Abstract
Bone marrow mononuclear cells (BMMNCs) increase capillary density and reduce fibrosis in rodents after myocardial infarction, resulting in an overall improvement in left ventricular function. Little is known about the effectiveness of BMMNC therapy in hypertensive heart disease. In the current study, we show that delivery of BMMNCs from hypertension protected SS-13BN/MCWi donor rats, but not BMMNC from hypertension susceptible SS/MCWi donor rats, resulted in 57.2 and 83.4% reductions in perivascular and interstitial fibrosis, respectively, as well as a 60% increase in capillary-to-myocyte count in the left ventricles (LV) of hypertensive SS/MCWi recipients. These histological changes were associated with improvements in LV compliance and relaxation (103 and 46.4% improvements, respectively). Furthermore, improved diastolic function in hypertensive SS/MCWi rats receiving SS-13BN/MCWi derived BMMNCs was associated with lower clinical indicators of heart failure, including reductions in end diastolic pressure (65%) and serum brain natriuretic peptide levels (49.9%) with no improvements observed in rats receiving SS/MCWi BMMNCs. SS/MCWi rats had a lower percentage of endothelial progenitor cells in their bone marrow relative to SS-13BN/MCWi rats. These results suggest that administration of BMMNCs can prevent or reverse pathological remodeling in hypertensive heart disease, which contributes to ameliorating diastolic dysfunction and associated symptomology. Furthermore, the health and hypertension susceptibility of the BMMNC donor are important factors influencing therapeutic efficacy, possibly via differences in the cellular composition of bone marrow.
Keywords: consomic, rat, progenitor, cardiac failure, diastolic dysfunction
the diagnosis of heart failure with preserved ejection fraction (HFPEF) has increased in prevalence in past decades (19). It is generally believed that the etiology of HFPEF involves pathology in mechanisms underlying diastolic function of the heart, including altered myocyte relaxation dynamics and reduced compliance of the left ventricular (LV) tissue (13). Hypertension significantly increases risk of HFPEF, and diastolic dysfunction is highly prevalent among patients with elevated blood pressure.
While blood pressure reduction is a major treatment goal in hypertensive heart disease, reducing ischemia and reversing pathological remodeling are key to preventing mortality and morbidity due to diastolic dysfunction (29). Recent evidence from work using bone marrow mononuclear cells (BMMNC) to treat myocardial infarction has shown a robust proangiogenic and antifibrotic effect of exogenous BMMNC therapy in ischemic hearts (8). Similar remodeling benefits have also been observed in models of myocarditis and dilated cardiomyopathy (11, 27) and in pressure-overload caused by aortic banding (25). There are currently no studies that have examined specific benefits of BMMNC administration to prevent or reverse diastolic dysfunction during the complex systemic condition of hypertension. In clinical trials investigating treatment with BMMNC following myocardial infarction mixed results and overall weak treatment effects have been observed (20), suggesting that additional preclinical research is necessary to better understand the mechanisms underlying beneficial effects of BMMNC.
The Dahl salt-sensitive (DahlS) rat is a rodent model of human genetic salt-sensitive hypertension (4). When placed on an 8% salt diet, DahlS rats rapidly develop hypertension, leading to pronounced LV hypertrophy and eventually heart failure and death (10). One study to date has examined the effect of cell therapy for treating heart failure in the DahlS rat on 8% NaCl diet. Skeletal myoblasts isolated from DahlS donor rats reduced fibrosis and increased fractional shortening (FS) in hypertensive DahlS recipient rats (24). The effect of myoblasts on capillary density and diastolic function was not tested. Furthermore, skeletal myoblasts injection may increase risk of adverse side effects, as evidence suggests they may not integrate into the functional syncytium of the heart and, therefore, may contribute to arrhythmias (7). Thus, additional research is needed to explore the effect of other autologously derived cells such as BMMNCs on remodeling and diastolic dysfunction in DahlS hearts.
The SS/MCWi rat has been inbred from the DahlS parent strain at the Medical College of Wisconsin and exhibits both salt-sensitive hypertension and microvascular rarefaction (3, 5). Recent work has also shown that BMMNC derived from the SS/MCWi rat are not capable of inducing neovascularization in a hindlimb angiogenesis model (6). Genetic introgression of chromosome 13 from the nonhypertensive Brown Norway (BN) rat onto the SS/MCWi background (SS-13BN/MCWi rat) rescues the SS/MCWi rat from salt-sensitive hypertension and vessel rarefaction (5) and also restores the capability of BMMNC to promote skeletal muscle angiogenesis (6). Thus there is a gene or genes located on chromosome 13 important for the therapeutic efficacy of BMMNC for neovascularization in the hindlimb. As mentioned above, there is a need for additional preclinical studies into the mechanisms that explain the benefit of BMMNC therapy as well as the reduced efficacy of cells derived from patients with cardiovascular risk factors such as hypertension. Comparisons between SS/MCWi and SS-13BN/MCWi rats could provide a valuable tool for pursuing such questions, though it is currently unclear whether exogenous administration of BMMNC from either SS/MCWi or SS-13BN/MCWi rats would be beneficial in the diseased heart. Based on the aforementioned rational, our study had two main purposes: to determine whether injection of BMMNC from the hypertension protected and immuno-compatible SS-13BN/MCWi donors would improve fibrosis, capillary density, and diastolic dysfunction in the hearts of hypertensive SS/MCWi rats and to examine whether BMMNCs from hypertension susceptible SS/MCWi donor rats have impaired therapeutic efficacy relative to SS-13BN/MCWi BMMNCs.
MATERIALS AND METHODS
Animal procedures.
Male SS/MCWi rats (n = 42) were fed 0.4% NaCl chow (Dyets, Bethlehem, PA) from weaning (3 wk old). At 8 wk of age, most animals were switched to an 8% NaCl chow (Dyets) for 2 wk, and then salt content was reduced to 1% NaCl chow (Purina, St. Louis, MO) until the termination of the study when the animals were between 13 and 14 wk of age. A separate group of rats were maintained at 0.4% NaCl for the entire study duration. Between 11 and 12 wk of age (3–4 wk on high-salt diet), four separate groups of animals received tail vein injections of either phosphate-buffered saline (PBS) vehicle or BMMNCs from 10 wk old SS/MCWi or SS-13BN/MCWi donor rats. Study groups were as follows: 1) Low-salt diet (0.4% NaCl) + PBS vehicle (n = 9); 2) High-salt diet + PBS vehicle (n = 9); 3) High-salt diet + SS-13BN/MCWi BMMNC (n = 12); 4) High-salt diet + SS/MCWi BMMNC (n = 12). For BMMNC-injected groups, 3 × 107 cells suspended in 500 μl of PBS were injected via a 23-gauge needle directly into the tail vein during isoflurane anesthesia. Vehicle-treated rats received 500 μl of PBS with no cells. Following injections, rats were monitored for an additional 2 wk before terminal functional assessment and death. At time of death, the lungs, liver, kidneys, and heart of each rat were excised and weighed. Tibia length was measured to serve as a control for body size. All animal experiments were approved by the Medical College of Wisconsin Animal Care Committee.
Isolation of BMMNC.
Tibiae and femurs of 10 wk old male SS-13BN/MCWi or SS/MCWi donor rats were flushed with MCDB131 media (US Biologicals, Swampscott, MA) supplemented with 2% fetal bovine serum (GIBCO by Invitrogen, Carlsbad, CA). Marrow tissue was dissociated into a single cell solution by gentle passage through an 18-gauge needle and filtered through a 100 μm cell strainer. Mononuclear cell fractions from whole bone marrow solutions were obtained by layering cell solutions onto Histopaque 1083 (Sigma-Aldrich, St. Louis, MO) followed by centrifugation at 400 g for 30 min and collection of the opaque middle layer. Mononuclear cell fractions containing the BMMNC were rinsed once in PBS, followed by two washes in PBS + 2% bovine serum albumin (Sigma), and counted on a hemocytometer. The desired cell dose (3 × 107) was aliquoted for each recipient animal, rinsed once and suspended in 500 μl of PBS vehicle for injection.
Noninvasive echocardiography to assess LV structure and function.
Echocardiographic assessments were performed at baseline, 2 wk after start of high-salt diet, prior to injection, and 2 wk after cell injection on a GE Vivid 7 ultrasound machine (GE Healthcare, Waukesha, WI) equipped with a 10 mHz transducer. Rats were anesthetized with anesthesia cocktail: 70 mg/kg Ketamine (Phoenix Pharmaceuticals, St. Joseph, MO), 4 mg/kg Xylazine (Lloyd laboratories, Shenandoah, IA), 1 mg/kg Acepromazine (Boehringer Ingelheim Vetmedica, St. Joseph, MO), and echocardiographic images were recorded in the apical four-chamber, parasternal long axis (PLA) and short axis (PSA) views. Motion mode (M-mode) was used to calculate left atrial chamber diameter in PLA view. Left ventricular posterior wall thickness and chamber dimensions in both systole (LVDs) and diastole (LVDd) were calculated in m-mode from PSA images. %FS was calculated as %FS = (LVDd-LVDs)/LVDd * 100. Diastolic function was assessed in apical four-chamber view using pulse wave Doppler measurement of blood flow velocity at the mitral valve leaflets during early (E) and late (A) phases of diastole. Tissue Doppler measurements at the septal mitral valve annulus (E′) were also performed.
Pressure-volume assessments.
Following terminal echocardiography, a Millar SP-838 micromanometer and conductance catheter (Millar, Houston, TX) was introduced into the LV via the right carotid artery. Volume calibration was performed using hypertonic saline (50 μl, 15% NaCl) and cuvette conductance according to the manufacturer's instructions. Pressure-volume (PV) relationships were recorded at steady state (SS), and in response to preload reduction due to brief occlusion of the inferior vena cava (VCo). Both SS and VCo measurements were obtained at baseline and following a 5 min intravenous infusion of 10 and then 40 μg/kg per min of the adrenergic receptor agonist dobutamine-HCl (Sigma). From VCo measurements, the end-systolic pressure-volume relationship (ESPVR) was plotted and fit with a linear regression, while the end-diastolic pressure-volume relationship (EDPVR) was plotted and fit with an exponential regression equation. Relaxation time constants were calculated from SS loops using the equation of Weiss et al. (26).
Immunohistochemistry.
Following PV analysis, hearts were excised, weighed, and immersed briefly in a 500 mM KCl solution before being cut midventricle. The chamber of the upper (base) half was filled with Tissue Tek optimal cutting temperature freezing compound (Sakura, Torrance, CA) and snap-frozen in a dry ice slurry with 2-methylbutane (Sigma-Aldrich). Cross sections 5 μm thick were cut on a cryostat (Richard Allen Scientific, Kalamazoo, MI) and then stained for capillaries (mouse anti-CD31; BD Pharmingen, San Diego, CA) and myocytes (rabbit anti-Caveolin-3; Abcam, Cambridge, MA) as previously described (16). Four high-power fields (×40) were captured midventricle at the anterior, posterior, septal, and lateral LV wall. Using Metamorph software (Molecular Devices, Sunnyvale, CA), we traced myocyte borders to determine cross-sectional area, and the number of CD31+ capillaries adjacent to each individual myocyte was determined. Average capillary-to-myocyte ratios were determined for each animal.
To measure fibrosis, the lower (apical) half of the heart was fixed in 10% formalin solution in phosphate buffer and then paraffin embedded with an automatic tissue processor (Microm HMP 300). Embedded tissues were cut in 3 μm sections (Microm HM355S) and mounted on silanized/charged slides. Slides were stained with Gomori's One-Step Trichrome to visualize collagen fibrils. To assess interstitial fibrosis (IF) at least 25 ×20 images were captured from multiple regions of the heart, and data were analyzed with Metamorph image analysis software. Fibrosis was calculated as percentage of total tissue stained blue for collagen in each ×20 field, excluding any perivascular regions, measured with Metamorph image analysis software. Perivascular fibrosis (PVF) was quantified separately by capturing ×20 images of at least 10 cross-sectional vessels of varying size. Lumen area, vessel wall area, and area of PVF were traced separately in Metamorph software and PVF area was normalized to lumen area for each vessel. Total wall area, normalized to lumen area, was quantified to assess vessel wall thickening.
Biochemical assays.
As a clinical indicator of heart failure, serum brain natriuretic peptide (BNP) was assessed with an ELISA kit according to the manufacturer's instructions (AssayPro, St. Charles, MO). Assays of renal function were performed on the ACE Alera Clinical Chemistry System (Alfa Wasserman, Caldwell, NJ). Blood urea nitrogen (BUN) was measured indirectly via the enzymatic method described by Talke and Schubert (22). Creatinine was measured directly via the Jaffe reaction (1).
Labeling of BMMNC with quantum dots and detection of cells in heart tissue.
BMMNC from 10–11 wk old SS/MCWi or SS-13BN/MCWi donors were labeled with QD625 quantum dots (QD) (Invitrogen), according to the manufacturer's instructions with slight modification of QD labeling concentration (2 μl of quantum dots per 3 × 107 cells were used). QD labeling was confirmed with flow cytometry, and labeled BMMNC (3 × 107 per rat) were injected via tail vein into hypertensive SS/MCWi rats after 5 wk high-salt diet feeding. Three days following injection, rats were killed, their hearts excised and weighed, then cut into four cross sections from apex to base and frozen in a dry-ice slurry. For each section of each heart, at least four 10 μm thick slices were made, two serial sections and then two additional serial sections 200–300 μm deeper into the tissue. A total of 16 × 10 μm thick sections were analyzed for each heart, and the number of QD-positive cells was counted manually. Detection of QD-positive cells within lung, spleen, and kidney was also examined visually but not quantified. Bone marrow from QD-injected rats was analyzed for QD cell homing on an LSRII flow cytometer (BD Biosciences, San Jose, CA).
Endothelial progenitor cell quantification.
BMMNC from SS/MCWi and SS-13BN/MCWi donors were washed free of media and fixed in a 2% paraformaldehyde solution for 10 min. The cells were then permeabilized with a 90% methanol solution for 30 min on ice. After blocking the samples with serum, we incubated the samples with monoclonal antibodies against CD34 [Courtesy of Jan E. Schnitzer (23), Prism, San Diego, CA] followed by PE-conjugated secondary antibody (Invitrogen) and with a monoclonal antibody against FLK-1 (Santa Cruz Biotechnology) followed by FITC-conjugated secondary antibody (Invitrogen). Secondary antibody only labeled cells served as controls. Each analysis included 50,000 events and was run on the LSRII Flow Cytometer (Becton Dickinson).
Quantitative PCR.
Mononuclear cell fractions were obtained from 8–10 wk old male SS-13BN/MCWi or SS/MCWi rats as described earlier. Following density centrifugation with Histopaque 1083 (Sigma), cells were rinsed once in PBS, pelleted at 300 g for 8 min, and processed with the Qiagen RNeasy kit (Valencia, CA) per manufacturer's instructions. Purified RNA was analyzed and quantified on an Agilent 2100 Bioanalyzer with RNA 6000 Nano chips (Agilent, Santa Clara, CA). Samples were prepared for qPCR with the Taqman one-step RT-PCR Master Mix (Applied Biosystems) and PrimeTime Mini qPCR assays from Integrated DNA Technologies (IDT, Coralville, IA) for interleukin 10 (IL10, Rn.PT.49a.11620095), Fas ligand (FASLG, Rn.PT.49a.12307874), chemokine receptor type 4 (CXCR4, Rn.PT.49a.7414783), B-cell lymphoma 2 (BCL2, Rn.PT.49a.7362966), or the Taqman Ribosomal control kit (Applied Biosystems) prepared per manufacturer's instructions. Samples were run on a 7900HT real-time PCR machine (Applied Biosystems) and analyzed as described by Knoll et al. (15).
Statistical analysis.
One-way analysis of variance was used to assess differences in test variables across the four treatment groups studied. A significance value of P < 0.05 was used, and Student-Neumann-Keuls post hoc testing was performed for all pairwise comparisons. Statistics were performed using SigmaPlot (Systat software, Chicago, IL) statistical software, version 11.0. All results are plotted as means ± SE. Independent t-tests were performed in Microsoft Excel for qPCR, cell homing, and EPC enumeration comparisons.
RESULTS
Effects of BMMNC on salt-sensitive hypertension and heart failure symptomology.
All groups of SS/MCWi rats fed high-salt diet exhibited LV hypertrophy within the first 2 wk of the study (Fig. 1) and had significantly elevated mean arterial, systolic, and diastolic blood pressures (Table 1). Injection of BMMNC from either donor strain had no effect on blood pressure, LV hypertrophy, or renal function as indicated by serum creatinine and BUN (Table 1).
Fig. 1.
Development of left ventricular (LV) hypertrophy on high-salt diet. SS/MCWi rats developed marked hypertrophy after 2 wk on 8% NaCl diet. This hypertrophy was sustained after reducing to 1% NaCl and for the remainder of the 6 wk study. Injection of bone marrow mononuclear cells (BMMNCs) did not affect LV wall thickness. Data displayed as means + SE. Low salt control (◆, n = 9); high salt ± vehicle (▫, n = 9); high salt + SS-13BN/MCWi BMMNCs (△, n = 12); high salt + SS/MCWi BMMNCs (○, n = 12). *P < 0.05 vs. low salt control.
Table 1.
Indicators of hypertension and development of LVH
| Low Salt Vehicle | High Salt Vehicle | High Salt SS-13BN/MCWi MNC | High Salt SS/MCWi MNC | Anova (P value) | |
|---|---|---|---|---|---|
| Mean arterial pressure, mmHg | 109.8 ± 3.0 | 147.7 ± 5.5* | 139.5 ± 5.0* | 137.0 ± 6.6* | 0.03 |
| Systolic pressure, mmHg | 128.9 ± 3.7 | 184.0 ± 6.8* | 170.3 ± 6.2* | 168.7 ± 8.7* | <0.001 |
| Diastolic pressure, mmHg | 90.6 ± 3.8 | 123.3 ± 4.5* | 115.6 ± 4.3* | 112.4 ± 5.4* | <0.001 |
| Left atria diameter, mm | 4.1 ± 0.03 | 4.7 ± 0.03 | 4.0 ± 0.02 | 4.4 ± 0.02 | 0.24 |
| BNP, pg/ml | 174.2 ± 16.5 | 294.3 ± 45.6 | 234.4 ± 38.5 | 447.5 ± 60.8* | 0.012 |
| Tibia length, cm | 4.0 ± 0.02 | 3.9 ± 0.04* | 3.9 ± 0.03* | 3.9 ± 0.03* | 0.031 |
| Body weight, g/cm tibia length | 89.4 ± 1.3 | 94.5 ± 3.1 | 93.3 ± 2.5 | 95.8 ± 1.6 | 0.05 |
| Heart weight, g/cm tibia length | 0.35 ± 0.01 | 0.42 ± 0.01* | 0.41 ± 0.01* | 0.42 ± 0.01* | <0.001 |
| Liver weight, g/cm tibia length | 3.73 ± 0.11 | 4.19 ± 0.18 | 4.12 ± 0.14 | 4.2 ± 0.14 | 0.12 |
| Lung weight, g/cm tibia length | 0.37 ± 0.01 | 0.42 ± 0.02 | 0.37 ± 0.02 | 0.41 ± 0.01 | 0.08 |
| Kidney weight, g/cm tibia length | 0.41 ± 0.01 | 0.59 ± 0.03* | 0.53 ± 0.02 | 0.52 ± 0.05 | 0.01 |
| Serum creatinine, mg/dl | 0.59 ± 0.01 | 0.71 ± 0.09 | 0.66 ± 0.04 | 0.63 ± 0.00 | 0.34 |
| BUN, mg/dl | 27.4 ± 1.24 | 30.2 ± 0.33 | 33.0 ± 3.38 | 29.7 ± 1.69 | 0.41 |
Values are expressed as means ± SE. LVH, left ventricular hypertrophy; BNP, brain natriuretic peptide; BUN, blood urea nitrogen.
Significant vs. low salt.
Improvements in diastolic function following BMMNC administration.
Several complementary indices were used to assess cardiac function (Table 2). Echocardiography was used to monitor diastolic function over time. The typical indices of diastolic impairment, E/A ratio and E/E′, were not different across any groups, including low- vs. high-salt animals. In contrast, closer analysis of E-wave dynamics revealed an increased deceleration slope and reduced E-wave duration in high salt diet-fed animals. Treatment with SS-13BN/MCWi BMMNC, but not SS/MCWi cells resulted in 13 and 22% improvements in E-wave slope and E-wave duration, respectively, relative to high salt vehicle-treated animals. No differences in FS were observed across any groups.
Table 2.
LV function
| Low Salt Vehicle | High Salt Vehicle | High Salt SS-13BN/MCWi MNC | High Salt SS/MCWi MNC | Anova (P value) | |
|---|---|---|---|---|---|
| Fractional shortening, % | 46.9 ± 2.3 | 45.6 ± 2.2 | 45.0 ± 1.7 | 44.3 ± 1.7 | 0.82 |
| E/A | 1.46 ± 0.09 | 1.36 ± 0.05 | 1.25 ± 0.05 | 1.42 ± 0.08 | 0.19 |
| E/E′ | 21.5 ± 1.0 | 23.0 ± 1.3 | 22.3 ± 0.9 | 25.1 ± 1.3 | 0.18 |
| E slope, m/s2 | 21.1 ± 1.1 | 26.0 ± 1.5* | 22.9 ± 0.8 | 25.9 ± 1.7* | 0.04 |
| E time/E vel, ms/m | 48.1 ± 3.0 | 37.7 ± 2.7* | 43.0 ± 2.1 | 40.7 ± 2.6* | 0.03 |
| End systolic volume, μl | 238.9 ± 38 | 357.1 ± 31 | 310.1 ± 33 | 269.1 ± 38 | 0.15 |
| End diastolic volume, μl | 361.9 ± 43 | 444.9 ± 46 | 411.1 ± 38 | 385.7 ± 40 | 0.58 |
| End systolic pressure, mmHg | 108.7 ± 6.2 | 148.5 ± 7.7* | 133.7 ± 8.5 | 124.7 ± 6.3 | 0.01 |
| End diastolic pressure, mmHg | 5.1 ± 1.3 | 9.1 ± 1.8* | 6.5 ± 0.8 | 10.0 ± 1.9* | 0.02 |
| +dP/dt, mmHg/s | 7732 ± 780 | 8718 ± 606 | 8018 ± 1059 | 7960 ± 1174 | 0.98 |
| −dP/dt, mmHg/s | −7136 ± 826 | −8031 ± 797 | −7161 ± 1164 | −7002 ± 979 | 0.95 |
| ESPVR, mmHg/μl | 0.43 ± 0.05 | 0.50 ± 0.07 | 0.42 ± 0.08 | 0.50 ± 0.08 | 0.82 |
| +dP/dt reserve† | 2.4 ± 0.2 | 1.8 ± 0.1 | 2.1 ± 0.2 | 1.9 ± 0.2 | 0.21 |
Values are expressed as means ± SE.
Significant vs. low salt;
fold change from baseline after 5 min infusion of 40 μg/kg dobutamine.
Terminal PV analyses showed an increase in end diastolic pressure (EDP) in vehicle and SS/MCWi BMMNC-treated hypertensive rats, which was significantly lower in SS-13BN/MCWi BMMNC-treated rats (Table 2). Concomitant to EDP, we observed an improved tau relaxation index and a reduction in the volume-independent stiffness coefficient derived from EDPVR analysis among SS-13BN/MCWi BMMNC-treated rats relative to vehicle-treated rats (Fig. 2). Hypertensive rats injected with SS/MCWi BMMNCs were not different from vehicle-treated rats. Overall, these data suggest improved diastolic function in the LV of hypertensive rats receiving SS-13BN/MCWi, but not SS/MCWi BMMNCs. Assessments of SS (ESPVR, dP/dt) or stressed (dP/dt reserve) systolic function were not different between treatment groups (Table 2) as expected in HFPEF.
Fig. 2.
Pressure-volume analysis of LV diastolic function. A: hypertensive SS/MCWi rats on high-salt diet had significantly prolonged relaxation dynamics (tau) relative to low-salt counterparts. SS-13BN/MCWi BMMNCs resulted in improved tau to a level not significantly different from normal low-salt group, while SS/MCWi BMMNC treated showed no improvement. B: similar to trends observed with Tau, the end-diastolic pressure-volume relationship stiffness coefficient was elevated in hypertensive SS/MCWi rats relative to vehicle, improved by SS-13BN/MCWi BMMNC injection but not SS/MCWi BMMNC injection. *P < 0.05 vs. low-salt control.
Changes in fibrosis and microvessel density.
Representative images of histological data are presented in Fig. 3. High-salt animals injected with vehicle had increased PVF and IF, which was significantly reduced among SS-13BN/MCWi, but not SS/MCWi, BMMNC-treated rats (Fig. 4, A and B). The same trend was observed when we examined capillary density. The number of CD31+ capillaries adjacent to individual LV myocytes was reduced in high salt vehicle-treated rats (Fig. 4C). Animals injected with SS-13BN/MCWi BMMNCs had a significantly improved capillary-to-myocyte ratio relative to vehicle-treated rats, whereas SS/MCWi BMMNC-treated animals were not different from vehicle control.
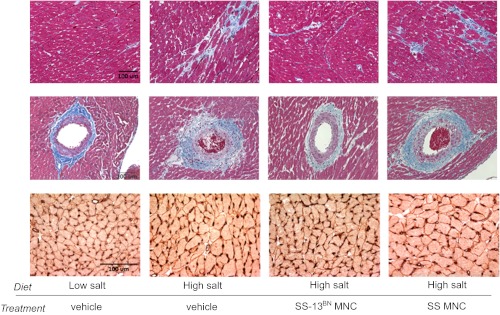
Fig. 3.
Representative images of histological data. Top two rows provide examples of Gomori trichrome-stained LV sections with blue staining for interstitial (top) and perivascular (middle) fibrosis. In the bottom row, red caveolin-3-stained myocyte borders were traced and black CD31 stained capillaries adjacent to each myocyte were counted. Scale bars represent 100 μm.
Fig. 4.
Quantification of LV fibrosis and capillary density. Interstitial (A) and perivascular (B) fibrosis were reduced in SS-13BN/ MCWi BMMNC-treated rats, but not SS/MCWi BMMNC-treated rats, relative to vehicle treatment. C: capillary-to-myocyte ratios were reduced in SS/MCWi rats with hypertension and significantly improved by treatment with SS-13BN/MCWi but not SS/MCWi BMMNCs. *P < 0.05 vs. low salt, #P < 0.05 vs. high salt + vehicle.
Detection of BMMNC in the heart after intravenous delivery.
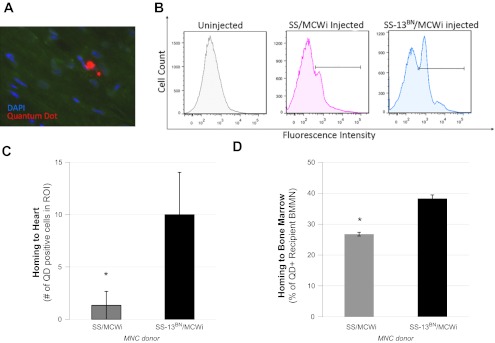
We used QD-labeled cells to investigate if intravenously injected BMMNC home to the hearts of our hypertensive recipient rats (Fig. 5). We detected 5, 7, and 18 QD-positive cells in 16 sections each of three separate hearts from SS-13BN/MCWi BMMNC-injected animals, and 4, 0, and 0 QD-positive cells in hearts from SS/MCWi BMMNC-injected animals (Fig. 5C). For both treatments, there were occasional QD-positive cells found in lung sections, a very small occurrence of positive cells in kidney, with the most QD-positive cells detected in the spleen (data not shown). We also evaluated rehoming of BMMNC from either donor strain into recipient bone marrow and found that a significantly higher fraction of BMMNC derived from SS-13BN/MCWi donors, rats relative to SS/MCWi donors, had localized back into bone marrow of hypertensive recipients (38.3 ± 2.9% vs. 26.7 ± 3.8% of all recipient cells, respectively) (Fig. 5D).
Fig. 5.
Tracking of intravenously injected quantum dot (QD)-labeled BMMNCs. A: representative image of positive QD cells in the heart. B: examples of QD detection in bone marrow samples using flow cytometry. Horizontal lines represent fluorescence intensity considered “positive” for the QD signal. Quantification of QD-positive BMMNCs homing to the heart (C) and bone marrow (D) of SS/MCWi recipient rats. Significantly more QD-positive cells were observed in animals injected with SS-13BN/MCWi BMMNCs relative to SS/MCWi BMMNCs. *P < 0.05 relative to SS/MCWi injected.
Expression of selected chromosome 13 genes.
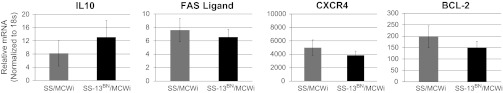
Based on the observation of reduced function and apparent homing to recipient tissues of SS/MCWi relative to SS-13BN/MCWi BMMNC we employed Ingenuity Pathway Analysis to screen the 936 annotated genes on rat chromosome 13 (rat genome database, http://rgd.mcw.edu) for possible functional roles in BMMNC survival and homing. From this analysis, three genes known to be involved in bone marrow cell survival and apoptosis (BCL2, FASLG, IL10) and one gene involved in BMMNC homing (CXCR4) were selected for comparison of expression between SS/MCWi and SS-13BN/MCWi-derived BMMNC, with no expression differences detected for any candidate genes (Fig. 6).
Fig. 6.
Expression of selected genes on chromosome 13. Gene ontology was used to select 4 genes from rat chromosome 13 related to bone marrow cell homing, survival, or other functions related to cardiac therapy for quantitative polymerase chain reaction analysis. No significant difference in expression of these 4 genes between strains was detected.
Enumeration of putative endothelial progenitor cells in bone marrow.
In a final experiment, we hypothesized that the reduced therapeutic efficacy of SS/MCWi BMMNC may be associated with reduction in a key subtype of cells within the bone marrow known to be involved in the promotion of angiogenesis in damaged myocardium, the CD34+/VEGFR2+ endothelial progenitor cell (EPC). Flow cytometry experiments indicated significantly fewer putative EPCs within the bone marrow of SS/MCWi relative to SS-13BN/MCWi rats (Fig. 7).
Fig. 7.
Enumeration of putative endothelial progenitor cells within the BMMNC fraction. A lower percentage of cells expressing both the vascular endothelial growth factor type 2 and CD34 proteins (i.e., putative endothelial progenitors) were detected in the bone marrow of SS/MCWi rats relative to SS-13BN/MCWi rats. *P < 0.05.
DISCUSSION
In this study we have shown that delivery of competent BMMNCs to hypertensive rats can improve diastolic function and reduce clinical signs of heart failure. We observed elevated mean, systolic, and diastolic blood pressures, significant ventricular hypertrophy, PVF and IF, elevated levels of circulating BNP, reduced E-wave duration, and increased E-wave deceleration slope, increases in end diastolic left ventricular pressure, a reduced tau relaxation, and reductions in capillary density in the hypertensive SS/MCWi rats. Treatment with SS-13BN/MCWi but not SS/MCWi derived BMMNCs specifically improved nearly every cardiac phenotype including BNP, E wave dynamics, end diastolic left ventricular pressure, tau and capillary density, without affecting blood pressure or renal function.
This is the first study to investigate the use of BMMNC to improve remodeling and function in heart disease caused specifically by complex systemic hypertension. Furthermore, we have focused on an early stage of hypertensive heart disease characterized by diastolic dysfunction with essentially normal systolic function. This approach, while less common in studies investigating hypertensive heart disease in salt-sensitive rats where end-stage heart failure is a more common end point, could ultimately have clinically relevant implications for prevention of progressive end-stage heart damage caused by hypertension. HFPEF is of increasing clinical importance. Diastolic dysfunction is quite prevalent, affecting as many as 30% of adults in the general population. In fact, Owan et al. (19) report that at least 50% of all heart failure cases are diagnosed as HFPEF.
We assessed diastolic dysfunction with several complimentary parameters. We observed the same trends between our groups across nearly all of our indicators of diastolic dysfunction. Reductions in E-wave deceleration time and increased E-wave slope observed in echocardiographic analyses indicate a more restrictive filling pattern and increased filling pressures (2), and these indicators were improved in animals that received SS-13BN/MCWi BMMNC. While we did not see corroborating evidence of restrictive filling in our E/A or E/E′ measurements, the animals may have progressed to a “pseudonormal” stage of diastolic dysfunction where the impact of restrictive filling is counteracted by increased filling pressures. The tendency for E- and A-waves to overlap in rodents due to high heart rates (2) also may have confounded E/A and E/E′ calculation. Large-scale studies in humans have suggested that E deceleration time and E-slope may be more sensitive indicators of diastolic dysfunction than E/A (18), which is a proposal that warrants further investigation. In support of our echo data, results of PV analysis clearly show that end diastolic pressure, LV compliance, and relaxation dynamics were all impaired during early hypertensive heart disease and improved upon treatment with SS-13BN/MCWi BMMNCs. These functional observations were further supported by our histological analysis.
Numerous studies have investigated possible underlying mechanisms of action for BMMNCs when administered to rats after myocardial infarction (14, 17, 30). Evidence for both direct integration of cells, to revascularize and regenerate damaged muscle tissue, as well as indirect paracrine actions, such as the secretion of proangiogenic and/or antifibrotic growth factors and cytokines, has been found. The studies generating this evidence have predominantly come from models of myocardial infarction, where large regions of cardiac tissue are completely destroyed and thus myocyte regeneration and extensive revascularization are necessary. The current experiments are some of the first to examine BMMNC effects in hypertensive heart disease, where myocyte regeneration is not a likely candidate for therapeutic benefit. Therefore, we hypothesize that during hypertension and diastolic dysfunction, the mechanism of action of BMMNC likely involves enhanced vascularization of hypertrophied tissue, which prevents tissue ischemia and subsequent fibrosis. Our observations of increased capillary density and reduced fibrosis in the hearts of SS-13BN/MCWi BMMNC-treated hypertensive SS/MCWi rats are consistent with this hypothesis.
We observed a very small number of cells that actually integrated into heart tissue, with the majority of injected cells detected in spleen and bone marrow of recipient rats. We cannot rule out loss of QD signal, either via exocytosis of the QD label or dilution of the signal subsequent to cell division and proliferation, and therefore results of these cell tracking experiments should be interpreted with caution. Assuming the QD data accurately represent cell homing, these data suggest that in our model BMMNC do not appear to localize in great numbers to the heart and, therefore, may mediate their beneficial effect from a site distal to the heart (21). Under that scenario, injected cells could secrete cytokines and other factors from the spleen, bone marrow, or other tissues that then act distally to effect angiogenesis and cardiac function. It will be important for following studies to identify the molecular factors mediating the therapeutic effects identified in the current paper.
An additional and important finding is that BMMNC derived from prehypertensive SS/MCWi donor rats failed to evoke the same improvements as cells derived from normotensive SS-13BN/MCWi rats. This observation is consistent with previous reports that bone marrow-derived progenitor cells, specifically EPCs, from hypertensive humans and animals are functionally impaired and are reduced in number (9, 28). Reduced efficacy of autologous donor cells is a major obstacle for ultimate clinical application of cell-based therapy. Impaired function in BMMNC derived from diseased patients has prompted a call for more thorough understanding of the molecular mechanisms that are crucial to efficacy in cell-based cardiac therapy. In a preliminary attempt to identify potential molecular players underlying our clear differences in therapeutic efficacy, we compared expression of selected genes present on rat chromosome 13 that are annotated in the ingenuity pathway analysis database as involved in BMMNC motility (CXCR4), as well as overall therapeutic competence (IL10) and survival (FASLG, BCL2), and found no differences in expression between any of these candidates. Interpretation of these expression differences in BMMNC is potentially confounded, however, by the heterogeneous nature of this tissue. The mononuclear fraction of bone marrow is composed of a mix of many different cell types, dominated by cells involved in immune function (e.g., B cells, T cells, macrophages, etc.). The subset of cells thought to mediate beneficial remodeling in cardiac disease, namely EPCs and/or mesenchymal stem cells, represent a very small fraction of the overall population of BMMNC (<1–2% by most estimates) (12). Therefore, global expression of potential therapeutic mediators in mixed BMMNC could be misleading, as expression patterns specific to the relevant cell type for cardiac remodeling benefits may be masked by expression of a given target in irrelevant but more abundant cells. This heterogeneity is reflected in the high degree of variability in gene expression within each of our treatment groups. Thus, to ultimately understand the mechanisms underlying the beneficial effects of BMMNC therapy it will be necessary to identify and target the specific cells responsible. Along these lines, we detected a smaller percentage of EPCs in SS/MCWi bone marrow relative to SS-13BN/MCWi. Lower numbers of proangiogenic EPCs in SS/MCWi may explain our observations of reduced capillary density and subsequent failure of BMMNC to improve function in SS/MCWi BMMNC-treated animals. Future studies are necessary to test whether EPCs are the mediating cells driving the improved remodeling and function observed in the current study. If this is found to be true, then additional work characterizing gene and protein expression between therapeutically competent SS/MCWi rats and therapeutically incompetent SS-13BN/MCWi rats may be very fruitful toward understanding the mechanisms driving the beneficial effect of BMMNC therapy for hypertensive heart disease.
The main purpose of this study was to determine whether BMMNC administration would be beneficial in animals exhibiting LV hypertrophy and diastolic dysfunction due exclusively to hypertension without additional ischemic insult. We have shown that administration of BMMNCs from a nonhypertensive donor is associated with beneficial remodeling and improved diastolic function in the LV of recipient rats with high salt-induced hypertension. In addition, we have identified the SS/MCWi rat as a genetic model of impaired BMMNC therapeutic efficacy in hypertensive heart disease, exhibiting reduced numbers of proangiogenic EPCs. With the model now characterized in the context of heart disease, future experiments capitalizing on the genetic similarities and differences between therapeutically competent SS-13BN/MCWi and incompetent SS/MCWi bone marrow-derived cells, particularly EPCs, may lead to significant advances toward understanding key questions about the mechanisms of donor cell survival, engraftment, and ultimate therapeutic effect in regard to bone marrow cell-derived therapy for heart failure.
GRANTS
This work was funded National Heart, Lung, and Blood Institute Grants HL-082798 and HL-007852, as well as by a generous donation from Drs. Robert D. and Patricia E. Kern.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.P. conception and design of research; S.J.P., D.N.D., J.R.K., T.J.S., and B.E. performed experiments; S.J.P., D.N.D., and T.J.S. analyzed data; S.J.P., J.R.K., and A.S.G. interpreted results of experiments; S.J.P., D.N.D., and J.R.K. prepared figures; S.J.P. and T.J.S. drafted manuscript; S.J.P., D.N.D., T.J.S., B.E., and A.S.G. edited and revised manuscript; A.S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Carol Bobrowitz for assistance with histology.
REFERENCES
- 1.Chromý V, Rozkošná K, Sedlák P. Determination of serum creatinine by Jaffe method and how to calibrate to eliminate matrix interference problems. Clin Chem Lab Med 46: 1127–1133, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Coatney RW. Ultrasound imaging: principles and applications in rodent research. Inst Lab Anim Res J 42: 233–247, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Dahl LK, Knudsen KD, Heine MA, Leitl GJ. Modification of experimental hypertension in the rat by variations in the diet. Circ Res 22: 11–18, 1968 [DOI] [PubMed] [Google Scholar]
- 5.de Resende MM, Amaral SL, Moreno C, Greene AS. Congenic strains reveal the effect of the renin gene on skeletal muscle angiogenesis induced by electrical stimulation. Physiol Genomics 33: 33–40, 2008 [DOI] [PubMed] [Google Scholar]
- 6.de Resende MM, Stodola TJ, Greene AS. Role of the renin angiotensin system on bone marrow-derived stem cell function and its impact on skeletal muscle angiogenesis. Physiol Genomics 42: 437–444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouts K, Fernandes B, Mal N, Liu J, Laurita KR. Electrophysiological consequence of skeletal myoblast transplantation in normal and infarcted canine myocardium. Heart Rhythm 3: 452–461, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol 37: 1726–1732, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension 55: 1389–1397, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Inoko M, Kihara Y, Morii I, Fujiwara H, Sasayama S. Transition from compensatory hypertrophy to dilated, failing left ventricles in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 267: H2471–H2482, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Ishida M, Tomita S, Nakatani T, Fukuhara S, Hamamoto M, Nagaya N, Ohtsu Y, Suga M, Yutani C, Yagihara T, Yamada K, Kitamura S. Bone marrow mononuclear cell transplantation had beneficial effects on doxorubicin-induced cardiomyopathy. J Heart Lung Transplant 23: 436–445, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 47: 126–131, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kass DA, Bronzwaer JGF, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 94: 1533–1542, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kinnaird T, Stabile E, Burnett MS, Epstein SE. Bone marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res 95: 354–363, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Knoll KE, Pietrusz JL, Liang M. Tissue-specific transcriptome responses in rats with early streptozotocin-induced diabetes. Physiol Genomics 21: 222–229, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kriegel AJ, Greene AS. Substitution of Brown Norway chromosome 16 preserves cardiac function with aging in a salt-sensitive Dahl consomic rat. Physiol Genomics 36: 35–42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 50: 280–289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra RK, Galloway JM, Lee ET, Best LG, Russell M, Roman MJ, Devereux RB. The ratio of mitral deceleration time to E-wave velocity and mitral deceleration slope outperform deceleration time alone in predicting cardiovascular outcomes: the Strong Heart Study. J Am Soc Echocardiogr 20: 1300–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature 451: 937–942, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol 296: H1888–H1897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talke H, Schubert GE. Urease/GLDH system applied to a somogyl deproteinized supernate. Klin Wochenschr 43: 174, 1965 [DOI] [PubMed] [Google Scholar]
- 23.Testa JE, Chrastina A, Oh P, Li Y, Witkiewicz H, Czarny M, Buss T, Schnitzer JE. Immunotargeting and cloning of two CD34 variants exhibiting restricted expression in adult rat endothelia in vivo. Am J Physiol Lung Cell Mol Physiol 297: L251–L262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toh R, Kawashima S, Kawai M, Sakoda T, Ueyama T, Satomi-Kobayashi S, Hirayama S, Yokoyama M. Transplantation of cardiotrophin-1-expressing myoblasts to the left ventricular wall alleviates the transition from compensatory hypertrophy to congestive heart failure in Dahl salt-sensitive hypertensive rats. J Am Coll Cardiol 43: 2337–2347, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Hu Q, Mansoor A, Lee J, Wang Z, Lee T, From AHL, Zhang J. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol 290: H1393–H1405, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest 58: 751–760, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner L, Deutsch V, Barshack I, Miller H, Keren G, George J. Transfer of endothelial progenitor cells improves myocardial performance in rats with dilated cardiomyopathy induced following experimental myocarditis. J Mol Cell Cardiol 39: 691–697, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Yao EH, Fukuda N, Matsumoto T, Katakawa M, Yamamoto C, Han Y, Ueno T, Kobayashi N, Matsumoto K. Effects of the antioxidative beta-blocker celiprolol on endothelial progenitor cells in hypertensive rats. Am J Hypertens 21: 1062–1068, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Yip GW, Fung JWH, Tan YT, Sanderson JE. Hypertension and heart failure: a dysfunction of systole, diastole or both? J Hum Hypertens 23: 295–306, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Yoon CH, Koyanagi M, Iekushi K, Seeger F, Urbich C, Zeiher AM, Dimmeler S. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation 121: 2001–2011, 2010 [DOI] [PubMed] [Google Scholar]