all five S1PRs were detected in adult rat spinal cord (11). These findings are consistent with our observations regarding expression of all five subtypes in the DRG and single isolated sensory neurons. Compatible with a lowering of S1P levels, prior intrathecal administration of n,n-dimethylsphingosine (an established sphingosine kinase inhibitor) significantly decreased the withdrawal latency in the hot plate test, suggesting that reduced S1P levels were associated with a heightened sensitivity to noxious thermal stimulation (11). However, two recent reports present findings that are in contrast to those of Coste et al. (11). First, morphine-induced thermal hyperalgesia exhibited a significant behavioral change after 3 days, and associated with the decreased thermal latency (i.e., enhanced sensitivity) was a fourfold increase in the S1P content of the dorsal horn of the spinal cord (33). This increase in S1P and the development of thermal hyperalgesia were blocked by pretreatment with n,n-dimethylsphingosine. Second, in a tibial nerve transection model of neuropathic pain, the levels of ceramides (both d16:0 and d18:1), sphinganine, sphingosine, as well as n,n-dimethylsphingosine were significantly elevated 21 days postinjury (38). Interestingly, there was a significant increase in mechanical sensitivity within 24 h of intrathecal delivery of n,n-dimethylsphingosine (38). These authors suggest that DMS produced mechanical allodynia independently of its capacity to inhibit sphingosine kinase as the dosage used was well below the value of the inhibitory constant for kinase inhibition. Also, this idea is supported by their findings that neither the levels of S1P in the dorsal horn after intrathecal DMS (1.6 μg) or the levels of sphingosine in isolated astrocytes were changed after DMS treatment. The contrasting actions observed in these three studies clearly indicate that our current understanding of S1P and the lysophospholipid signaling cascade in neuronal function is quite poor. Similar to the findings of Coste et al. (11), antinociceptive observations were obtained in another investigation wherein the latency of a thermally evoked tail-flick was significantly increased 20 min after intracerebroventricular injection of S1P (48). These authors showed that injection of SEW2871 also produced antinociceptive effects and the actions of both S1P and SEW2871 were reversed by pretreatment with VPC44116, suggesting that S1PR1 may be involved. It is difficult to assess the specific brain target of this S1P response since intracerebroventricular injection also produced catalepsy, hypothermia, and locomotor inhibition. Taking these results together, it appears that central S1P may be antinociceptive, whereas peripheral S1P may be pronociceptive. However, the mechanisms giving rise to these differences are presently unknown. Clearly, this is an area for future studies to resolve the role of S1P in neuronal function.
Previous studies have used Northern blot or in situ hybridization to assess expression of S1PRs in a variety of tissues. Northern blot analysis of S1PR expression in adult mouse brain indicated that S1PR1 was more highly expressed than S1PR5, whereas the levels of S1PR2/3/4 were much lower (57, 58). In the primate brain, in situ hybridization studies indicated that S1PR1 and R3 were expressed primarily in neurons; S1PR2 appeared to be more widespread, whereas S1PR4 was not detected (1). Expression of S1PR4 in neuronal tissues appears to be controversial. The mRNA for S1PR4 was detected in the spinal cord (11) and the DRG (Ref. 28 and the present report). However, in acutely isolated DRG neurons, S1PR4 mRNA was no longer detected, suggesting its expression was limited to nonneuronal cells (28). Our present study indicates that S1PR4 dmRNA was expressed in single identified neurons of the DRG. However, this observation must be tempered by our finding that the raw qPCR curve was abnormal and required adjustments to the baseline to obtain any results regarding the values of the Cq; thus caution is warranted regarding a meaningful determination of the levels of S1PR4 mRNA. In lung tissue from the mouse, Northern blot measurements of S1PR mRNA expression showed that S1PR1 exceeded that of R2 and R3 (which were similar to one another), whereas S1PR4/5 were quite weak (57, 58). These expression profiles exhibited in mouse brain and lung are consistent with our qPCR results obtained in rat (see Fig. 3, A and B). In addition, S1P-induced 35 S-GTPγS labeling was used to determine the localization of S1PRs in the brain wherein the labeling was quite variable from region to region. For example, labeling was high in the molecular layer of the cerebellum, whereas it was low in the thalamus (48, 54).
In conclusion, our work indicates that S1P significantly augments the excitability of small-diameter sensory neurons through enhancement of a TTX-resistant sodium current and inhibition of total potassium currents (59). When applied peripherally, S1P appears to be pronociceptive (12, 28), whereas centrally it may be antinociceptive (11, 48). In hippocampal neurons of the central nervous system, exposure to S1P appears to enhance the excitability and augments the release of glutamate (22–24), whereas in isolated neonatal cortical neurons, S1P appears to suppress transmitter release (48). To our knowledge, these are the only reports that have examined the functional impact of S1PR activation in nerve tissues. Based on the emerging role of S1P/S1PR-mediated signaling in neurons and glia and their potential interaction with immune cells, it is important to enhance our knowledge of the contributions of S1PRs to neuronal function.
GRANTS
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06 RR015481-01 from the National Center for Research Resources, National Institutes of Health. This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-060853.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S.K., C.L., and G.D.N. conception and design of research; J.S.K. and C.L. performed experiments; J.S.K. and G.D.N. analyzed data; J.S.K. and G.D.N. interpreted results of experiments; J.S.K. and G.D.N. drafted manuscript; J.S.K., C.L., and G.D.N. edited and revised manuscript; J.S.K., C.L., and G.D.N. approved final version of manuscript; G.D.N. prepared figures.
REFERENCES
- 1. Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396– 15401, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Anelli V, Bassi R, Tettamanti G, Viani P, Riboni L. Extracellular release of newly synthesized sphingosine-1-phosphate by cerebellar granule cells and astrocytes. J Neurochem 92: 1204– 1215, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol 15: 457– 465, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Beer MS, Stanton JA, Salim K, Rigby M, Heavens RP, Smith D, Mcallister G. EDG receptors as a therapeutic target in the nervous system. Ann NY Acad Sci 905: 118– 131, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453– 21457, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377: 428– 431, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci 32: 16– 24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chi XX, Nicol GD. The sphingosine 1-phosphate receptor, S1PR1, plays a prominent but not exclusive role in enhancing the excitability of sensory neurons. J Neurophysiol 104: 2741– 2748, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol 160: 5037– 5044, 1998 [PubMed] [Google Scholar]
- 10. Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature 387: 505– 508, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Coste O, Brenneis C, Linke B, Pierre S, Maeurer C, Becker W, Schmidt H, Gao W, Geisslinger G, Scholich K. Sphingosine 1-phosphate modulates spinal nociceptive processing. J Biol Chem 283: 32442– 32451, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Doyle T, Finley A, Chen Z, Salvemini D. Role for peroxynitrite in sphingosine-1-phosphate-induced hyperalgesia in rats. Pain 152: 643– 648, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goetzl EJ, Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest 114: 1531– 1537, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11: 946– 958, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139– 150, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359: 31– 46, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol 359: 47– 63, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol 15: 513– 520, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology 76: S3– S8, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem 265: 9308– 9313, 1990 [PubMed] [Google Scholar]
- 21. Jiang X, Zhang YH, Clark JD, Tempel BL, Nicol GD. Prostaglandin E2 inhibits the potassium current in sensory neurons from hyperalgesic Kv1.1 knockout mice. Neuroscience 119: 65– 72, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol 27: 3429– 3440, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanno T, Nishizaki T. Endogenous sphingosine 1-phosphate regulates spontaneous glutamate release from mossy fiber terminals via S1P(3) receptors. Life Sci 89: 137– 140, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Kanno T, Nishizaki T, Proia RL, Kajimoto T, Jahangeer S, Okada T, Nakamura S. Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience 171: 973– 980, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum 54: 742– 753, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Aδ or Aα/β-fibres. J Exp Physiol 87: 239– 244, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci 8: 2394– 2405, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mair N, Benetti C, Andratsch M, Leitner MG, Constantin CE, Camprubí-Robles M, Quarta S, Biasio W, Kuner R, Gibbins IL, Kress M, Haberberger RV. Genetic evidence for involvement of neuronally expressed S1P1receptor in nociceptor sensitization and inflammatory pain. PLoS One 6: e17268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346– 349, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355– 360, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta 1768: 923– 940, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci 19: 1844– 1854, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muscoli C, Doyle T, Dagostino C, Bryant L, Chen Z, Watkins LR, Ryerse J, Bieberich E, Neumman W, Salvemini D. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J Neurosci 30: 15400– 15408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okada T, Kajimoto T, Jahangeer S, Nakamura S. Sphingosine kinase/sphingosine 1-phosphate signalling in central nervous system. Cell Signal 21: 7– 13, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol 174: 1153– 1158, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Olivera A, Rivera J. An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease. Adv Exp Med Biol 716: 123– 142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316: 295– 298, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Patti GJ, Yanes O, Shriver LP, Courade JP, Tautenhahn R, Manchester M, Siuzdak G. Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nat Chem Biol 8: 232– 234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rasmussen R. Quantification on the LightCycler. In: Rapid Cycle Real-time PCR, Methods and Applications, edited by Meuer S, Wittwer C, Nakagawara K. Heidelberg, Germany: Springer, 2001, p. 21– 34 [Google Scholar]
- 41. Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 8: 753– 763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol 5: 560– 570, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 78: 743– 768, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Roviezzo F, Brancaleone V, De Gruttola L, Vellecco V, Bucci M, D'Agostino B, Cooper D, Sorrentino R, Perretti M, Cirino G. Sphingosine-1-phosphate modulates vascular permeability and cell recruitment in acute inflammation in vivo. J Pharmacol Exp Ther 337: 830– 837, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Roviezzo F, Del Galdo F, Abbate G, Bucci M, D'Agostino B, Antunes E, De Dominicis G, Parente L, Rossi F, Cirino G, De Palma R. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci USA 101: 11170– 11175, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem 92: 913– 922, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309: 1735– 1739, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Sim-Selley LJ, Goforth PB, Mba MU, Macdonald TL, Lynch KR, Milstien S, Spiegel S, Satin LS, Welch SP, Selley DE. Sphingosine-1-phosphate receptors mediate neuromodulatory functions in the CNS. J Neurochem 110: 1191– 1202, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397– 407, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Swan DJ, Kirby JA, Ali S. Vascular biology: the role of sphingosine 1-phosphate in both the resting state and inflammation. J Cell Mol Med 14: 2211– 2222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60: 181– 195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol 142: 229– 240, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci 10: 3470– 3478, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Waeber C, Chiu ML. In vitro autoradiographic visualization of guanosine-5'-O-(3-[35S]thio)triphosphate binding stimulated by sphingosine 1-phosphate and lysophosphatidic acid. J Neurochem 73: 1212– 1221, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Weigert A, Weis N, Brüne B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology 214: 748– 760, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Xie W, Strong JA, Kays J, Nicol GD, Zhang JM. Knockdown of the sphingosine-1-phosphate receptor S1PR1 reduces pain behaviors induced by local inflammation of the rat sensory ganglion. Neurosci Lett 515: 61– 65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang AH, Ishii I, Chun J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim Biophys Acta 1582: 197– 203, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Zhang G, Contos JJ, Weiner JA, Fukushima N, Chun J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene 227: 89– 99, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol 96: 1042– 1052, 2006 [DOI] [PubMed] [Google Scholar]